Abstract
Background: Ceramic and polymer composite scaffolds are widely used in tissue engineering for bone tissue regeneration. Composite of β-tricalcium phosphate (β-TCP) and poly L-lactic acid (PLLA), due to its biocompatibility and biodegradability, is widely used in bioengineering. However, optimal ratio, porosity and pore size of this kind of scaffolds were not very clear yet. Materials and methods: We cultured osteoblastic induced rMSCs on β-TCP/PLLA scaffolds to investigate the optimum construction, which owned better properties for supporting cells growth, proliferation and differentiation. A total of 24 mice were divided into three groups: rMSCs + β-TCP/PLLA, osteoblastic rMSCs + β-TCP/PLLA and β-TCP/PLLA without cells. 8 rude mice were implanted with rMSCs + β-TCP/PLLA in the left thighs and β-TCP/PLLA without cells in the right thighs. 8 rude mice were implanted with osteoblastic rMSCs + β-TCP/PLLA in the left thighs and the same treatments in the right thighs as the above. After 8 and 12 weeks, the mice were sacrificed and implants with the surrounding tissues were harvested together. Paraffin sections were got and HE stain and Masson-Goldner stain were employed to observe the ectopic bone formation. Results: The scaffolds of β-TCP/PLLA = 2:1 significantly increased osteocalcin production of the cells. In addition, scaffolds with NaCl = 70 wt%, pore size 200~450 μm showed better compatibility to these seeding cells. A significantly larger area of bone formation in the osteoblastic rMSCs and β-TCP/PLLA composite than that in rMSCs/scaffold and in the scaffold without cells in vivo. Conclusion: compounds of osteoblastic induced rMSCs and the scaffold with β-TCP/PLLA = 2:1, NaCl = 70 wt%, pore size = 200-450 μm had good properties as a kind of bone substitute.
Keywords: Mesenchymal stem cells, osteoblastic induced, bone tissue engineering, poly-L-lactic acid (PLLA), β-tricalcium phosphate (β-TCP)
Introduction
One type of produces for bone tissue engineering combines seed cells, growth factors and scaffolds. Currently, many composite scaffolds for bone tissue engineering were made to repair and reconstruct damaged bone tissue function. The composite scaffold should provide have favorable weight ratio of compound, porosity, pore size and mechanical properties. Ceramic and polymer composite scaffolds are widely used in tissue engineering for bone tissue regeneration [1]. As a absorbable ceramic material, β-tricalcium phosphate (β-TCP) was suitably used in bone reconstruction implants for many years. However, these β-TCP implants with low stress levels have a brittle fracture behavior in the clinical applications. Biodegradable polymers, such as poly L-lactic acid (PLLA), have a modulus of elasticity closer to that of natural cortical bone, and can retain sufficient mechanical strength for providing stabilization of bone implants [2,3].
To overcome the shortcomings of these different materials, composites of β-TCP and PLLA, due to its biocompatibility and biodegradability, have been widely developed and used in bioengineering. However, optimal ratio, porosity and pore size of this kind of scaffolds were not very clear yet. In addition, the seed cell, especially the stem cell, is one of the critical factors among those three components of bone tissue engineering. The deal bio-active composite scaffold should adequate bioactivity for stem cells attachment, proliferation, migration and differentiation. So, in this study, we cultured rat bone marrow mesenchymal stem cells (rMSCs) in vitro on β-TCP/PLLA scaffolds with different ratio, different pore size and different porosity. The aim is to investigate the optimum construction of β-TCP/PLLA porous scaffold for cells growth, proliferation and differentiation, and to explore the probability of osteogenesis of the composite in vivo.
Materials and methods
Synthesis and preparation of scaffolds
The process which consists of solvent casting, compression molding and leaching stage had been used to fabricate scaffold of β-TCP/PLLA composite. Scaffolds were fabricated according to weight ratio of β-TCP/PLLA = 1:1, 1:2 and 2:1; the porosity of the composites ranges from 50~75% when the porogen NaCl added is 60 wt%, 70 wt% and 80 wt% respectively; the pore size ranges from 200 to 950 μm by adding different crystal porogen NaCl.
rMSCs culture and osteoblastic induced
Bone marrow mesenchymal stem cells were isolated and cultured according to the method of Han [4]. Briefly, the Spraque-Dawley (SD) rats 3 months old were cervical dislocated and the femurs were aseptically excised. The proximal head of the femur was clipped off and the marrow was flushed with essential primary media (DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin) by a 20 gauge needle. The suspension was spun down at 1000 rpm for 10 min, the supernatant was aspirated, and the pellet was re-suspended in fresh primary media and added into Percoll separating medium (GIBCOBRL company). The monolayer cells were harvested and washed by essential primary media. The cells were cultured in flasks in an incubator at 37°C, 5% CO2, and 95% humidity. The medium was exchanged every 3 days and the cells were passaged when they were overgrowing in the flasks. Flow cytometry was used to assay the phenotype of rMSCs. To induce spontaneous differentiation into osteoblastic cells, we prepared a differentiation medium by addition of 50 μg/ml ascorbic acid, 10 mM β-glycerophosphate and 100 nM dexamethasone to the growth medium [5-7]. Then, we detected the proliferation and differentiation of osteoblastic rMSCs in different phases [8-12].
Cell morphology and proliferation study
When rMSCs were osteoblastic induced for 10 days, the cells were digested and seeded onto φ = 10 mm β-TCP/PLLA scaffolds placed in 24 wells with 1×105 cells per ml. Fluorescence microscope and scanning electron microscope (SEM) were used to investigate the cellular morphology and attachment. MTT assay was applied to detect the proliferation of the cells on the scaffolds.
Alkaline phosphatase (ALP) activity and osteocalcin assay
ALP activity was assessed by a commercial kit (IFCC Company); measuring optical density was at 405 nm. Results equaled to the amount of ALP dividing the amount of total protein. Osteoblastic rMSCs without materials were the control group.
At 7 d and 14 d, we collected the media from the wells and used the method of radioactive immunoassay (RIA) to test the osteocalcin. Two days prior to this assaying, 10-8 M 1, 25 (OH) 2-VD3 was added to the media [13-15].
Ectopic bone formation by implantation and degradation test in vivo
All the animal experiments were approved by the Animal Research and Care Committee of the China. The mice were divided into three groups: rMSCs + β-TCP/PLLA, osteoblastic rMSCs + β-TCP/PLLA and β-TCP/PLLA without cells. All the disk scaffolds seeded with 8×105 cells [16]. 24 Male rude mice (5 weeks old) were anesthetized and placed in a prone position. The bilateral thighs were accessed via a lateral approach and intramuscular pouches were created. 8 rude mice were implanted with rMSCs + β-TCP/PLLA in the left thighs and β-TCP/PLLA without cells in the right thighs. 8 rude mice were implanted with osteoblastic rMSCs + β-TCP/PLLA in the left thighs and the same treatments in the right thighs as the above. After 8 and 12 weeks, the mice were sacrificed and implants with the surrounding tissues were harvested together. Paraffin sections were got and HE stain and Masson-Goldner stain were employed to observe the ectopic bone formation.
Statistical analysis
The software SPSS 16.0 was used to do statistical analysis. The metering data was represented by mean ± standard deviation ( x̅± s). The comparison among multiple groups was analyzed with one-way ANOVA while the comparison between two groups was detected with LSD method, with P < 0.05 considered statistically significant.
Results
Study in vitro
Through observing the cells morphology and assaying the cells surface antigen by flow cytometry (FCM), we found that the cells were CD44 positive and CD45 negative (Figure 1), which were some characters of bone marrow mesenchymal stem cells [17-21].
Figure 1.
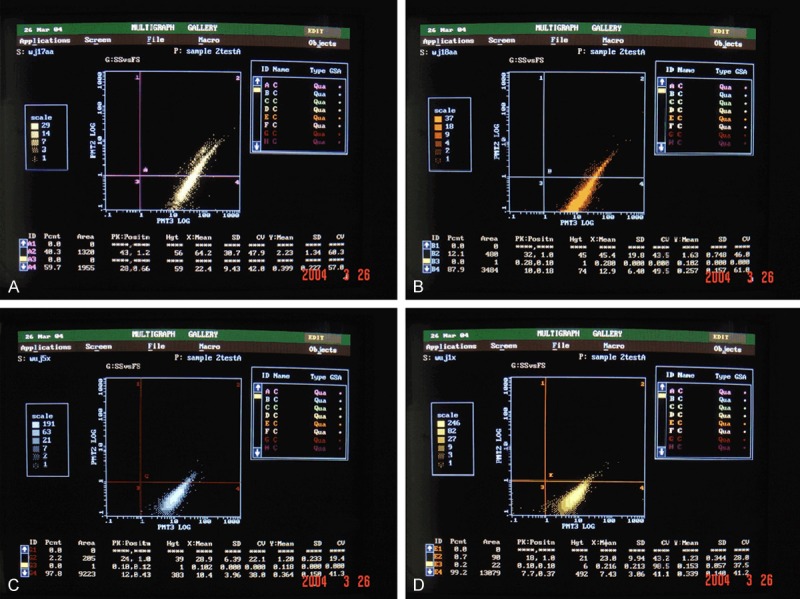
Rat bone marrow mesenchymal stem cells were CD44+ and CD45- by FCM. A: rMSCsP0; B: rMSCsP1; C: rMSCsP3; D: rMSCsP5.
Fluorescence microscope and SEM showed that osteoblastic rMSCs could attach and proliferate on the scaffolds with different ratio, different porosity and different pore size (Figures 2, 3). However, MTT assay showed that there were more osteoblastic cells grew on β-TCP/PLLA =2:1 after the 5th day (Figure 4; Table 1). On the other hand, the cell density increased significantly (P < 0.01) on β-TCP/PLLA = 2:1 compared with other ratio scaffolds (Figure 5; Table 2).
Figure 2.
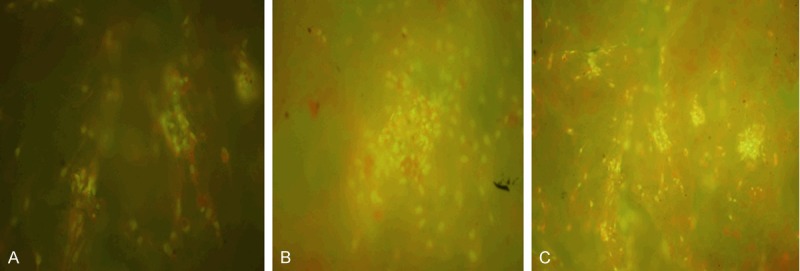
Fluorescence photomicrographs of osteoblastic rMSCs cocultured with β-TCP/PLLA of different ratio. 20×. A: β-TCP/PLLA = 1:1; B: β-TCP/PLLA = 1:2; C: β-TCP/PLLA = 2:1.
Figure 3.
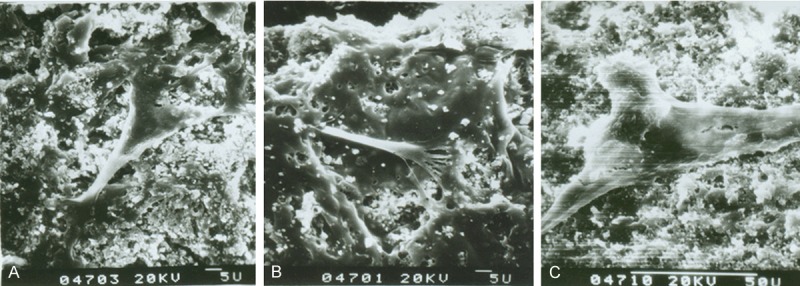
SEM of osteogenic rMSCs seeding on β-TCP/PLLA scaffolds for 4 d. A: β-TCP/PLLA = 1:1; B: β-TCP/PLLA = 1:2; C: β-TCP/PLLA = 2:1.
Figure 4.
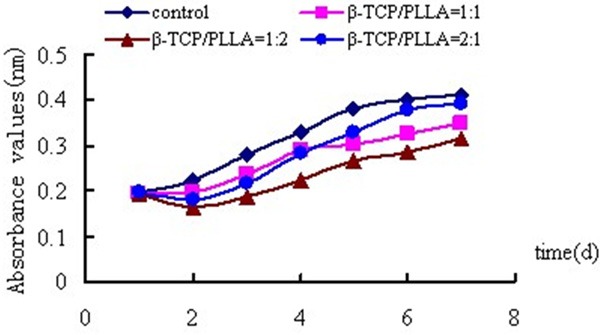
Absorbance values of osteogenic rMSCs cultured on scaffolds with different ratio (Porosity = 60%, d = 100-400 μm).
Table 1.
ALP activity of osteogenic rMSCs on β-TCP/PLLA composite with different weight percent of porogen-NaCl (β-TCP/PLLA = 2:1, d = 200-450 µm) (x̅ ± s, n=8)
| Groups | ALP (U/mg) 7 d | ALP (U/mg) 14 d |
|---|---|---|
| 60 wt% | 0.13 ± 0.01* | 0.20 ± 0.03 |
| 70 wt% | 0.15 ± 0.00 | 0.21 ± 0.03 |
| 80 wt% | 0.10 ± 0.01**,## | 0.13 ± 0.01*,# |
P < 0.05 vs NaCl = 70 wt%;
P < 0.01 vs NaCl = 70 wt%.
P < 0.05 vs NaCl = 60 wt%;
P < 0.01 vs NaCl = 60 wt%.
Figure 5.
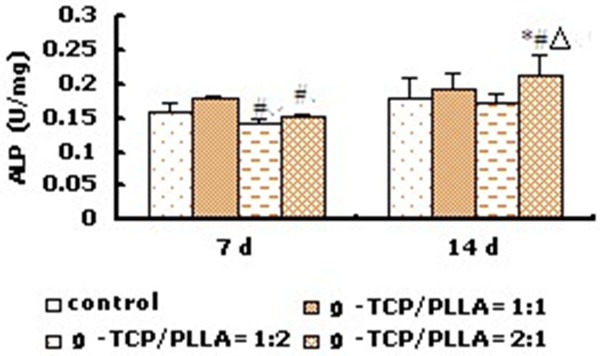
ALP activity of osteogenic rMSCs on β-TCP/PLLA composite with different ratio (Porosity = 60%, d = 100-400 μm) (n=8). *P < 0.05 vs corresponding control group; ΔP < 0.01 vs β-TCP/PLLA = 1:2; #P < 0.05 vs β-TCP/PLLA = 1:1.
Table 2.
ALP activity of osteogenic rMSCs on β-TCP/PLLA composite with different pore size (β-TCP/PLLA = 2:1, porosity = 70%) (x̅ ± s, n = 8)
| Groups | ALP (U/mg) 7 d | ALP (U/mg) 14 d |
|---|---|---|
| d=200-450 µm | 0.15 ± 0.00 | 0.21 ± 0.03 |
| d=450-900 µm | 0.15 ± 0.01 | 0.18 ± 0.01 |
Osteocalcin (OCN) production assay showed that all the composite could promote cell osteocalcin production which manifested that the compound had good osteoinductive. In detail, cells on β-TCP/PLLA = 1:2 produced less osteocalcin than that of β-TCP/PLLA = 1:1 and β-TCP/PLLA = 2:1 (P < 0.05) (Figure 6). The cells on scaffold with NaCl = 70 wt% could produce more OCN than that with NaCl = 80 wt% (Table 3) and the cells on scaffold with the pore size = 200-450 µm could produce more OCN than that with 450-900 µm (Table 4).
Figure 6.
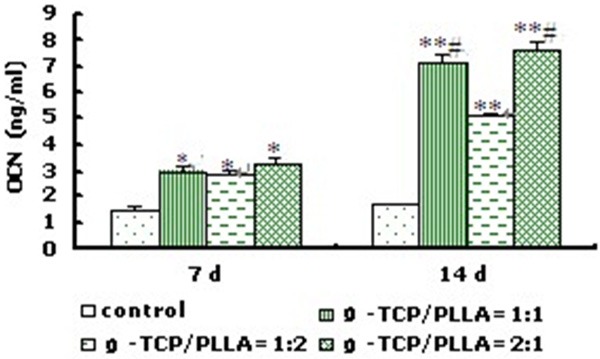
OCN production of osteogenic rMSCs on β-TCP/PLLA composite with different ratio (Porosity = 60%, d = 100-400 μm) (n=8). *P < 0.05, **P < 0.01 vs control group; #P < 0.05 vs β-TCP/PLLA = 1:2.
Table 3.
OCN production of osteogenic rMSCs on β-TCP/PLLA composite with different porosity (β-TCP/PLLA = 2:1, d = 200-450 µm) (x̅ ± s, n=8)
| Groups | OCN (ng/ml) 7 d | OCN (ng/ml) 14 d |
|---|---|---|
| 60 wt% | 2.79 ± 0.11 | 6.21 ± 0.11* |
| 70 wt% | 3.26 ± 0.29 | 7.60 ± 0.32 |
| 80 wt% | 2.47 ± 0.10* | 4.88 ± 0.67* |
P < 0.05 vs NaCl = 70 wt%.
Table 4.
OCN production of osteogenic rMSCs on β-TCP/PLLA composite with different pore size (β-TCP/PLLA = 2:1, porosity =70%) (x̅ ± s, n = 8)
| Groups | OCN (ng/ml) 7 d | OCN (ng/ml) 14 d |
|---|---|---|
| d=200-450 µm | 3.3 ± 0.29 | 7.6 ± 0.32 |
| d=450-900 µm | 3.1 ± 0.16 | 6.6 ± 0.30* |
P < 0.05 vs d = 200-450 µm.
Study in vivo
Before the designated time points after surgery, all the animals had no inflammation. The scanning electron microscope (Figure 7) and histological study (Figure 8) found that at 8 weeks and 12 weeks post-implantation, there were few cells existed in the scaffolds which had not been seeded by the cells. In addition, there were some fibrous connective tissue surrounded the scaffolds. In rMSCs plus β-TCP/PLLA group, there were some cell interconnections forming honeycomb appearance. On the brink of the scaffolds, a great quantity cells aggregated and there were some zones of necrosis. There were a few osteoblastic cells growing along with the pore. Few micro-capillary could be seen and the material was degraded slowly in this group. In the osteoblastic induced rMSCs + β-TCP/PLLA group, a layer of osseous precursor cells on the surface of the constructs, some cuboidal-shaped active osteoblasts were seen in the pores. Also, plenty of blood vessels were seen in the pores without fibrous tissue. At 12 weeks, some multinuclear cells and blood vessels were found and the material of the scaffold was degraded quickly in this group. Masson-Goldner staining showed collagenous structures on the surface of the constructs, which supported bone formation progressively.
Figure 7.
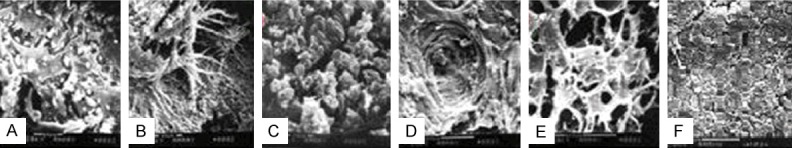
Scanning electron micrographic of ObI/scaffold, rMSCs/scaffoldand scaffold without cells transplanted intramuscular of nude mice for 8 weeks and 12 weeks. Noted: ObI/scaffold (A: 8 weeks, 2000×) (D: 12 weeks, 600×); rMSCs/scaffold (B: 8 weeks, 2000×) (E: 12 weeks, 600×); scaffold without cells (C: 8 weeks, 2000×) (F: 12 weeks, 600×). nude mice for 8 weeks and 12 weeks. Noted: composite of osteogenic rMSCs and β-TCP/PLLA scaffold (A: 8 weeks, 200×, D: 12 weeks, 100×); composites of rMSCs and β-TCP/PLLA scaffold ( B: 8 weeks, 200×, E: 12 weeks, 100×); β-TCP/PLLA scaffold (C: 8 weeks, 200×, F: 12 weeks, 100×), (ABC: HE stain; DEF: Masson stain), the arrow in A shows osteoclast and “bv” means blood vessels.
Figure 8.
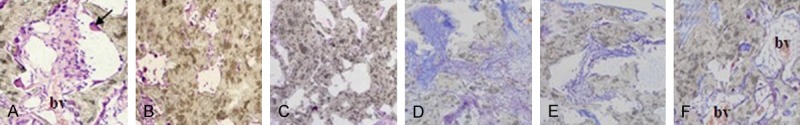
Histological section photographs of different groups transplanted intramuscular of nude mice for 8 weeks and 12 weeks. Noted: composite of osteogenic rMSCs and β-TCP/PLLA scaffold (A: 8 weeks, 200×, D: 12 weeks, 100×); composites of rMSCs and β-TCP/PLLA scaffold (B: 8 weeks, 200×, E: 12 weeks, 100×); β-TCP/PLLA scaffold (C: 8 weeks, 200×, F: 12 weeks, 100×), (ABC: HE stain; DEF: Masson stain), the arrow in A shows osteoclast and “bv” means blood vessels.
Discussion
Ideal bio-active compound requires a number of factors: cells that can undergo differentiation to form osteoblasts; biological factors that control the growth and differentiation of these cells; and osteoconductive bioresorbable scaffolding matrices that promote cellular attachment, migration, and proliferation. Composite of β-tricalcium phosphate (β-TCP) and poly L-lactic acid (PLLA) is widely used in bioengineering. However, optimal ratio, porosity and pore size of this kind of scaffolds were not very clear yet. In this study, we cultured osteoblastic induced rat bone marrow mesenchymal stem cells (rMSCs) on β-TCP/PLLA scaffolds to investigate the optimum construction, which owned better properties for supporting cells growth, proliferation and differentiation.
In studying in vitro, results found that the cells were CD44 positive and CD45 negative, which were some characters of bone marrow mesenchymal stem cells. The results suggested that the osteoblastic rMSCs came into proliferative phase, matrix synthesis phase and mineralization phase after 10, 14 and 20 days induced respectively. To evaluate the biocompatibility of the scaffold with different technical parameter and to build new type bio-active scaffold, we chose passage 2 osteoblastic induced rMSCs as seeding cells in the latter study [10-12].
In studying in vivo, the scanning electron microscope and histological study found that at 8 weeks and 12 weeks post-implantation, there were few cells existed in the scaffolds which had not been seeded by the cells. In addition, there were some fibrous connective tissue surrounded the scaffolds. This phenomenon was in according with simple β-TCP or PLLA implantation [26-31]. In rMSCs plus β-TCP/PLLA group, there were some cell interconnections forming honeycomb appearance. In the osteoblastic induced rMSCs + β-TCP/PLLA group, At 12 weeks, some multinuclear cells and blood vessels were found and the material of the scaffold was degraded quickly in this group. Masson-Goldner staining showed collagenous structures on the surface of the constructs, which supported bone formation progressively.
The results showed that, 1. The osteoblastic rMSCs came into proliferation, matrix synthesis and mineralization phase respectively after 10, 14 and 20 days induction. 2. The scaffolds of β-TCP/PLLA = 2:1 could not only promote the cells proliferation, but also significantly increased osteocalcin production of the cells. In addition, scaffolds with NaCl = 70 wt%, pore size 200~450 μm showed better compatibility to these seeding cells. 3. A significantly larger area of bone formation in the osteoblastic rMSCs and β-TCP/PLLA composite than that in rMSCs/scaffold and in the scaffold without cells in vivo. The results demonstrated that osteoblastic induced rMSCs and the scaffold with β-TCP/PLLA = 2:1, NaCl = 70 wt%, pore size = 200-450 µm had good properties as a kind of bone substitute, which could form a bone -like tissue in vivo.
Acknowledgements
The National Natural Science Foundation of China for The Youth (NO. 81301336); National Natural Science Foundation of China (Grant No. 11262020); Natural Science Foundation of Xinjiang Uygur Autonomous Region (No. 201233146-9).
Disclosure of conflict of interest
None.
References
- 1.Johnson EO, Troupis T, Soucacos PN. Tissue-engineered vascularized bone grafts: basic science and clinical relevance to trauma and reconstructive. Microsurgery. 2011;31:176–182. doi: 10.1002/micr.20821. [DOI] [PubMed] [Google Scholar]
- 2.Zhu ZJ, Shen H, Wang YP, Jiang Y, Zhang XL, Yuan GY. Effect of beta-tricalcium phosphate/poly-l-lactide composites on radial bone defects of rabbit. Asian Pac J Trop Med. 2013;6:753–6. doi: 10.1016/S1995-7645(13)60132-7. [DOI] [PubMed] [Google Scholar]
- 3.Carulli C, Matassi F, Civinini R, Innocenti M. Tissue engineering applications in the management of bone loss. Clin Cases Miner Bone Metab. 2013;10:22–5. doi: 10.11138/ccmbm/2013.10.1.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao YS, Ding H, Xie XT, Zhang CQ. Osteogenic induction protects rat bone marrow-derived mesenchymal stem cells against hypoxia-induced apoptosis in vitro. J Surg Res. 2013;184:873–879. doi: 10.1016/j.jss.2013.03.082. [DOI] [PubMed] [Google Scholar]
- 5.Hengameh A, Reyhaneh D, Nima MM, Hamed H. Effects of two bioactive materials on survival and osteoblastic differentiation of human mesenchymal stem cells. J Conserv Dent. 2014;17:349–353. doi: 10.4103/0972-0707.136509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavery K, Swain P, Falb D, Alaoui-Ismaili MH. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem. 2008;283:20948–20958. doi: 10.1074/jbc.M800850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SB, Hasegawa U, van der Vlies AJ, Sung MH, Uyama H. Preparation of poly(γ-glutamic acid)/hydroxyapatite monolith via biomineralization for bone tissue engineering. J Biomater Sci Polym Ed. 2014;2:1–16. doi: 10.1080/09205063.2014.953404. [DOI] [PubMed] [Google Scholar]
- 8.Arahira T, Todo M. Effects of Proliferation and Differentiation of Mesenchymal Stem Cells on Compressive Mechanical Behavior of Collagen/β-TCP Composite Scaffold. J Mech Behav Biomed Mater. 2014;39:218–230. doi: 10.1016/j.jmbbm.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Jian GY, Hu JG. Research of mesenchymal stem cells differentiates to osteoblast cells. Dentistry Foreign Medical Science. 2004;4:57–59. [Google Scholar]
- 10.Kasten P, Luginbühl R, van Griensven M, Barkhausen T, Krettek C, Bohner M, Bosch U. Comparison of human bone marrow stromal cells seeded on calcium deficient hydroxyapatite, β-tricalcium phosphate and demineralized bone matrix. Biomaterials. 2003;24:2593. doi: 10.1016/s0142-9612(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 11.Shindo K, Kawashima N, Sakamoto K, Yamaguchi A, Umezawa A, Takagi M, Katsube K, Suda H. Osteogenic differentiation of the mesenchymal progenitor cells, Kusa is suppressed by Notch signaling. Exp Cell Res. 2003;290:370–380. doi: 10.1016/s0014-4827(03)00349-5. [DOI] [PubMed] [Google Scholar]
- 12.Lieb E, Vogel T, Milz S, Dauner M, Schulz MB. Effects of transforming growth factor beta1 on bonelike tissue formation in three-dimensional cell culture. II: Osteoblastic differentiation. Tissue Eng. 2004;10:1414–1425. doi: 10.1089/ten.2004.10.1414. [DOI] [PubMed] [Google Scholar]
- 13.Yang M, Zhu SS, Chen Y. Studies on bone marrow stromal cells affinity of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) Biomaterials. 2004;25:1365–1373. doi: 10.1016/j.biomaterials.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Atmani H, Chappard D, Basle MF. Proliferation and differentiation of osteoblasts and adipocytes in rat bone marrow stromal cell cultures: effects of dexametha-sone and calcitriol. J Cell Biochem. 2003;89:364–372. doi: 10.1002/jcb.10507. [DOI] [PubMed] [Google Scholar]
- 15.Laure GA, Audrey R, Céline C, Hulin P, Louarn G, Heymann D, Trichet V, Layrolle P. Osteoblastic and osteoclastic differentiation of human mesenchymal stem cells and monocytes in a miniaturised three-dimensional culture with mineral granules. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.08.033. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Woodbury D, Reyno lds K, Black IB. Adult bone marrow stromal stem cell express germ line, ectodermal, endodermal, mesodermal gene prior to neurogenesis. J Neurosci Res. 2002;69:908–917. doi: 10.1002/jnr.10365. [DOI] [PubMed] [Google Scholar]
- 17.Tejeda-Montes E, Smith KH, Rebollo E. Bioactive membranes for bone regeneration applications: effect of physical and biomolecular signals on mesenchymal stem cell behavior. Acta Biomater. 2014;10:134–141. doi: 10.1016/j.actbio.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Jorgensen C, Djouad F, Apparailly F, Noël D. Engineering mesenchymal stem cells for immunotherapy. Gene Ther. 2003;10:928–931. doi: 10.1038/sj.gt.3302019. [DOI] [PubMed] [Google Scholar]
- 19.Bukharova TB, Logovskaya LV, Volkov AV, Garas MN, Vikhrova EB, Logunov DY, Makhnach OV, Shmarov MM, Gol’dshtein DV. Adenoviral transduction of multipotent mesenchymal stromal cells from human adipose tissue with bone morphogenetic protein BMP-2 gene. Bull Exp Biol Med. 2013;156:122–126. doi: 10.1007/s10517-013-2294-y. [DOI] [PubMed] [Google Scholar]
- 20.Roccanova L, Ramphal P. The role of stem cells in the evolution of longevity and its application to tissue therapy. Tissue and Cell. 2003;35:79–81. doi: 10.1016/s0040-8166(02)00104-0. [DOI] [PubMed] [Google Scholar]
- 21.Campoccia D, Arciola CR, Cervellati M, Maltarello MC, Montanaro L. In vitro behaviour of bone marrow-derived mesenchymal cells cultured on hydroxyapatite-coated substrata with different roughness. Biomaterials. 2003;24:587–596. doi: 10.1016/s0142-9612(02)00373-3. [DOI] [PubMed] [Google Scholar]
- 22.Lee JW, Kim YH, Park KD, Jee KS, Shin JW, Hahn SB. Importance of integrin b1-mediated cell adhesion on biodegradable polymers under serum depletion in mesenchymal stem cells and chondrocytes. Biomaterials. 2004;25:1901–1909. doi: 10.1016/j.biomaterials.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Deligianni DD, Katsala N, Ladas S, Sotiropoulou D, Amedee J, Missirlis YF. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials. 2001;22:1241–51. doi: 10.1016/s0142-9612(00)00274-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang CY, Duan YR, Markovic B, Barbara J, Howlett CR, Zhang X, Zreiqat H. Phenotypic expression of bone-related genes in osteoblasts grown on calcium phosphate ceramics with different phase compositions. Biomaterials. 2004;25:2507–2514. doi: 10.1016/j.biomaterials.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 25.Wang YH, Zhang Y, Yin GF, et al. Study on the preparation of β-TCP using octacalcium phosphate precursor. AMME. 2004;17:266–269. [Google Scholar]
- 26.Mao K, Cui F, Li J, Hao L, Tang P, Wang Z, Wen N, Liang M, Wang J, Wang Y. Preparation of combined β-TCP/α-CSH artificial bone graft and its performance in a spinal fusion model. J Biomater Appl. 2012;27:37–45. doi: 10.1177/0885328210391919. [DOI] [PubMed] [Google Scholar]
- 27.Matsushita N, Inoue H, Miyamoto S, Terai H, Okada T, Nozaki K, Takaoka K. A new bone inducing biodegradable porous β-tricalcium phosphate. J Biomed Mater Res A. 2004;70:450–458. doi: 10.1002/jbm.a.30102. [DOI] [PubMed] [Google Scholar]
- 28.Bessho K, Carnes DL, Cavin R, Ong JL. Experimental studies on bone induction using low-molecular-weight poly (DL-lactide-coglycolide) as a carrier for recombinant human bone morphogenetic protein-2. J Biomed Mater Res. 2002;61:61–65. doi: 10.1002/jbm.10169. [DOI] [PubMed] [Google Scholar]
- 29.Liang G, Yang Y, Oh S, Ong JL, Zheng C, Ran J, Yin G, Zhou D. Ectopic osteoinduction and early degradation of recombinant a-human bone morphogenetic protein-2-loaded porous β-tricalcium phosphate in mice. Biomaterials. 2005;26:4265–4271. doi: 10.1016/j.biomaterials.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Prins HJ, Braat AK, Gawlitta D, Dhert WJ, Egan DA, Tijssen-Slump E, Yuan H, Coffer PJ, Rozemuller H, Martens AC. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem Cell Res. 2014;12:428–440. doi: 10.1016/j.scr.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Dinarvand P, Seyedjafari E, Shafiee A, Jandaghi AB, Doostmohammadi A, Fathi MH, Farhadian S, Soleimani M. New approach to bone tissue engineering: simultaneous application of hydroxyapatite and bioactive glass coated on a poly (L-lactic acid) scaffold. ACS Appl Mater Interfaces. 2011;3:4518–4524. doi: 10.1021/am201212u. [DOI] [PubMed] [Google Scholar]


