Abstract
Neutrophil to Lymphocyte Ratio (NLR) was recently demonstrated as a useful index in predicting the prognosis of Non-Small Cell Lung Cancer (NSCLC). Thus, a meta-analysis was performed to demonstrate the relationship between NLR and overall survival (OS), progress-free survival (PFS) or disease free survival (DFS) in patients with NSCLC. We searched for relevant literatures in PubMed, EMBASE and Cochrane library and pooled the eligible studies and synthesized hazard ratios (HRs) using Stata 12.0. Final analysis of NSCLC patients from 12 eligible studies was performed. Combined HR suggested that high NLR had an unfavorable effect on patients’ OS (n=1700 in 11 studies; HR= 1.43, 95% CI: 1.25-1.64; I^2=80.2%, P<0.01) and PFS (n=664 in 5 studies, HR=1.37, 95% CI: 1.07-1.74; I^2=70.8%, P=0.004). Subgroup analysis based on cutoff shown that, compared with other subgroups, the subgroup with a cutoff of 5 had a significantly poorer survival (HR=1.87, 95% CI 1.49-2.34) with less heterogeneity (I^2=21.3%, P=0.28). However, subgroup analysis based on treatment method indicated that the “surgery” subgroup seemed to have not a significant impact on survival (HR=1.32, 95% CI 0.99-1.77; I^2=80.0%, P=0.063) compared with the chemotherapy subgroup (HR=1.61, 95% CI 1.24-2.10; I^2=82.6%, P<0.01). Additionally, combined odds ratio (OR) suggested high NLR was associated inversely with response to treatment (n = 276 in 2 studies; OR = 1.73, 95% CI: 1.04-2.88; I^2=0%, P=0.40). This study suggests high NLR (especially with a cutoff of 5) seems to be associated with a worse prognosis in patients with NSCLC as well as a worse response to treatments.
Keywords: Neutrophil to lymphocyte ratio, NLR, non-small cell lung cancer, NSCLC, overall survival, progress-free survival, treatment response
Introduction
Lung cancer is the leading cause of cancer-related death world-wide, accounting for about 1.1 million deaths per year [1]. Non-small cell lung cancer (NSCLC) is responsible for 85% of the total cases [2]. Although great progress made in the past decade, especially the emergence of molecular targeting therapy, has revolutionized the treatment of lung cancer, the prognosis of lung cancer is still unsatisfactory, with a five year overall survival rate of about 15% [2]. So it is necessary to identify potential prognostic indicators, especially the easy-to-access laboratory indexes for oncology doctors to well manage this strong fatal disease.
In recent years, an increasing number of studies have indicated that NLR is a useful biomarker to measure the inflammatory status of immune system [3]. The latest research suggested that NLR had a prognostic value in predicting the survival of patients with NSCLC [4-7]. Additionally, growing evidence showed that the neutrophils located in tumor stroma predict unfavorable outcomes, while tumor related lymphocytes have been associated with a better prognosis [8-10]. All taken into consideration, we deduce that NLR might have be a practical prognostic index for patients with NSCLC and performed a meta-analysis to verify it.
Methods
Search strategy
We searched PubMed, EMBASE and Cochrane library for relevant articles until March, 2014. Articles are identified using the following terms: ((lung OR pulmonary) AND (neoplasm OR cancer OR carcinoma OR tumor)) AND (neutrophil lymphocyte OR neutrophil to lymphocyte OR NLR). We restricted the above words/phrases in title/abstract and reviewed the references of included literatures for potential eligible articles.
Selection criteria
All eligible articles focusing on correlation between NLR and OS or PFS and/or clinicopathological features and treatment response in patients with NSCLC were included in this meta-analysis. All retrieved articles were carefully reviewed to identify potential relevant studies. Articles included in our meta-analysis should meet the following criteria as follows: 1) patients pathologically diagnosed as non-small cell lung cancer; 2) NLR was measured by serum based methods and should be detected before treatments; 3) information between neutrophil-lymphocyte ratio (NLR) and overall survival (OS), progress-free survival (PFS) or disease-free survival (DFS), and/or clinicopathological figures and treatment response; 4) sufficient information provided to estimate hazard ratio (HR) and 95% confidence interval (CI) of OS, PFS or DFS; 5) If multiple studies investigated the same patients or partially overlapping patients, only the most complete single study was selected. We excluded literatures, such as letters, reviews, case reports, conference abstracts editorials and articles with sample size less than 20.
Data extraction
Two reviewers (Peng and Wang) independently scanned the title and abstract of the potentially eligible articles. All candidate articles with full-text were retrieved to review, if articles could not be categorized based on title and abstract. Articles were independently read and checked according to the inclusion/exclusion criteria in this study. Any disagreement in the course of quality assessment was discussed and resolved together.
Quality assessment
In this study, we adopted the Newcastle-Ottawa Scale (NOS) which was designed for retrospective and prospective studies, to assess study quality (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). The Scale includes three parts: selection (4 points), comparability (2 points), and outcome assessment (3 points). The maximum value is 9 points.
Statistical analysis
In this meta-analysis, OR was only used to estimate the association between NLR and treatment response, since there was no sufficient studies focusing on the relationship between NLR and Clinicopathological characteristics. Therefore, the number of events (no treatment response) compared to the total number of patients in each group was subjected for the analysis of each variable.
As for OS and PFS, HR with its 95% CI was used to evaluate the significance of NLR on them. For survival data presented as Kaplan-Meier curves, we extracted and calculated HR and its 95% CI according to the methods described by Parmar [11]. Kaplan-Meier curves were read by Engauge Digitizer version 4.1 (http://digitizer.sourceforge.net/). When statistical parameters were displayed as both multivariate analysis and univariate analysis, we chose the former for data combination. In very rare cases, when a study had multiple cut-offs, we use the HR of “top versus bottom group” for pooling [12]. Heterogeneity was tested by using the Cochran’s test (Chi squared test) and by quantifying the inconsistency (I^2). A fixed-effect model was employed when heterogeneity was not detected (P:0.10); otherwise, a random-effect model was used. Robustness of the pooled results was verified by one-way sensitivity analysis, of which, every single study was deleted and the pooled HR and 95 % CI as well as the tests for heterogeneity of the remaining studies were calculated. Funnel plots were presented to visualized publication bias. All statistical calculations including graphical presentations were performed by Stata 12.0 (STATA Corporation, College Station, TX, USA).
Results
Characteristics of studies
The initial search through PubMed, EMBASE and Cochrane library yielded 146 studies (Figure 1). One hundred and twelve studies were excluded by title or abstract. Upon full text review of the remaining 34 articles, another 22 studies had to be excluded as they were either articles unrelated with the topic (n=2), duplicate publication (n=3), conference abstracts without full text (n=12), reports without associating NLR with survival parameters, such as OS/DFS/PFS (n=2), or articles without sufficient data (n=3). Finally, twelve studies with a total number of 2377 patients were recruited into our meta-analysis to determine the value of NLR as prognostic and clinicopathological markers in NSCLC [4-7,13-20]. Among the twelve articles included, one article could be split into two “sub-studies”, given that it provided the survival data between NLR and PFS based on two different treatment methods.
Figure 1.
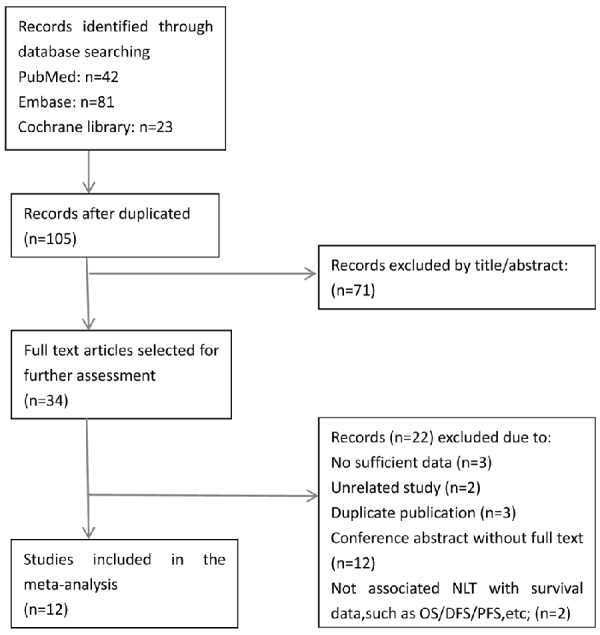
Flow chart representing the process of literature search and study selection.
Study demographics and quality
All articles were retrospective studies directly investigated NLR as a prognostic factor. Of the twelve eligible articles, four were performed in Turkey, two in both UK and Japan, and one in China, Korea, US and Spain, respectively. The median value of the mean age was 60.5 (range 57-68). Males accounted for 60.5% of patients from twelve reported studies. Advanced stage (stage III + IV) was observed in majority of patients with a weighted average of 74.4%. Histology was described in three categories as squamous cell carcinoma (28.6%), adenocarcinoma (56.0%) or other types (15.4%) in ten reported studies. Patients from nine studies underwent chemotherapy, while those from three studies underwent surgery. Eight studies were estimated by multivariate analysis, while four studies were estimated by univariate analysis. Five studies had a NLR cutoff of 5, three between 3 and 4, two between 2 and 3, one applied tertiles and one used quartiles. OS was reported or estimated in eleven studies, whereas PFS was only provided in five studies. The scores of study quality assessed by Newcastle-Ottawa quality assessment scale ranged from 5 to 7. The basic features of the twelve studies were summarized in Table 1.
Table 1.
Clinical and methodological characteristics of included studies
| Author | Years | Country | No. of patients | Gender (M/F) | Mean age (y) | UICC-stages (III/IV vs I/II) | Histology (SCC/ADC/others) | treatment methods | follow-up median (mon) | Cut-off value | survival analysis | HR estimate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KM Sarraf | 2009 | UK | 177 | NA | 63 | 49/128 | 56/86/35 | surgery | 29 | tertile | OS | MV |
| S Teramukai | 2009 | Japan | 388 | 276/112 | 65 | 388/0 | 76/274/38 | Chemo | 19 | quartile | OS/PFS | MV |
| M Tomita | 2011 | Japan | 284 | 178/106 | 67 | 54/230 | ?/208/76 | surgery | 60.7-131.7 | 2.5 | OS | MV |
| S Cedres | 2012 | Spain | 171 | 143/28 | 63 | 171/0 | 31/69/71 | Chemo | 9.1 | 5 | OS/PFS | MV/UV |
| Y Lee | 2012 | Korea | 199 | 17/182 | 57 | 199/0 | 0/199/0 | Chemo | 36 | 3.25 | OS/PFS | MV/MV |
| C Botta(1) | 2013 | USA | 73 | 55/18 | 58 | 73/0 | 11/58/4 | chemo+beva | 15 | 4 | PFS | UV |
| C Botta(2) | 2013 | USA | 39 | 26/13 | 68 | 39/0 | 11446 | Chemo | 15 | 4 | PFS | UV |
| D Unal | 2013 | Turkey | 94 | 88/6 | 58 | 85/9 | 66/15/13 | Chemo | NA | 3.44 | OS | UV |
| M Yildirim | 2013 | Turkey | 95 | 77/18 | 59 | 95/0 | NA | Chemo | 14 | 5 | OS | UV |
| V Kaya | 2013 | Turkey | 156 | 80/76 | 60 | 156/0 | 85/44/21 | Chemo | 12.5 | 5 | OS | UV |
| YW yao | 2013 | China | 182 | 119/63 | 59 | 182/0 | 48/128/6 | Chemo | NA | 2.63 | OS/PFS | MV/MV |
| DJ Pinato | 2014 | UK | 220 | 110/110 | 65 | 29/191 | 53/132/35 | surgery | NA | 5 | OS | MV |
| T Kacan | 2014 | Turkey | 299 | 270/29 | 61 | 250/49 | 124/71/104 | NA | NA | 5 | OS | MV |
HR: hazard ratio, NA: not available, Mon: month, SCC: squamous cell carcinoma, ADC: adenocarcinoma, others: other types of lung cancer, MV: multivariate analysis, UV: univariate analysis, OS: overall survival, PFS: progress-free survival, Chemo: chemotherapy, beva: bevacizumab.
Meta-analysis
Nine out of the twelve studies had provided the follow up time. For the overall population, high NLR was found to be significantly associated with poor OS (HR=1.43, 95% CI: 1.25-1.64; I^2=80.2%, P<0.01) and PFS (HR=1.37, 95% CI: 1.07-1.74; I^2=70.8%, p=0.004) (Figure 2A and 2B). Furthermore, the studies were stratified to evaluate HR of OS by study region, statistical method to estimate HR, cutoff value and treatment method (shown in Table 2). To analyze study region on evaluating HR, the studies from Asia countries showed a statistically significant HR of 1.56 (95% CI 1.24-1.96, P<0.01), however, the studies from Western countries almost had no significance (HR=1.53, 95% CI 0.97-2.40, P=0.065). In analyzing statistical method on evaluating HR, combined HR of studies by multivariate analysis was 1.27 (95% CI 1.12-1.43, P<0.01), while combined HR by univariate analysis was 1.96 (95% CI 1.54-2.50, P<0.01). A subgroup analysis was also performed for studies with respect to cutoff value in predicting OS. The results indicated that both cutoff value =5 and cutoff value between 2 and 3 showed statistically significant HR of 1.87 (95% CI 1.49-2.34, P<0.01) and 1.40 (95% CI 1.07-1.82, P=0.014), respectively. Whereas cutoff value between 3 and 4 and multiple cut-offs were not statistically significant with a pooled HR of 1.32 (95% CI 0.78-2.23, P=0.31) and 1.25 (95% CI 0.90-1.74, P=0.185), respectively. When studies were st-ratified by treatment method, combined HR of studies with chemotherapy showed a prognostic HR of 1.61 (95% CI 1.24-2.10, P<0.01), while surgery subgroup showed non-statistically significant HR of 1.32 (95% CI 0.99-1.77, P=0.063). Given the small number of studies for PFS, the subgroup analysis was abandoned.
Figure 2.
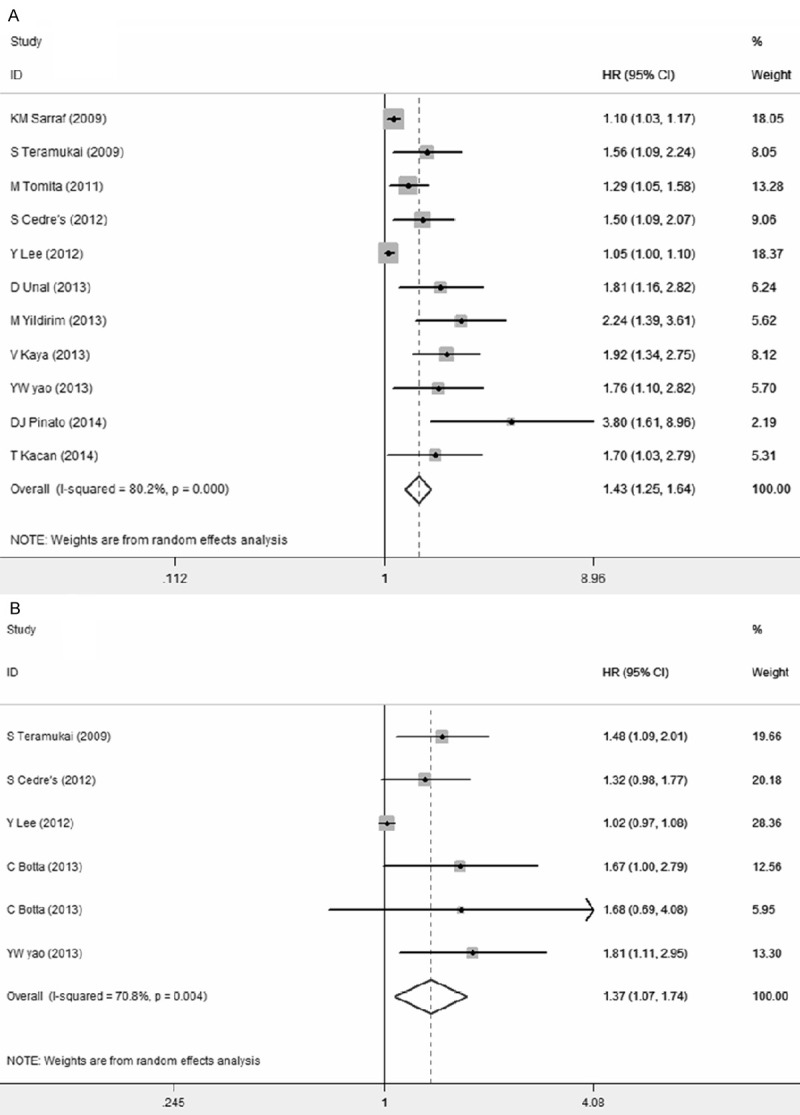
Meta-analysis of the association between NLR and OS (A), and PFS (B) in patients with NSCLC. Results are presented as the individual and summarized Hazard ratios (HR) with 95% confidence interval (CI).
Table 2.
Subgroup analysis of summarized Hazard ratios (HRs) reflecting the association between Neutrophil to Lymphocyte Ratio (NLR) and overall survival (OS) in NSCLC
| Subgroup | NO. Of studies | Cases | Pooled-data-(random) | Test-for-heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| HR | 95 % CI | P value | Chi^2 | P-value | I^2 (%) | |||
| Area | ||||||||
| Asia | 8 | 1697 | 1.56 | 1.24-1.96 | <0.01 | 39.16 | <0.001 | 82.1 |
| Western | 3 | 568 | 1.53 | 0.97-2.40 | 0.065 | 11.23 | 0.004 | 82.2 |
| Survival analysis | ||||||||
| Multivariate | 8 | 1920 | 1.27 | 1.12-1.43 | <0.01 | 27.85 | <0.001 | 74.9 |
| Univariate | 3 | 345 | 1.96 | 1.54-2.50 | <0.01 | 0.44 | 0.80 | 0 |
| Cutting value | ||||||||
| =5 | 5 | 941 | 1.87 | 1.49-2.34 | <0.01 | 5.08 | 0.28 | 21.3 |
| 3-4 | 2 | 293 | 1.32 | 0.78-2.23 | 0.31 | 5.75 | 0.016 | 82.6 |
| 2-3 | 2 | 466 | 1.40 | 1.07-1.82 | 0.014 | 1.41 | 0.236 | 28.9 |
| Multiple cut-offs | 2 | 565 | 1.25 | 0.90-1.74 | 0.185 | 3.51 | 0.061 | 71.5 |
| Treatment | ||||||||
| Surgery | 3 | 681 | 1.32 | 0.99-1.77 | 0.063 | 9.98 | 0.007 | 80.0 |
| Chemotherapy | 8 | 1584 | 1.61 | 1.24-2.10 | <0.01 | 40.28 | <0.001 | 82.6 |
In addition, we investigated the association between NLR and treatment response with a pooled OR of 1.73 (95% CI 1.04-2.88, P=0.035; I^2=0%, P=0.40), suggesting that NLR has a potential prognostic value (Figure 3).
Figure 3.
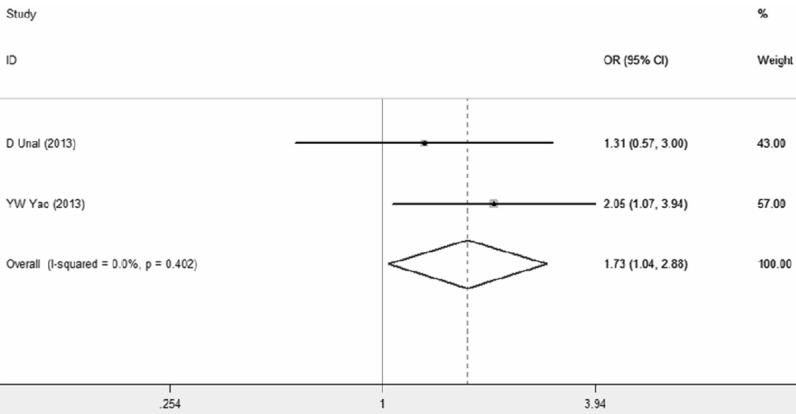
Meta-analysis of studies investigating the association between NLR and treatment response. Forest plot present odds ratio (OR) and 95% confidence interval (CI) calculated by a fixed effect model.
Publication bias
Egger’s test for publication bias suggested that there was statistically significant for OS (P<0.01) (Figure 4).
Figure 4.
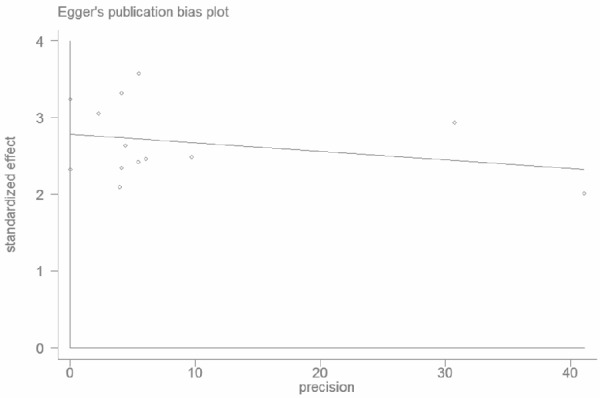
Egger’s funnel plot for detecting publication bias of OS. There is publication bias detected (P<0.01).
Sensitivity analysis
Sensitivity analysis was conducted to test the robustness of the result of OS by removing one study each time. The combined HR and its 95% CIs were not significantly altered when any study was excluded, suggesting that any single study had not significant impact on the combined result and confirmed the robustness of the outcome of this study.
Discussion
Although great progress has been made in the treatment of NSCLC, the overall survival still has enough space for improvement. So, potential biomarkers for predicting or improving the outcomes have been explored. In recent years, the prognosis of NLR has been studied in NSCLC, so we conducted a meta-analysis to clarify the role of pretreatment NLR in the prognostic significance of patients with NSCLC. The pooled HR indicated that elevated NLR was associated with poor OS and PFS. Besides, high NLR predicted worse response to chemotherapy. Subgroup analysis suggested that a higher NLR implied a much worse OS. Subgroup by treatment method showed that elevated NLR had a prognostic significance in patients treatment by chemotherapy than surgery, however, more studies were needed to consolidate or overthrow the conclusion since only three studies were conducted based on the method of surgery. We did not make a meta-analysis on the correlation between NLR and clinicopathological characteristics due to a lack of adequate studies with sufficient data. In addition, subgroup analysis for PFS based on study region had suggested that elevated NLR had an unfavorable impact on patients with NSCLC in western countries compared with Asian countries.
Apart from NSCLC, some other solid tumors including hepatocellular carcinoma, colorectal cancer, and gastric cancer showed similar conclusion based on pooled analysis [21,22]. The relationship between inflammation and tumor has been studied for several decades. The exact mechanism between the elevated NLR and poor prognosis in these cancer patients was still undefined. Evidence has shown that neutrophils are implicated in the promotion of metastasis in patients with pulmonary carcinoma and are prognostic indicator for patients with advanced NSCLC [23]. On the contrary, decreased lymphocyte has been demonstrated as biomarker of poor survival for patients with terminal cancer due to the key role of lymphocytes in killing cancer cells and regulating the proliferation, apoptosis, angiogenesis and metastasis of cancer by secreting cytokine [24-26]. Overall, NLR reflects the balance between tumor destruction and tumor protection.
The limitations in this meta-analysis should be addressed. Obviously, the heterogeneity was significant in both pooled HR of OS (I^2=80.2%, P<0.001) and PFS (I^2=70.8%, P=0.004). There were many possible causes to explain the heterogeneity, such as histology type, UICC stage differences, study country, treatment method, method to detect the laboratory index (such as neutrophil count and lymphocyte count), NLR cutting value, HR estimate method, quality of study and potential publication bias. To explore the possible causes of heterogeneity, subgroup was divided according to the mentioned items above. However, subgroup analysis did not eliminate the high heterogeneity based on study region and treatment method. Further analysis showed that the subgroup with a cut-off of 5 had a decreased heterogeneity (I^2=21.3%, P=0.28), while other subgroups nearly had no obvious change. Even so, we still could not rule out the heterogeneity caused by cut-off. Additionally, from the point of clinical practice, it is necessary to unify the cut-off standard. In our meta-analysis, the included studies most set the cut-off based on the previous studies or mean of NLR, and only three studies set the cutting value by ROC. One study calculated the HR based on analysis of regression with tertiles, and another study calculated the HR based on quartiles. The method to calculate HR also contributed to the heterogeneity of pooled HR. Another disadvantage of our studies was that the publication bias was evident, and the possible causes could attribute to language restriction in the inclusion criterion or selective publication. It is worth mentioning that a total of 12 conference abstracts without full text excluded in this meta-analysis probably had a significant impact on the publication bias. By the way, because of insufficient data, we did not conduct subgroup analysis based on histology type and UICC stage differences.
In conclusion, the NLR is a useful clinical index to predict the survival and treatment response of patients with NSCLC. Subgroup analysis indicated that the patients with NSCLC treated by chemotherapy, especially those patients with a NLR more than 5 had a significant prognostic value. In future, more studies with better design are needed to confirm the conclusion.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 4.Tomita M, Shimizu T, Ayabe T, Yonei A, Onitsuka T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. 2011;31:2995–2998. [PubMed] [Google Scholar]
- 5.Lee Y, Kim SH, Han JY, Kim HT, Yun T, Lee JS. Early neutrophil-to-lymphocyte ratio reduction as a surrogate marker of prognosis in never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. J Cancer Res Clin Oncol. 2012;138:2009–2016. doi: 10.1007/s00432-012-1281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaya V, Yildirim M, Demirpence O, Yildiz M, Yalcin AY. Prognostic significance of basic laboratory methods in non- small-cell-lung cancer. Asian Pac J Cancer Prev. 2013;14:5473–5476. doi: 10.7314/apjcp.2013.14.9.5473. [DOI] [PubMed] [Google Scholar]
- 7.Kacan T, Babacan NA, Seker M, Yucel B, Bahceci A, Eren AA, Eren MF, Kilickap S. Could the Neutrophil to Lymphocyte Ratio be a Poor Prognostic Factor for Non Small Cell Lung Cancers? Asian Pac J Cancer Prev. 2014;15:2089–2094. doi: 10.7314/apjcp.2014.15.5.2089. [DOI] [PubMed] [Google Scholar]
- 8.Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, Huang XW, Fan J, Zhou J. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012;56:2242–2254. doi: 10.1002/hep.25907. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Margolin K. Tumor-infiltrating lymphocytes in melanoma. Curr Oncol Rep. 2012;14:468–474. doi: 10.1007/s11912-012-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz EC, Qu B, Hoth M. Calcium, cancer and killing: the role of calcium in killing cancer cells by cytotoxic T lymphocytes and natural killer cells. Biochim Biophys Acta. 2013;1833:1603–1611. doi: 10.1016/j.bbamcr.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 13.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 14.Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, Nakano K, Tsuboi M, Shibata K, Furuse K, Fukushima M. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: An analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer. 2009;45:1950–1958. doi: 10.1016/j.ejca.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Cedres S, Torrejon D, Martinez A, Martinez P, Navarro A, Zamora E, Mulet-Margalef N, Felip E. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. 2012;14:864–869. doi: 10.1007/s12094-012-0872-5. [DOI] [PubMed] [Google Scholar]
- 16.Botta C, Barbieri V, Ciliberto D, Rossi A, Rocco D, Addeo R, Staropoli N, Pastina P, Marvaso G, Martellucci I, Guglielmo A, Pirtoli L, Sperlongano P, Gridelli C, Caraglia M, Tassone P, Tagliaferri P, Correale P. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol Ther. 2013;14:469–475. doi: 10.4161/cbt.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unal D, Eroglu C, Kurtul N, Oguz A, Tasdemir A. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev. 2013;14:5237–5242. doi: 10.7314/apjcp.2013.14.9.5237. [DOI] [PubMed] [Google Scholar]
- 18.Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunology, Immunotherapy. 2013;62:471–479. doi: 10.1007/s00262-012-1347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yildirim M, Yildiz M, Duman E, Goktas S, Kaya V. Prognostic importance of the nutritional status and systemic inflammatory response in non-small cell lung cancer. J BUON. 2013;18:728–732. [PubMed] [Google Scholar]
- 20.Pinato DJ, Shiner RJ, Seckl MJ, Stebbing J, Sharma R, Mauri FA. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer. 2014;110:1930–5. doi: 10.1038/bjc.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue TC, Zhang L, Xie XY, Ge NL, Li LX, Zhang BH, Ye SL, Ren ZG. Prognostic Significance of the Neutrophil-to-Lymphocyte Ratio in Primary Liver Cancer: A Meta-Analysis. PLoS One. 2014;9:e96072. doi: 10.1371/journal.pone.0096072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23:31–39. doi: 10.1016/j.suronc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Wislez M, Antoine M, Rabbe N, Gounant V, Poulot V, Lavole A, Fleury-Feith J, Cadranel J. Neutrophils promote aerogenous spread of lung adenocarcinoma with bronchioloalveolar carcinoma features. Clin Cancer Res. 2007;13:3518–3527. doi: 10.1158/1078-0432.CCR-06-2558. [DOI] [PubMed] [Google Scholar]
- 24.Lissoni P, Brivio F, Fumagalli L, Messina G, Ghezzi V, Frontini L, Giani L, Vaghi M, Ardizzoia A, Gardani GS. Efficacy of cancer chemotherapy in relation to the pretreatment number of lymphocytes in patients with metastatic solid tumors. Int J Biol Markers. 2004;19:135–140. doi: 10.1177/172460080401900208. [DOI] [PubMed] [Google Scholar]
- 25.Eerola AK, Soini Y, Paakko P. A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin Cancer Res. 2000;6:1875–1881. [PubMed] [Google Scholar]
- 26.Eerola AK, Soini Y, Paakko P. Tumour infiltrating lymphocytes in relation to tumour angiogenesis, apoptosis and prognosis in patients with large cell lung carcinoma. Lung Cancer. 1999;26:73–83. doi: 10.1016/s0169-5002(99)00072-0. [DOI] [PubMed] [Google Scholar]


