Abstract
Hemangiopericytoma (HPC) and meningioma, for their morphology immunohistochemical markers similarity, were usually confused especially before surgery. This study aimed to develop a panel of biomarkers to differentiate HPC from meningioma. Real-time PCR and immunohistochemical staining were employed to determine the levels of p53, bcl-2, c-myc, vimentin, CD34, FVIIIa, MGMT and reticular fiber in 15 meningiomas, HPCs and their normal controls. We found that, in the mRNA expression level, both Bcl-2 and c-myc were high in HPC and meningiomas, but bcl-2 was higher in HPC than in meningiomas, c-myc was lower in HPC than in meningiomas. In protein expression level, reticular fibers were around most HPC tumor cells but observed null in meningiomas; CD34 and FVIIIa were both found positive in HPCs but negative in meningiomas; MGMT was weak focal in HPC but strong diffuse in meningiomas. In conclusion, bcl-2, c-myc, and MGMT could be employed as the new panels of biomarkers for distinguishing HPC from meningiomas.
Keywords: Meningioma, hemangiopericytoma, immunohistochemistry
Introduction
Meningiomas originated from the meningothelial (arachnoidal) cells, which accounts for about 24-30% of primary intracranial neoplasms [1,2]. Meningeal hemangiopericytoma (HPC), once classified as ‘angioblastic’ variant of meningioma and divided from the meningioma by WHO in 1993, is now recognized as meningeal interstitial source tumor and located mostly in the musculoskeletal system and the skin, while intracranial localization is rare, representing 2% to 4% of large series of meningeal tumors and accounts for less than 1% of all intracranial tumors. The WHO classification (2000) of Central Nervous System tumors distinguishes HPC as an entity of its own, and classified it into the group of “mesenchymal, non-meningothelial tumors” [3-5].
Meningiomas and HPC are two tumors with characteristic histological patterns that can be distinguished clearly in classical way. HPCs tend to recur and metastasize where the local recurrence and extraneural metastasis rates were 48.2% and 6%, respectively. However, occasional cases display sufficient morphologic overlap so that ancillary tests are necessary to distinguish between them. Histological and immunohistochemical examinations are important for diagnosis of intracranial HPCs. HPCs is distinguished from meningiomas by the absence of whorl or syncytium formation, which are characteristic of meningiomas. Immunohistochemically, HPCs are positive for CD34, and negative for EMA and S-100 protein, for which meningiomas are positive. Currently, the most useful immunohistochemical and histochemical stains to distinguish them are as follows: (1) epithelial membrane antigen (EMA) [6-8], which is typically positive in meningiomas and usually negative in HPCs; (2) a characteristic individual cell FVIIIa staining pattern seen in HPCs and not in benign meningiomas [9,10]; (3) reticulin, which shows a dense network of intercellular deposition in HPCs, with most meningiomas being relatively reticulin poor. CD34 was initially considered to be specific for HPCs [11], but there were also some opposite theories [12,13] and studies have shown that in contrast to the strong diffuse staining seen in solitary fibrous tumors, with 60% of meningiomas being similarly positive [9,10]. In contrast to meningiomas, HPCs currently do not have any well-characterized or signature genetic alterations. So, we explored the issue in these two tumor types, adding several recently developed biomarkers such as MGMT and bcl-2. MGMT has been reported to be frequently positive in HPCs, and bcl-2 is strongly positive in the closely related solitary fibrous tumor (SFT) [9,14].
Materials and methods
Patients and tissue samples
15 paired surgical specimens of HPC (1999 to 2012), meningiomas and normal tissues (2012) from patients suffered from brain trauma and decompression of the cerebral hemorrhage were obtained from patients who had undergone curative surgical treatment in Nanjing Brain Hospital affiliated to Nanjing Medical University. Each tissue specimen was divided into two parts after resection. For total RNA extraction, samples was immediately frozen in liquid nitrogen and stored at -80°C until extraction. The other parts were processed for pathological examination.
HPC patients were 7 females and 8 males whose case histories were 2 days to 2 years. The age of patients varied from 37 to 73 (mean age=56.2 years). Meningiomas patients were 6 females and 9 males, case histories were 1 week to 3 years. The age of patients varied from 36 to 74 (mean age=54.4 years). Normal tissues were 6 females and 9 males, with the case history from 1 h to 7days. The age of patients varied from 35 to 75 (mean age=53.3 years) (Table 1). All procedures mentioned above have been approved by the local Ethical Committee and the patients.
Table 1.
Patient’s information
| Class | Number | Male | Female | Age | Mean age | Develop | Therapy |
|---|---|---|---|---|---|---|---|
| HPC | 15 | 7 | 8 | 37-73 | 56.2 | 2 d-2 y | Operation |
| Meningiomas | 15 | 6 | 9 | 36-74 | 54.4 | 1 w-3 y | Operation |
| Normal | 15 | 6 | 9 | 35-75 | 53.3 | 1 h-7 d | Operation |
RT-PCR and real-time RT-PCR
Total RNA was isolated using TRIzol Total RNA Isolation kit (Invitrogen, NY 14072, USA) according to the manufacturer’s protocol. Reverse transcription was performed using the Superscript III RT kit (Invitrogen, NY 14072, USA) according to the manufacturer’s protocols. Real-time PCR amplification was performed using the SYBR Green master mix (Applied Biosystems, Foster City CA, 94404 United States) and the Prism 7500 Real-time PCR Detection System (Applied Biosystems, Foster City CA, 94404 United States). Cycling conditions were 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Primers for Real-time PCR were designed by Primer3 software and synthesized at the University of Utah (Table 2). Relative amounts of mRNA were normalized by GAPDH and calculated using the delta-delta method from threshold cycle numbers.
Table 2.
Primers for real-time PCR
| Gene | Accession No. | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|---|
| c-myc | NM_002467.4 | GTCACCACCCAAATCCTTAT | ATCTACTGCCTGGAGACCTT |
| Bcl-2 | NM_000633.2 | CCGAGGTGGTTTTCATCTGT | CGAGGTCTTTTTGGTTTTCC |
| P53 | NM_000546.5 | GGGAATGGGACAAAAAGACA | CTTCAGGGGCAACACGAA |
Immunohistochemistry
Immunohistochemistry was performed in order to establish the levels of expression and cellular localization of bcl-2 (Anti-bcl-2, Santa Cruz Biotechnology, CA. 95060, USA), vimentin (Anti-vimentin, Santa Cruz Biotechnology, CA. 95060, USA), CD34 (Anti-CD34, Biogenics, USA), FVIIIa (Anti-FVIIIa, Calbiochem, USA) and MGMT (Anti-MGMT, Santa Cruz Biotechnology, CA. 95060, US) proteins. The samples were formalin fixed, paraffin embedded, and 4-um thick sections were placed on capillary gap microscope slides (DakoCytomation, Denmark). Deparaffinized and rehydrated sections were microwaved in Dako Target Retrieval Solution (Dako Corporation, USA) thrice for 5 min at 800 W to unmask epitopes. To block endogenous peroxidase activity, cells were fixed in methanol containing 3% H2O2. Nonspecific binding was blocked by incubating it in normal mouse serum for 30 min in a humid chamber. Slides were blotted and primary antibodies at optimized dilutions were applied for 30 min at room temperature. After incubation, the slides were washed thrice in phosphate-buffered saline/goat serum and incubated in secondary antibody for 25 min. The washing was repeated, and the slides were incubated with streptavidin horseradish peroxidase for 25 min. Negative controls were samples that underwent same staining procedure with the exclusion of the primary antibodies. The analysis of the labeling was performed by two independent observers, i.e. blinded pathologists.
Statistical analysis
All data were presented as means ± SE. Statistical analysis was performed using one-way ANOVA followed by Bonferroni’s test with the SPSS 11.5 software. P<0.05 was considered as statistically significant.
Results
Morphology of HPCs and meningiomas
Microscopically, the meningomas fulfilled WHO criteria [10] -tumor cells was the same size and arranged lobulatedly with a few of collagen fiber at interval with the key characteristic feature of arachnoid string. The HPCs contained monomorphic oval to spindled cells arranged as dense hypercellular sheets, often interrupted by pale hypocellular islands, as well as abundant thin-walled, gaping vessels, some with the characteristic arborizing (“staghorn”) pattern (Figure 1).
Figure 1.
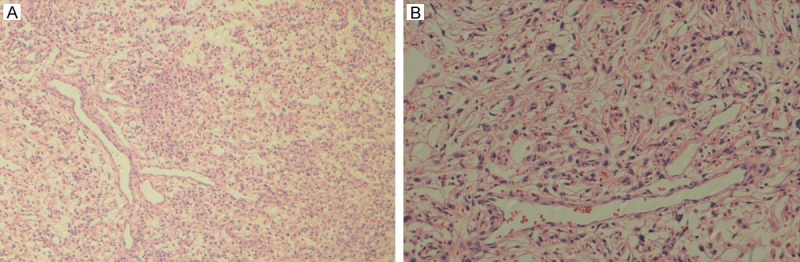
Representative hematoxylin and eosin staining showed Hemangiopericytoma with identical monomorphic cells and a single dilated “staghorn” vessel. (original magnification, A 100×, B 400×).
Gene expression levels of selected biomarkers in HPCs and meningiomas
Genetically, p53 expression was decreased in both meningomas and HPC, especially in the former ones. Bcl-2 and c-myc expression levels were both increased in meningomas and HPC. The levels of Bcl-2 were higher in HPC than in meningiomas, which suggest that bcl-2 related apoptosis might involve in HPC progression. However, the levels of c-myc in HPC were lower than in meningiomas, which may due to the amount of tumor samples were small (Figure 2).
Figure 2.
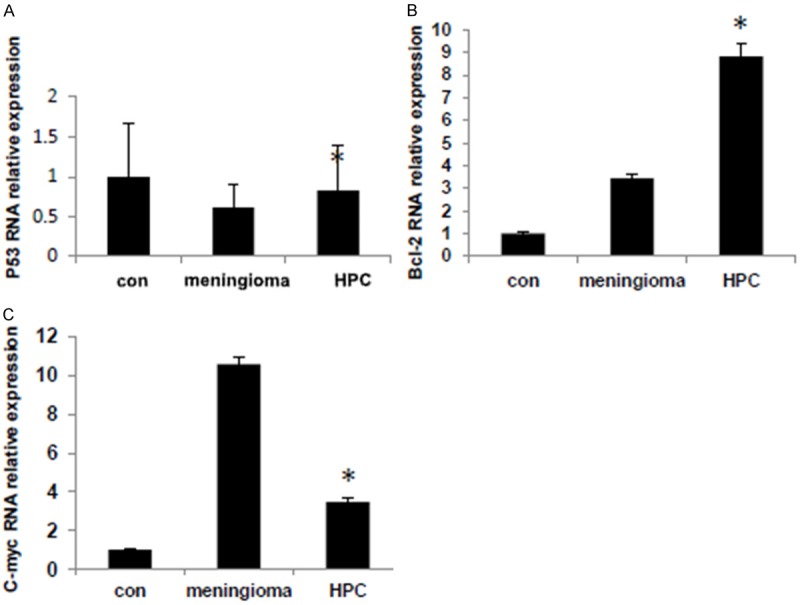
A. P53 was 0.613, 0.825 times higher than the control in meningioma and HPC, respectively. B. Bcl-2 was 3.44, 8.81 times higher than the control in meningioma and HPC, respectively. C. C-myc was 10.59, 3.49 times higher than the control in meningioma and HPC, respectively. *P<0.05 versus control.
Protein expression levels of selected biomarkers in HPCs and meningiomas
Reticular fiber staining showed reticular fibers were around most tumor cells in HPCs while absent in meningiomas (Figure 3). MGMT was locally positive from 10%-95%, and Bcl-2 was positive in a few tumor tissues which were contrary to the RT-PCR analysis (Figure 4). CD34, vimentin and FVIIIa were positive in all HPC tissues (Figure 5). We analyzed that our samples were only 15 and the variation between the same groups of samples. In meningomas, vimnetin and MGMT were positive; CD34, FVIIIa and bcl-2 were negative in almost all tissues (Figure 6). The detail information was showed in Table 3.
Figure 3.
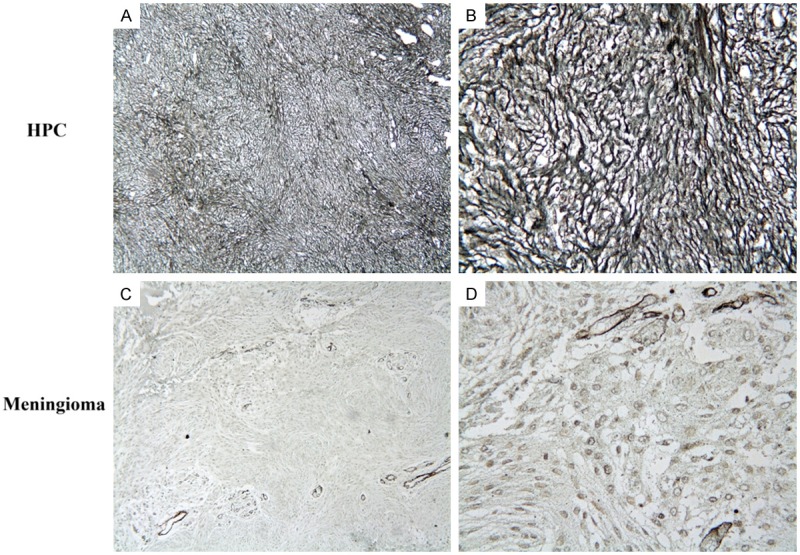
Reticular fiber staining showed reticular fibers were around most tumor cells in HPCs while not in meningiomas (A, C 100×, B, D 400×).
Figure 4.
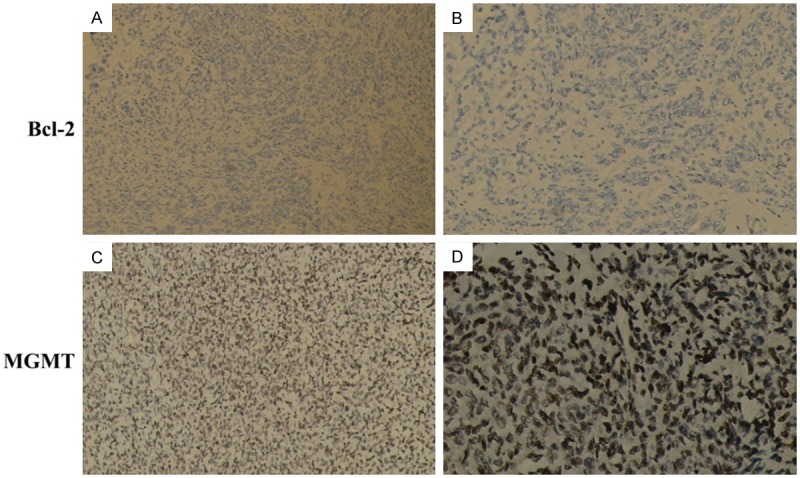
Bcl-2 was positive in little tumor tissue (original magnification, A 100×, B 400×); MGMT was 95% positive in one HPC tissue (original magnification, C 100×, D 400×).
Figure 5.
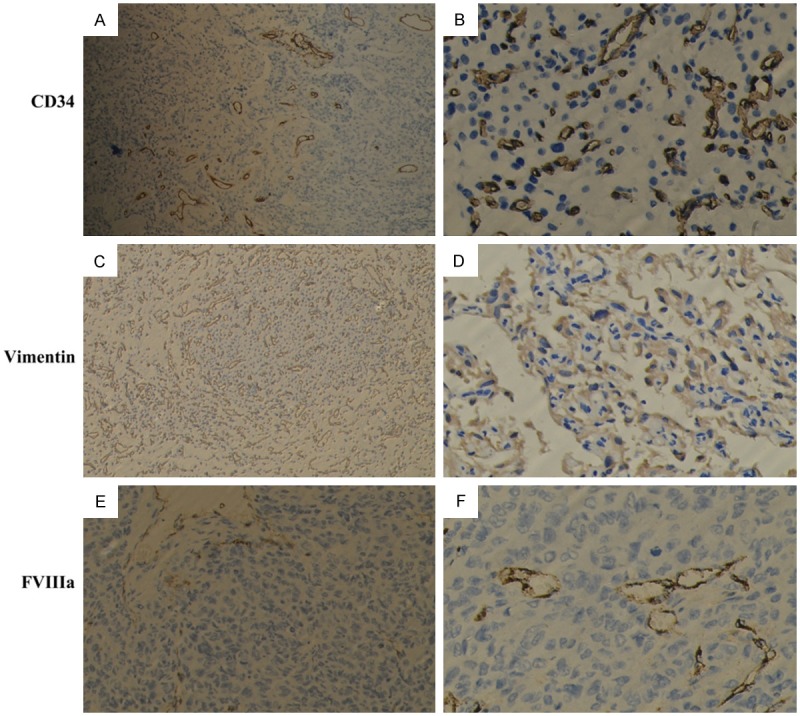
CD34 (original magnification, A 100×, B 400×), vimentin (Original magnification, C 100×, D 400×), FVIIIa (original magnification, E 100×, F 400×) were positive in all HPC tissues.
Figure 6.
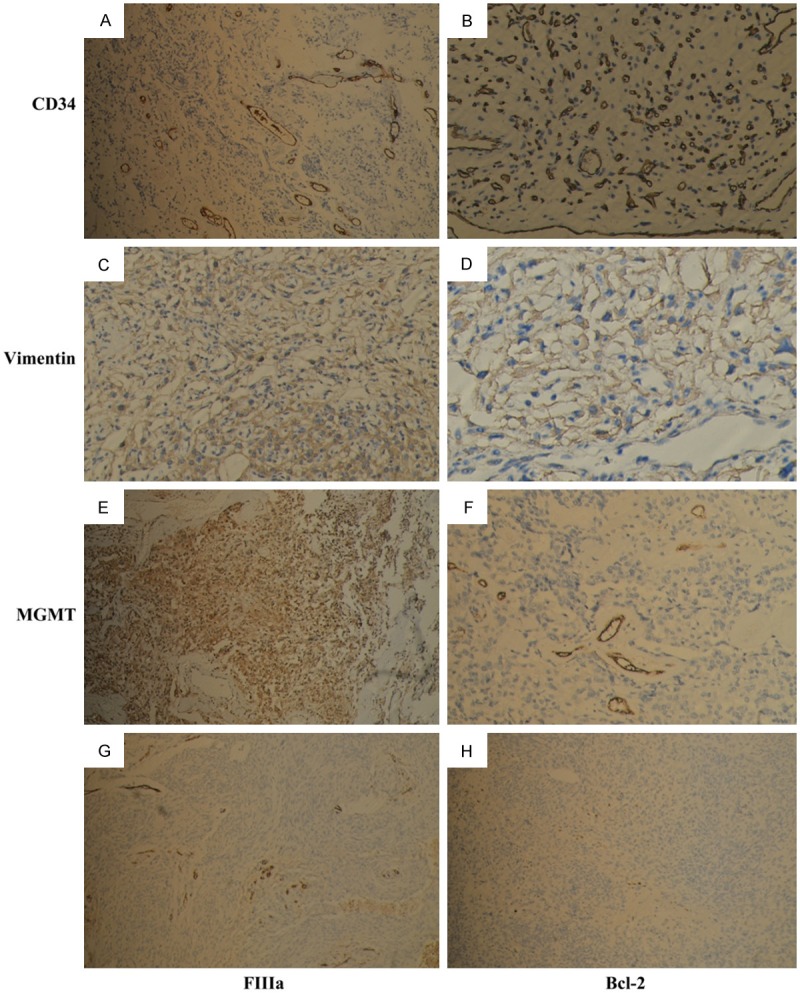
CD34 (original magnification, A 100×, B 400×) was positive in vessels but not in tumor cells in meningiomas, vimentin (original magnification, C 100×, D 400×), MGMT (original magnification, E 100×, F 400×) were positive in all meningioma tissues, FVIIIa (original magnification, G 100×), bcl-2 (original magnification, H 100×) were negative in almost meningiomas tissues.
Table 3.
Staining level of different markers in HPCs and meningiomas
| Variable | Vimentin (H/M*) | CD34 (H/M*) | FVIIIa (H/M*) | Bcl-2 (H/M*) | MGMT (H/M*) |
|---|---|---|---|---|---|
| Negative | 0/0 | 0/15 | 0/15 | 12/13 | 0/0 |
| Weak focal | 0/0 | 0/0 | 0/0 | 3/2 | 11/0 |
| Weak diffuse | 0/0 | 0/0 | 0/0 | 1/0 | 0/0 |
| Strong focal | 0/0 | 0/0 | 0/0 | 0/0 | 3/1 |
| Strong diffuse | 15/15 | 15/0 | 15/0 | 0/0 | 1/14 |
H, HPC; M, meningioma.
Discussion
Genetic variation and its relationship with tumor have attracted more and more attention in the occurrence and development of HPC. Proto-oncogenes, code for proteins that help to regulate cell growth and differentiation, are often involved in signal transduction and execution of mitogenic signals, usually through their protein products. Upon activation, a proto-oncogene (or its product) becomes a tumor-inducing agent, an oncogene C-myc is such an example oncogene. C-myc is activated mainly by amplification and chromosome translocation and rearrangement [15]. Previous studies have suggested that c-myc gene expression is as high as 63% in meningiomas, and five times higher than that in normal brain tissue, which may interact with other onco-factors and play an important role in the formation and development of meingiomas. However, Durand A et al. [16], showed that c-myc had no direct relation with high degree of malignant meningiomas but with the prognosis of low degree ones which reminds us that it may be regarded as a predictor of recurrence of benign meningioma after surgery. Our study found out that it was also higher in HPCs than normal but lower than meningiomas. Whether it plays a role in HPC development needs to be further studied.
Bcl-2 is an anti-apoptosis gene and is located on mitochondria, endoplasmic reticulum and nuclear membrane. It prolongs cell life span and cell accumulation by inhibiting apoptosis. It had been confirmed that bcl-2 could block p53-mediated apoptosis but p53 translocation and p53-mediated growth retardation which may be explained by that bcl-2 blocks activated apoptosis signaling. Uzum et al. [17] found that bcl-2 gene was expressed widely in meningioma and related with malignant degree, and the mechanisms involved has not been defined. We found bcl-2 expression was nearly 9 times higher than the control which was higher than in meningiomas. Thus, we could explore bcl-2 related apoptosis in HPC development in the future.
TP53, an important tumor suppressor, could inhibit cell proliferation, induce cell apoptosis and maintain genome stability. TP53 gene mutation is correlated with tumor formation and development. More than 50% of tumor has p53 mutation which leads to its inactivation. Kheirollahi M et.al. [18] showed that p53 expressed significantly different. Additionally, it was thought that p53 could be an indicator to estimate the meningioma malignant degree in Ohba S [19] and Kantha R [20] studies. Consistently, we also found the expression of p53 gene was lower in meningioma than that in normal tissue, but with no difference with that in HPC which may due to the variation between the same groups of samples.
Pathology is the golden criteria to discriminate meningiom a from HPC. In our research, we not only detected classical HPC immunohistochemical markers: CD34, vimentin and FVIIIa [21-23], but also explored some probable markers, such as bcl-2 and MGMT. Immunohistochemical examination demonstrated that tumor cells were positive for vimentin staining, a mesenchymal marker, and CD34, which stained the vascular endothelium in HPC which showed negative in some studies [24]. CD34 is expected to be positive in both neoplastic cells and endothelial cells with vimentin and factor FVIIIa immunoreactivity in HPC and it is also a marker that usually positive in SFTs, and is expressed in up to 60% of fibrous meningiomas [25]. Angiogenesis seems to be significantly associated with a high growth fraction, development of recurrences and shorter overall survival of meningiomas, Neoangiogenesis can be quantified in tissues by the evaluation of micro-vessel density (MVD). Methodological differences in the assessment of MVD may involve the different sensitivities of the endothelial marker (factor VIII, CD31, CD34 and endoglin) used for vessel identification, the different cutoff values as well as microvessel counting techniques. In most studies concerning meningiomas, MVD has been evaluated by using pan-endothelial markers, such as factor VIII, CD31 or CD34 [26,27]; Nevertheless, the expression of these markers is not restricted to the newly formed vessels, but also presented in the endothelial cells of pre-existing vessels. Additionally, the DNA-repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) is a key factor in resistance to alkylating agents [28]. MGMT promoter methylation has been investigated as an independent favorable prognostic factor for glioblastoma [29]. In this study, we found that MGMT was all locally positive in HPC and prompted us to confirm our hypothesis. However, bcl-2 gene expression was contrary to the immunohistochemical staining. Bcl-2 expression has been previously reported in proliferative phase and late secretory phase endometrial stroma, ectopic endometriumand adenomyosis but, to our knowledge, never been reported in endometrial stromal sarcoma [30,31]. As reported the HPC in some study were diffusely positive with bcl-2 [32], which may suggest an etiologic role for this anti-apoptotic gene in tumor progression.
In conclusion, we found that the gene alteration in bcl-2 especially its relation to apoptosis may be involved in HPC prophase molecular mechanism but the samples amount was small and the subtype of HPC might affect the results.
Acknowledgements
We are highly indebted to Prof. Xu Jun for his kindly help, to the neurosurgery team of Nanjing Brain Hospital for providing necessary information regarding the research & also for their support in completing the research. The research programs in the authors’ laboratories are supported by grants from the Youth Training Program of Nanjing (No. QRX11009), Nanjing Medical Science and Technique Development Foundation, Nanjing Department of Health (No. ZKX13035) (No. QRX11197), a nd National Natural Science Foundation of China (81271003).
Disclosure of conflict of interest
None.
References
- 1.Monz D, Munnia A, Comtesse N, Fischer U, Steudel WI, Feiden W, Glass B, Meese EU. Novel tankyrase-related gene detected with meningioma-specific sera. Clin Cancer Res. 2001;7:113–119. [PubMed] [Google Scholar]
- 2.Zhu HD, Xie Q, Gong Y, Mao Y, Zhong P, Hang FP, Chen H, Zheng MZ, Tang HL, Wang DJ, Chen XC, Zhou LF. Lymphoplasmacyte-rich meningioma: our experience with 19 cases and a systematic literature review. Int J Clin Exp Med. 2013;6:504–515. [PMC free article] [PubMed] [Google Scholar]
- 3.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. discussion 226-219. [DOI] [PubMed] [Google Scholar]
- 4.Spatola C, Privitera G. Recurrent intracranial hemangiopericytoma with extracranial and unusual multiple metastases: case report and review of the literature. Tumori. 2004;90:265–268. doi: 10.1177/030089160409000222. [DOI] [PubMed] [Google Scholar]
- 5.Alen JF, Lobato RD, Gomez PA, Boto GR, Lagares A, Ramos A, Ricoy JR. Intracranial hemangiopericytoma: study of 12 cases. Acta Neurochir (Wien) 2001;143:575–586. doi: 10.1007/s007010170062. [DOI] [PubMed] [Google Scholar]
- 6.Nemes Z. Differentiation markers in hemangiopericytoma. Cancer. 1992;69:133–140. doi: 10.1002/1097-0142(19920101)69:1<133::aid-cncr2820690124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Moss TH. Immunohistochemical characteristics of haemangiopericytic meningiomas: comparison with typical meningiomas, haemangioblastomas and haemangiopericytomas from extracranial sites. Neuropathol Appl Neurobiol. 1987;13:467–480. doi: 10.1111/j.1365-2990.1987.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 8.Iwaki T, Fukui M, Takeshita I, Tsuneyoshi M, Tateishi J. Hemangiopericytoma of the meninges: a clinicopathologic and immunohistochemical study. Clin Neuropathol. 1988;7:93–99. [PubMed] [Google Scholar]
- 9.Alawi F, Stratton D, Freedman PD. Solitary fibrous tumor of the oral soft tissues: a clinicopathologic and immunohistochemical study of 16 cases. Am J Surg Pathol. 2001;25:900–910. doi: 10.1097/00000478-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Perry A, Scheithauer BW, Nascimento AG. The immunophenotypic spectrum of meningeal hemangiopericytoma: a comparison with fibrous meningioma and solitary fibrous tumor of meninges. Am J Surg Pathol. 1997;21:1354–1360. doi: 10.1097/00000478-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Yuri T, Kanematsu S, Lei YC, Kuwata M, Oishi M, Tsubura A. Solitary fibrous tumor of soft tissue: a case report and immunohistochemical study. Med Mol Morphol. 2010;43:60–64. doi: 10.1007/s00795-009-0451-1. [DOI] [PubMed] [Google Scholar]
- 12.Tihan T, Viglione M, Rosenblum MK, Olivi A, Burger PC. Solitary fibrous tumors in the central nervous system. A clinicopathologic review of 18 cases and comparison to meningeal hemangiopericytomas. Arch Pathol Lab Med. 2003;127:432–439. doi: 10.5858/2003-127-0432-SFTITC. [DOI] [PubMed] [Google Scholar]
- 13.Sundaram C, Uppin SG, Uppin MS, Rekha JS, Panigrahi MK, Purohit AK, Rammurti S. A clinicopathological and immunohistochemical study of central nervous system hemangiopericytomas. J Clin Neurosci. 2010;17:469–472. doi: 10.1016/j.jocn.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Chang IW, Lin JW, Wu YT. The status of MGMT protein expression is a prognostic factor for meningeal hemangiopericytoma: a clinicopathologic and immunohistochemical study of 12 cases at a single institution. J Neurooncol. 2011;105:563–572. doi: 10.1007/s11060-011-0620-7. [DOI] [PubMed] [Google Scholar]
- 15.Escamilla-Powers JR, Daniel CJ, Farrell A, Taylor K, Zhang X, Byers S, Sears R. The tumor suppressor protein HBP1 is a novel c-myc-binding protein that negatively regulates c-myc transcriptional activity. J Biol Chem. 2010;285:4847–4858. doi: 10.1074/jbc.M109.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M, Casimiro MC, Wang C, Shirley LA, Jiao X, Katiyar S, Ju X, Li Z, Yu Z, Zhou J, Johnson M, Fortina P, Hyslop T, Windle JJ, Pestell RG. p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc Natl Acad Sci U S A. 2009;106:19035–19039. doi: 10.1073/pnas.0910009106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durand A, Champier J, Jouvet A, Labrousse F, Honnorat J, Guyotat J, Fevre-Montange M. Expression of c-Myc, neurofibromatosis Type 2, somatostatin receptor 2 and erb-B2 in human meningiomas: relation to grades or histotypes. Clin Neuropathol. 2008;27:334–345. doi: 10.5414/npp27334. [DOI] [PubMed] [Google Scholar]
- 18.Uzum N, Ataoglu GA. Histopathological parameters with Ki-67 and bcl-2 in the prognosis of meningiomas according to WHO 2000 classification. Tumori. 2008;94:389–397. doi: 10.1177/030089160809400316. [DOI] [PubMed] [Google Scholar]
- 19.Kheirollahi M, Mehr-Azin M, Kamalian N, Mehdipour P. Expression of cyclin D2, P53, Rb and ATM cell cycle genes in brain tumors. Med Oncol. 2011;28:7–14. doi: 10.1007/s12032-009-9412-8. [DOI] [PubMed] [Google Scholar]
- 20.Ohba S, Yoshida K, Hirose Y, Ikeda E, Kawase T. Early malignant transformation of a petroclival meningothelial meningioma. Neurosurg Rev. 2009;32:495–499. doi: 10.1007/s10143-009-0207-3. [DOI] [PubMed] [Google Scholar]
- 21.Kantha R, Saffari HM, Suryati MY. The relationship of p53 protein in meninigioma grading and their various influencing factors amongst neurosurgical patients in Hospital Kuala Lumpur. Med J Malaysia. 2007;62:194–196. [PubMed] [Google Scholar]
- 22.Wang J, Vargas H, Gaal K, Wang X, Peng SK. Malignant hemangiopericytoma arising in neurofibromatosis: a case report with histological, immunohistochemical and ultrastructural studies. Sarcoma. 1999;3:135–139. doi: 10.1080/13577149977776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland H, Livrea M, Ahnert P, Koschny R, Kirsten H, Meixensberger J, Bauer M, Schober R, Fritzsch D, Krupp W. Intracranial hemangiopericytoma: Case study with cytogenetics and genome wide SNP-A analysis. Pathol Res Pract. 2011;207:310–316. doi: 10.1016/j.prp.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Binello E, Bederson JB, Kleinman GM. Hemangiopericytoma: collision with meningioma and recurrence. Neurol Sci. 2010;31:625–630. doi: 10.1007/s10072-010-0227-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Zhao JZ. Clinical and pathological characteristics of primary intraspinal hemangiopericytoma and choice of treatment. Chin Med J (Engl) 2007;120:115–119. [PubMed] [Google Scholar]
- 26.Hahn HP, Bundock EA, Hornick JL. Immunohistochemical staining for claudin-1 can help distinguish meningiomas from histologic mimics. Am J Clin Pathol. 2006;125:203–208. doi: 10.1309/G659-FVVB-MG7U-4RPQ. [DOI] [PubMed] [Google Scholar]
- 27.Yoo H, Baia GS, Smith JS, McDermott MW, Bollen AW, Vandenberg SR, Lamborn KR, Lal A. Expression of the hypoxia marker carbonic anhydrase 9 is associated with anaplastic phenotypes in meningiomas. Clin Cancer Res. 2007;13:68–75. doi: 10.1158/1078-0432.CCR-06-1377. [DOI] [PubMed] [Google Scholar]
- 28.Guevara P, Escobar-Arriaga E, Saavedra-Perez D, Martinez-Rumayor A, Flores-Estrada D, Rembao D, Calderon A, Sotelo J, Arrieta O. Angiogenesis and expression of estrogen and progesterone receptors as predictive factors for recurrence of meningioma. J Neurooncol. 2010;98:379–384. doi: 10.1007/s11060-009-0086-z. [DOI] [PubMed] [Google Scholar]
- 29.Ludlum DB. DNA alkylation by the haloethylnitrosoureas: nature of modifications produced and their enzymatic repair or removal. Mutat Res. 1990;233:117–126. doi: 10.1016/0027-5107(90)90156-x. [DOI] [PubMed] [Google Scholar]
- 30.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 31.Jones RK, Searle RF, Bulmer JN. Apoptosis and bcl-2 expression in normal human endometrium, endometriosis and adenomyosis. Hum Reprod. 1998;13:3496–3502. doi: 10.1093/humrep/13.12.3496. [DOI] [PubMed] [Google Scholar]
- 32.Mertens HJ, Heineman MJ, Evers JL. The expression of apoptosis-related proteins Bcl-2 and Ki67 in endometrium of ovulatory menstrual cycles. Gynecol Obstet Invest. 2002;53:224–230. doi: 10.1159/000064569. [DOI] [PubMed] [Google Scholar]


