Abstract
The role of resveratrol in cerebral ischemia/reperfusion injury is not well understood. The aim of this study was to investigate whether resveratrol modulates inflammation and oxidative stress in cerebral ischemia/reperfusion injury in mice. Rats were subjected to 2 h of transient middle cerebral artery occlusion (MCAO), followed by 24 h of reperfusion. Rats were randomly exposed to sham operation group, MCAO group and MCAO+ resveratrol group. The results demonstrated that compared to I/R, resveratrol reduced cerebral infarction area, brain water content, neuronal apoptosis, myeloperoxidase (MPO) levels, and cerebral TNF-α production. Our results suggested that resveratrol has protective effects against cerebral I/R injury in rats, which may be attributed to attenuating inflammation and apoptosis induced by cerebral ischemia reperfusion.
Keywords: Resveratrol, cerebral ischemia, stroke, inflammation, apoptosis, TNF-α
Introduction
Stroke is a leading cause of death and adult disability worldwide [1,2]. Ischemic stroke accounts for approximately 80% of all strokes, and occurs when a major cerebral artery is blocked by a thrombus or embolism [3]. This blockage leads to brain injury through a complex series of pathophysiological events leading to neuronal cell death and subsequent neurological dysfunction. Thrombolysis with intravenous administration recombinant tissue plasminogen activator (rt-PA) is the only approved treatment for acute stroke, but fewer than 5% of stroke patients are eligible for this therapy, primarily due to the narrow time window (< 3 h). Besides, it does not provide reperfusion and it increases the risk of symptomatic intracranial hemorrhage [4]. Thus, there is a compelling need to develop novel therapeutic options for patients with ischemic stroke.
Resveratrol (3,4,5-trihydroxy-trans-stilbene, Res) is a poly-phenol presented in plants, e.g., grapes, cranberries and peanuts, which possesses multiple biological qualities including anti-inflammatory and antioxidant effects [5,6]. Recently, a number of studies have demonstrated that Res exhibits neuroprotective effects in a variety of animal models of cerebral ischemic stroke [7,8]. The neuroprotective mechanisms of Res may be attributed to its NO-promoting properties [9-13], inhibition of excitatory synaptic transmission [14], or a combination of the two. However, the molecular mechanisms underlying the neuroprotective effects of Res are not fully understood. This study, therefore, aims to investigate the effect of Res on inflammation and oxidative stress in a rat model of middle cerebral artery occlusion and its underlying mechanisms.
Material and methods
Reagents
Resveratrol was purchased from Sigma Chemical Co. (St. Louis, MO, USA). MPO assay kit was purchased from Jiancheng Bioengineering Institute (Nanjing, China). TNF-α ELISA kit was purchased from R&D Corporation, (USA). BCA protein quantification kit was purchased from Bio-Rad (USA).
Animals
Forty adult male Sprague-Dawley rats (250-300 g) were purchased from the Center of Experimental Animal in Harbin Medical University, China. All animals used in this study were cared for in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health (NIH publication no. 85-23, revised 1996), and all procedures were approved by the Committee of Experimental Animals of Harbin Medical University.
Preparation of MCAO model
Rats were randomly assigned into three experimental groups: sham operation group (n = 13), MCAO group (n = 14) and Res +MCAO group (n = 13). After being anesthetized with chloral hydrate (350 mg/kg, i.p.), the rat was placed in a supine position and a midline skin incision was made to expose the right common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA). The MCAO rats were generated as described previously [15]. Briefly, a 4-0 monofilament nylon thread (Sunbio Biotech, Beijing, China) with a rounded tip was inserted into the ECA and gently advanced about 20 mm to block the blood flow of the right middle cerebral artery. After a 120-minute occlusion, the 4-0 filament was pulled out to permit reperfusion for 24 h. During the operation, the rectal temperature of rat was kept at 37°C by a heating pad. Rats in sham groups were subjected to the same procedure, but the middle cerebral artery was not occluded. For the MCAO+Res group, Res was administered at a dose of 30 mg/kg i.p. starting at 3 h after reperfusion and lasting for 4 days. Rats in the Res alone group underwent the same procedures as those in the Res +MCAO group, except for the occlusion of the middle cerebral artery. Resveratrol was dissolved in 2% dimethyl sulfoxide (DMSO). Sham group and MCAO group rats were injected solely with an equal concentration of DMSO.
Neurological evaluation
Neurological deficits were evaluated by an observer blinded to the treatment of animals after 24 h reperfusion according to the methods previously described [16]. Score 0: no neurologic deficit; Score 1: failure to extend left forepaw fully; Score 2: circling to the left; Score 3: falling to the left; Score 4: did not walk spontaneously and had a depressed level of consciousness. Rats that did not show neurological deficits after reperfusion (neurological score = 0) were excluded from the groups.
Assay of cerebral infarct volume
The animals were euthanized 24 h after reperfusion (n = 10 in each group) by chloral hydrate (350 mg/kg, i.p.) anesthesia overdose. The brains were rapidly dissected out and the forebrains were cut into six coronal sections (2 mm thick). The sections were stained by incubating them in a solution of 2% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich) at 37°C for 30 min. The crosssectional areas with or without infarction in each brain slice were measured using Image J analysis software (version 1.6 NIH).
Evaluation of cerebral edema
Twenty-four hours after MCAO, cerebral edema was determined by measuring the brain water content according to the wet-dry method [3]. These rats were killed and brains were immediately removed and placed on a frozen plate. Tissue samples were dissected out from infarct areas in ischemic rats and from corresponding areas in sham-operated and non-operated animals. Samples were immediately weighed to obtain wet weight. Then, samples were dried in a desiccating oven at 110°C for 24 h and weighed again to obtain the dry weight. Brain water content was calculated as follows: brain water content (%) = (wet weight - dry weight) × 100/wet weight.
Histological assessment
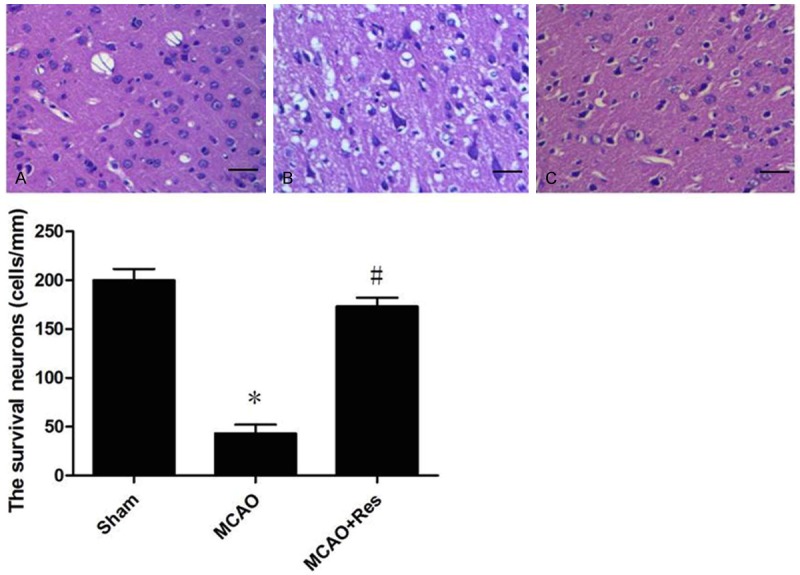
Twenty-four hours after reperfusion, rats (n = 10 in each group) were anesthetized and transcardially perfused with saline for 5 min, following by 4% paraformaldehyde (PFA) in phosphate-buffered saline. A tissue block spanning from the optic chiasm to 4 mm posterior to it was cut and sliced into 5 μm sections until use. Hematoxylin and eosin staining was performed to assess the histological injury. The damage was assayed by counting the number of surviving neurons per 1 mm of cortex cells with a light microscope.
Detection of neuronal apoptosis by Western blotting
The expressions of caspase-3, cleaved caspase3, Bcl-2, Bax, and β-actin were measured using Western blotting. Protein content was determined with BCA protein assay, and protein samples were separated by electrophoresis on SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membranes were blocked with 5% fat free milk and incubated overnight with the appropriate primary antibodies, respectively [anti-Bcl-2 (1:200), anti-Bax (1:200), anti-β-actin (1:5000) (SantaCruz Biotechnology), anti-caspase-3 (1:1000), anti-cleaved caspase3 (1:1000) (Cell Signaling Technology, Beverly, MA, USA)]. After being extensively rinsed with Tris-buffered saline containing 0.1% Triton × -100 buffer, the membranes were incubated with secondary antibodies (Santa Cruz Inc, Santa Cruz, CA; 1:2000) for 1 hour at room temperature, and then the bar graph depicts the ratios of semi- quantitative results obtained by scanning reactive bands and quantifying the optical density using Video densitometry (Bio-1D vers. 99 image software, Bio-Rad).
Determination of myeloperoxidase (MPO) level
After reperfusion, cortical tissue from the penumbral cortex was immediately isolated and stored at -80°C until use. Cortical tissue was homogenized in ice cold phosphate buffer to make a 10% homogenate. Then the homogenate was centrifuged at 3000 rpm for 15 min. MPO test kit was used to detect level of MPO in the cerebral tissue according to manufacturer’s instruction.
Detection of TNF-α level
After reperfusion, cortical tissue from the penumbral cortex was immediately isolated and stored at -80°C until use. Cortical tissue was homogenized in ice cold phosphate buffer to make a 10% homogenate. Then the homogenate was centrifuged at 3000 rpm for 15 min. The level of TNF-α in cerebral tissue was detected in strict accordance with manufacturer’s instructions.
Statistical analysis
Data is presented as means ± SD. The significance of differences among groups was evaluated by a Student’s t-test for unpaired data or Dunnett’s t-test for multiple comparisons preceded by one-way analysis of variance (ANOVA). For all test, P < 0.05 was considered as statistically significant.
Results
Effects of resveratrol on neurological deficits
No neurological deficits were seen in the sham group. When tested at 24 after reperfusion, MCAO rats displayed severe neurological deficits. Resveratrol treatment significantly improved the neurological score compared with the MCAO group (P < 0.05) (Figure 1).
Figure 1.
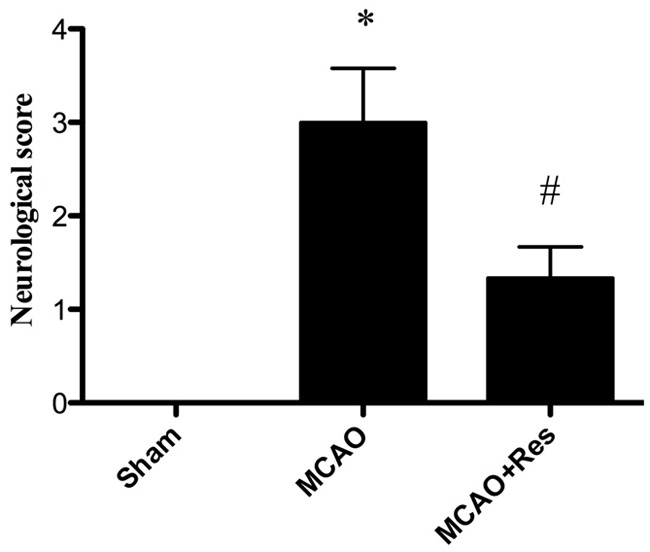
Effects of resveratrol on neurological deficits. Resveratrol (Res) significantly improved neurological recovery compared with the MCAO group at 24 h after reperfusion. Data were expressed as mean ± SD (n = 10 in each group); *P < 0.05 versus the sham group, #P < 0.05 versus the MCAO group.
Effects of resveratrol on cerebral infarct volume
No infarct area observed in the sham group, while in the MCAO group, a significant infarction was observed at 24 h after reperfusion. Under treatment with resveratrol, the infarct volume in the MCAO+Res group significantly decreased compared with that in the MCAO group (P < 0.05) (Figure 2).
Figure 2.
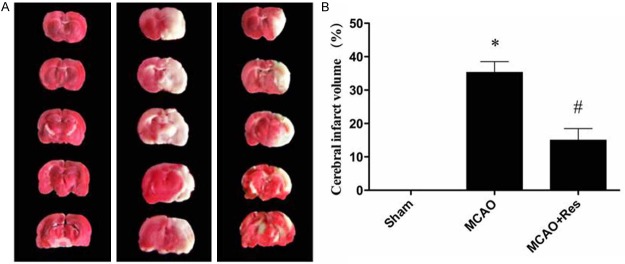
Resveratrol (Res) reduces the infarct volume in middle cerebral artery occlusion model. A: Representative pictures of stained cerebral sections in each group. The normal tissue stained dark red, while the infarct tissue was white; B: Quantitative analysis of infarct volume. Data were expressed as mean ± SD (n = 10 in each group). *P < 0.05 versus the sham group, #P < 0.05 versus the MCAO group.
Effects of resveratrol on brain edema formation
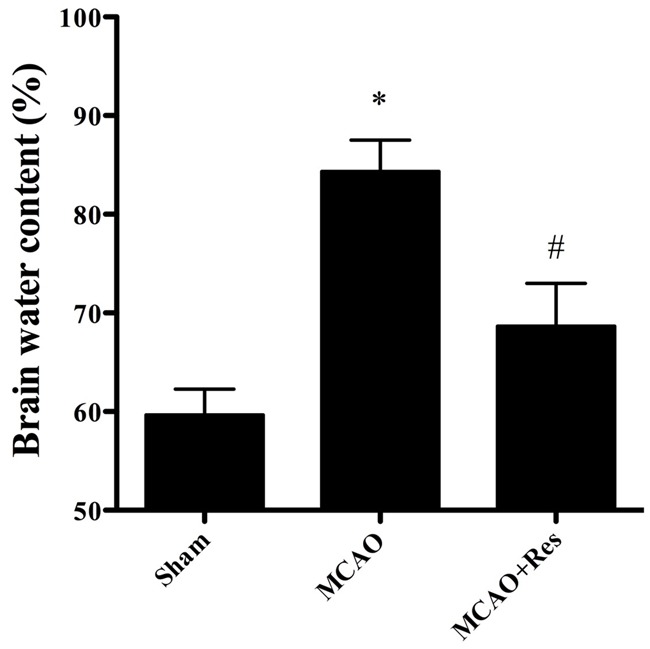
Twenty-four hours after MCAO, the brain water content in the ischemic area of the MCAO group was significantly higher than that of the sham group. Pretreatment with resveratrol significantly reduced brain edema compared with that in the MCAO group (P < 0.05) (Figure 3).
Figure 3.
Histological examinations of cortex neurons by hematoxylin and eosin staining (scale bar = 20 μm). A: sham group; B: MCAO group; C: MCAO+Res group. Resveratrol increased the number of surviving neurons and ameliorated the extent of damage in the cortex. Data were expressed as mean ± SD (n = 10 in each group). *P < 0.05 versus the sham group, #P < 0.05 versus the MCAO group.
Effects of resveratrol on histopathology
To evaluate the effects of resveratrol on cerebral IR injury, histological examination in each group was performed in HE stained sections. The sham group showed normal neurons with no pathological change. Compared with the sham and MCAO+Res group, most of the neurons in the MCAO group had disappeared. In the MCAO group, neuronal loss was severe and dying neurons showed shrunken cytoplasm and pyknotic nuclei. In the MCAO+Res group, the number of surviving neurons was markedly increased compared with that in the MCAO group (P < 0.05), and the extent of damage was significantly ameliorated as well (Figure 4).
Figure 4.
Resveratrol (Res) ameliorates oxidative stress after cerebral ischemia/reperfusion (IR) injury. A: Res significantly decreased the content of malondialdehyde (MDA); B: Res significantly enhanced superoxide dismutase (SOD) activity; C: Res significantly enhanced glutathione peroxidase (GSH-PX) activities. Scale bar = 20 μm. Data were expressed as mean ± SD (n = 10 in each group). *P < 0.05 versus the sham group, #P < 0.05 versus the MCAO group.
Effects of resveratrol on neuronal apoptosis
As shown in Figure 5, resveratrol increased Bcl-2 expression dramatically compare with MCAO group (P < 0.05). On the other hand, resveratrol attenuated the expression of Bax, cleaved caspase-3 and caspase-3 expressions significantly compared with MCAO group (P < 0.05).
Figure 5.
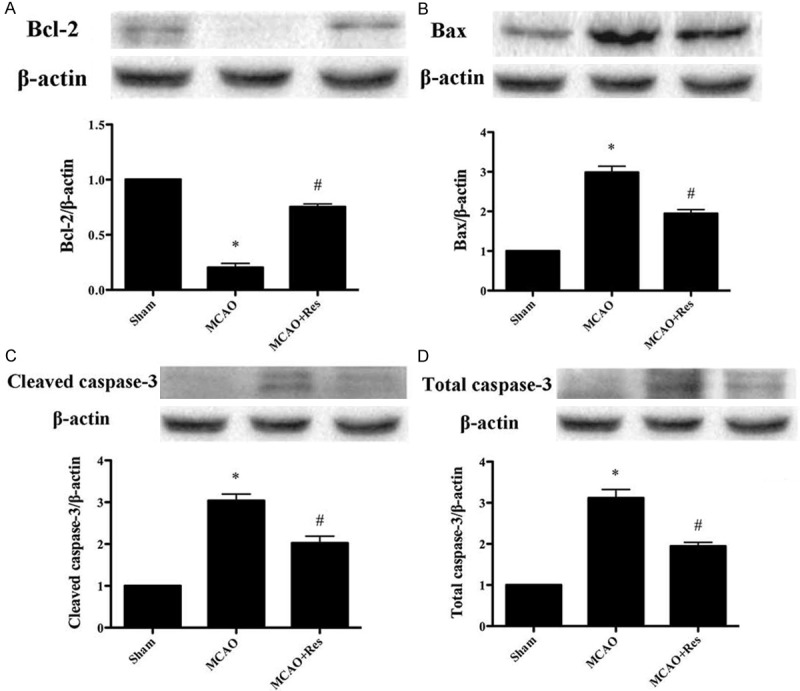
The effect of resveratrol on apoptotic signaling pathway. A: Bcl-2 expression; B: Bax expression; C: Cleaved caspase-3 expression; D: Total caspase-3 expression. Data were expressed as mean ± SD (n = 10 in each group). *P < 0.05 versus the sham group, #P < 0.05 versus the MCAO group.
Effects of resveratrol on MPO activity
Neutrophil contains a certain amount of myeloperoxidase (MPO), accounting for 5% of dry cell weight. So the activity of MPO in the cerebral tissue can be considered as the indication of neutrophil infiltration. As shown in Figure 6, the MPO activity in the sham group was relatively lower, while the MPO activity in the MCAO group was significantly increased (P < 0.05). Resveratrol significantly decreased cerebral MPO activity (P < 0.05).
Figure 6.
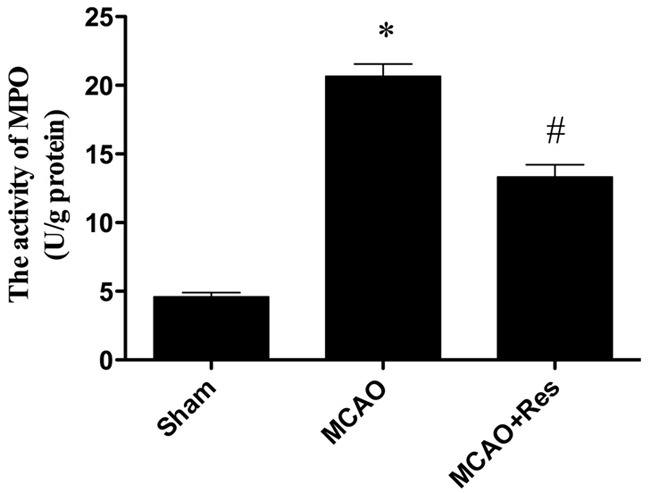
The comparison of MPO activity in each group. Compared with MCAO group, the MPO activity in MCAO+Res group reduced significantly. Data were expressed as mean ± SD (n = 10 in each group). *P < 0.05 versus the sham group, #P < 0.05 versus the MCAO group.
Effects of resveratrol on cerebral TNF-α level
The cerebral I/R injury results in production of large amount of TNF-α. Thus, cerebral TNF-α level was examined. In Figure 7, compared with the MCAO group, resveratrol significantly decreased the level of TNF-α in cerebral tissue (P < 0.05).
Figure 7.
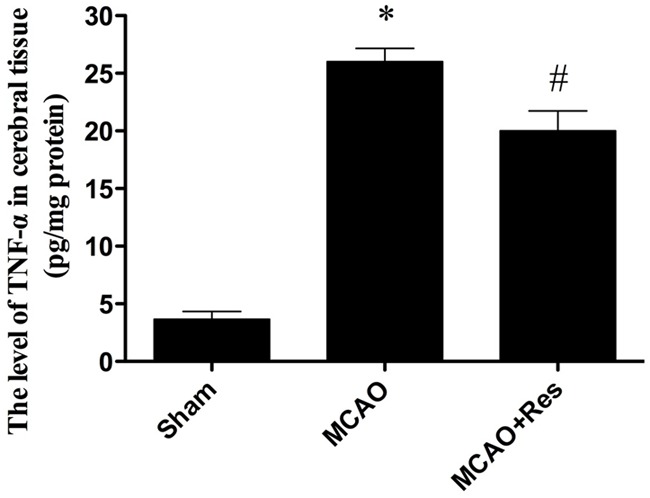
The comparison of levels of TNF-α in cerebral tissue in each group. Compared with MCAO group, resveratrol reduced TNF-α level significantly in MCAO+Res group. Data were expressed as mean ± SD (n = 10 in each group). *P < 0.05 versus the sham group, #P < 0.05 versus the MCAO group.
Discussion
The major findings in the present study are: (1) Resveratrol attenuates cerebral I/R injury through inhibiting neutrophil infiltration and TNF-α production. (2) Resveratrol attenuates cerebral I/R injury by attenuating neuronal apoptosis.
Accumulating evidence suggested that post-ischemic inflammation contributes to the development of neuronal injury and cerebral infarction. In the inflammation, endogenous mediators (cytokine and chemokines) and recruitment of circulating leukocytes are involved. Cytokines play a key role in the attraction of leukocytes as potent inducers of chemokines. Therefore, cytokines act as primary mediators and chemokines act as secondary mediators to attract leucocytes in the condition of inflammation [17]. In addition, activated astrocytes and microglia produced cytokines and chemokines [18]. These molecules appear to be responsible for the accumulation of inflammatory cells in injured brain tissue. TNF-α, IL-1β, and IL-6 are the potential cytokines which initiate inflammatory mediator and inflammatory reactions and induce expression of other cytokines after ischemia–reperfusion injury [19,20]. The ischemic brain was observed with increased levels of TNF-α, IL-6, and IL-1β. They are considered as a part of tissue damaging response in ischemia and reperfusion injury [19,20]. In this study, our results suggest that resveratrol has cerebroprotective action through anti-inflammatory effect, such as decreasing the level of TNF-α.
In summary, our data indicate that resveratrol has protective effects against cerebral IR injury in rats, and the neuroprotective effects may be attributed to inhibiting inflammation and attenuating oxidative stress. The findings provide further insight into the mechanism by which resveratrol exerts its neuroprotection and suggest that resveratrol might be of therapeutic value for the treatment of ischemic stroke.
Disclosure of conflict of interest
None.
References
- 1.Flynn RW, MacWalter RS, Doney AS. The cost of cerebral ischaemia. Neuropharmacology. 2008;55:250–256. doi: 10.1016/j.neuropharm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Okoro CA, Balluz LS, Campbell VA, Holt JB, Mokdad AH. State and metropolitan-area estimates of disability in the United States, 2001. Am J Public Health. 2005;95:1964–1969. doi: 10.2105/AJPH.2004.047308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Green AR, Shuaib A. Therapeutic strategies for the treatment of stroke. Drug Discov Today. 2006;11:681–693. doi: 10.1016/j.drudis.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Delmas D, Jannin B, Latruffe N. Resveratrol: preventing properties against vascular alterations and ageing. Mol Nutr Food Res. 2005;49:377–395. doi: 10.1002/mnfr.200400098. [DOI] [PubMed] [Google Scholar]
- 6.Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health--a comprehensive review of human clinical trials. Mol Nutr Food Res. 2011;55:1129–1141. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- 7.Gao D, Zhang X, Jiang X, Peng Y, Huang W, Cheng G, Song L. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006;78:2564–2570. doi: 10.1016/j.lfs.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 8.Huang SS, Tsai MC, Chih CL, Hung LM, Tsai SK. Resveratrol reduction of infarct size in Long-Evans rats subjected to focal cerebral ischemia. Life Sci. 2001;69:1057–1065. doi: 10.1016/s0024-3205(01)01195-x. [DOI] [PubMed] [Google Scholar]
- 9.Jagdeo J, Adams L, Lev-Tov H, Sieminska J, Michl J, Brody N. Dose-dependent antioxidant function of resveratrol demonstrated via modulation of reactive oxygen species in normal human skin fibroblasts in vitro. J Drugs Dermatol. 2010;9:1523–1526. [PubMed] [Google Scholar]
- 10.Sinha K, Chaudhary G, Gupta YK. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002;71:655–665. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- 11.Tsai SK, Hung LM, Fu YT, Cheng H, Nien MW, Liu HY, Zhang FB, Huang SS. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg. 2007;46:346–353. doi: 10.1016/j.jvs.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Valdecantos MP, Perez-Matute P, Quintero P, Martinez JA. Vitamin C, resveratrol and lipoic acid actions on isolated rat liver mitochondria: all antioxidants but different. Redox Rep. 2010;15:207–216. doi: 10.1179/135100010X12826446921464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 14.Gao ZB, Chen XQ, Hu GY. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res. 2006;1111:41–47. doi: 10.1016/j.brainres.2006.06.096. [DOI] [PubMed] [Google Scholar]
- 15.Mulcahy NJ, Ross J, Rothwell NJ, Loddick SA. Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br J Pharmacol. 2003;140:471–476. doi: 10.1038/sj.bjp.0705462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 17.Gouwy M, Struyf S, Proost P, Van Damme J. Synergy in cytokine and chemokine networks amplifies the inflammatory response. Cytokine Growth Factor Rev. 2005;16:561–580. doi: 10.1016/j.cytogfr.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasuda Y, Shimoda T, Uno K, Tateishi N, Furuya S, Tsuchihashi Y, Kawai Y, Naruse S, Fujita S. Temporal and sequential changes of glial cells and cytokine expression during neuronal degeneration after transient global ischemia in rats. J Neuroinflammation. 2011;8:70. doi: 10.1186/1742-2094-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ. Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res. 2006;3:327–337. doi: 10.2174/156720506778249515. [DOI] [PubMed] [Google Scholar]
- 22.Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int. 2013;62:712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Taylor JM, Crack PJ. Impact of oxidative stress on neuronal survival. Clin Exp Pharmacol Physiol. 2004;31:397–406. doi: 10.1111/j.1440-1681.2004.04017.x. [DOI] [PubMed] [Google Scholar]
- 24.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Candelario-Jalil E, Mhadu NH, Al-Dalain SM, Martinez G, Leon OS. Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci Res. 2001;41:233–241. doi: 10.1016/s0168-0102(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 26.Niizuma K, Yoshioka H, Chen H, Kim GS, Jung JE, Katsu M, Okami N, Chan PH. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim Biophys Acta. 2010;1802:92–99. doi: 10.1016/j.bbadis.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


