Abstract
Spinal cord injury (SCI) is not only devastating but also represents a public health burden for society. Endoplasmic reticulum stress (ERS) is implicated in secondary injury following damage to the SC. Hyperbaric oxygen (HBO) treatment can improve the recovery of motor function after SCI, but the effect of HBO on the ERS response is unknown. This study tested the hypothesis that HBO treatment protects against secondary SCI by inhibiting the ERS response via regulation of glucose-regulated protein (GRP) 78 and c-Jun N-terminal kinase (JNK) expression. Rats were randomly assigned to sham, SCI, and SCI + HBO groups and the extent of neuronal damage and neurological recovery were evaluated 1, 3, 7, and 14 days after surgery. GRP78 and JNK expression was evaluated by immunohistochemical, western blot, and real-time reverse transcription-polymerase chain reaction analyses, while caspase-3 activation was evaluated by enzyme-linked immunosorbent assay. SCI resulted in an upregulation in GRP78 and JNK expression compared to sham-operated animals. HBO treatment increased GRP78 level, but decreased that of JNK and suppressed caspase-3 activation as well as neuronal damage relative to the SCI group. In addition, hind limb motor function was improved by HBO treatment. HBO treatment reduces SCI-induced neuronal death and promotes the recovery of neurological function recovery by inhibiting the ERS response via modulation of GRP78 and JNK expression levels.
Keywords: Hyperbaric oxygen, spinal cord injury, glucose-regulated protein 78, c-Jun N-terminal kinase
Introduction
There are currently no effective therapeutic options for treating spinal cord injury (SCI) owing to the complexity of the pathophysiological process, which includes inflammation, hypoxia, excitotoxicity, blood brain barrier disruption, ischemia, and demyelination of axons [1]. It is essential to elucidate the molecular mechanisms underlying these events so that potential targets for therapeutic intervention can be identified.
Recent studies have reported the activation of the endoplasmic reticulum stress (ERS) response following contusive SCI in rats and mice [2,3], in which cells activate a self-protective mechanism known as the unfolded protein response (UPR) involving the induction of molecular chaperones such as glucose-regulated protein (GRP)78 in the ER, and translational inhibition and degradation of ER-associated proteins [4]. However, the ERS response can also activate apoptosis if ER function is not quickly restored, leading to massive cell death [5]. This is mediated in part by the phosphorylation of c-Jun N-terminal kinase (JNK), a major effector of the ERS response [6]. Therefore, targeting components of the ERS signaling pathway represents a potential therapeutic approach for mitigating the damage caused by SCI.
Hyperbaric oxygen (HBO) treatment can promote the recovery of neurological function and block pathological changes induced by SCI [7-10]. However, there have been no studies investigating the effect of HBO treatment on the ERS response. In this study, changes in GRP78 expression, JNK phosphorylation, and caspase-3 activation were assessed in a rat model of SCI. The effect of HBO treatment on the recovery of neurological function after SCI was also evaluated. The results clarify the molecular basis for the therapeutic effects of HBO and provide support for its use in treating SCI.
Materials and methods
Animals and surgery
Sprague-Dawley rats (n = 72), 8 weeks old, weighing 220-250 g, were obtained from the Center of Experimental Animals of Capital Medical University (Beijing, China). The rats were maintained on a 12:12-h light/dark cycle with free access to food and water, and divided into sham, SCI, and SCI + HBO groups using the randomization table method. Each group was subdivided into five subgroups of six animals each that were evaluated 1, 3, 7, and 14 days after injury.
The SCI rat model was generated using a previously described weight-drop method [11]. Dorsal laminectomy at the level of the 10th thoracic vertebra was carried out under anesthesia with 10% chloral hydrate (350 mg/kg by intraperitoneal injection). A contusion was created using the Multicenter Animal SCI Study impactor by dropping a 10-g rod from a distance of 25 mm onto the exposed SC. For sham surgery, animals underwent laminectomy and clips were placed on the vertebral spinal processes without causing impact injury. After surgery, animals were returned to their home cages and monitored for the development of postoperative infections or signs of discomfort. Animal protocols were approved by the Committee on the Ethics of Animal Experiments of Capital Medical University.
HBO treatment
Rats in the SCI + HBO group were placed in a hyperbaric chamber 6 h after surgery where they were exposed to 2.0 atmospheres absolute (ATA) of 100% oxygen once daily for 60 min. The chamber was initially flushed with 100% oxygen for 5 min; the pressure was then increased to 2.0 ATA for 10 min, followed by slow decompression over 15 min to normobaric air (21% O2). Rats in the sham and SCI groups were postoperatively treated with normobaric air at 1.0 ATA.
Assessment of motor function
The Basso, Beattie, and Bresnahan (BBB) locomotor rating scale [12] was used to evaluate the recovery of hindlimb motor function. The initial BBB score for each group was 21. SCI was characterized by dragging of the body on forelimbs, contact between the abdomen and dorsal side of the hind paws and the ground, and loss of hindlimb motor and weight-bearing functions and urine retention, with a BBB score of 0. Animals were blindly scored by two trained laboratory technicians 1, 3, 7, and 14 days after surgery.
Tissue sample preparation
Rats were anesthetized and transcardial perfusion was performed with saline followed by ice-cold 4% paraformaldehyde. SC tissue samples (4-6 mm) were collected from the center of the injury site and divided into two parts; one was fixed with 10% formaldehyde solution at 4°C for 1 week for histological evaluation, and the other was preserved at -80°C for molecular analysis.
Histological analysis
SC tissue samples were embedded in paraffin and sectioned at a thickness of 5 μm. Sections were stained with hematoxylin and eosin and examined by light microscopy at 200 × magnification (Eclipse E100; Nikon, Tokyo, Japan). Neuronal damage was evaluated by an observer blinded to the treatment groups, by counting the average number of uninjured neurons in the gray matter of the SC. Injured neurons were defined by shrinkage of the cell body; an eosinophilic cytoplasm with a loss of Nissl-positive granules; triangular and pyknotic nuclei.
Immunohistochemistry
Tissue sections were deparaffinized and rehydrated, and antigen retrieval was performed according to standard protocols. After treatment with 3% H2O2 in methanol and normal non-immune goat serum, sections were incubated first with primary antibodies against GRP78 (1:200) and phosphorylated (p-) JNK (1:1000) (both from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C, followed by biotinylated goat anti-rabbit IgG (Dako, Glostrup, Denmark) for 50 min at room temperature and streptavidin-peroxidase treatment (Dako). Phosphate-buffered saline (PBS) replaced primary antibody in the negative control. Diaminobenzidine was applied to visualize peroxidase activity, and sections were counterstained with hematoxylin. Five fields on each of three slides per animal were randomly selected for visualization by light microscopy. Analysis was performed using Image J software (National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
Frozen SC tissue was homogenized in ice-cold isolation buffer containing 250 mmol/L sucrose, 10 mmol/L triethanolamine, 1 mg/ml leupeptin, and 0.1 mg/ml phenylmethylsulfonyl fluoride. Homogenates were centrifuged at 15,000 × g for 10 min at 4°C, and the protein concentration in the supernatant was measured using a protein assay kit (Sunbio, Beijing, China). Total protein (50 µg) in each sample was resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes, which were incubated overnight at 4°C with primary antibodies against GRP78 (1:200), p-JNK (1:1000), and β-actin (1:2000) (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc.) diluted at 1:10,000 for 2 h at 37°C, and protein was visualized using an enhanced chemiluminescence kit (BestBio Inc., Shanghai, China). The film was scanned (Konica Minolta Medical Imaging, Inc., Wayne, NJ, USA) and protein expression was quantified with Adobe Photoshop (Adobe, Mountain View, CA, USA) and LabWorks (UVP, Upland, CA, USA) software.
RNA extraction and real-time (RT)-PCR
Total RNA (5 μg) was extracted from frozen SC tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and an extraction kit (Takara Biotechnology, Dalian, China) according to the manufacturer’s instructions. First strand cDNA was synthesized by reverse transcription using Moloney Murine Leukemia Virus reverse transcriptase (Bioer, Hangzhou, China) and real-time PCR was performed using BioEasy SYBR Green I Real Time PCR Kit (Sysmex UK Ltd., Milton Keynes, UK) on a Line-Gene sequence detector (Bioer). The amplification reaction was 45 cycles of 95°C for 20 s; 60°C for 25 s; and 72°C for 30 s. PCR products were detected by incorporation of SYBR green during the reaction, and were verified by generating an amplification curve and by gel electrophoresis. GRP78, p-JNK, and actin primer sequences are listed in Table 1. Amplification products were quantified using the 2-ΔΔCT method and were measured relative to level of glyceraldehyde-3-phosphate dehydrogenase.
Table 1.
Sequences of Primers
| Gene | Primer | Sequence |
|---|---|---|
| GRP78 | Primer (forward) | 5’-CATAGCCAACGATCAGGGCA-3’ |
| Primer(reverse) | 5’-TCATTCCAAGTGCGTCCGAT-3’ | |
| P-JNK | Primer (forward) | 5’-AAGGACCAGTTCCCAGAGGT-3’ |
| Primer (reverse) | 5’-CCAACTCTACCTGCTTCCCG-3’ | |
| Actin | Primer (forward) | 5’-GCAAGTTCAACGGCACAG-3’ |
| Primer (reverse) | 5’-CGCCAGTAGACTCCACGAC-3’ |
GRP78, glucose-regulated protein 78; p-JNK, Phospho-c-Jun-N-terminal kinase.
Enzyme-linked immunosorbent assay (ELISA)
Caspase-3 activity in tissue homogenates was detected by ELISA using a colorimetric caspase-3 assay kit (Sigma, St. Louis, MO, USA) according to the manufacturer’s instructions. Briefly, the synthetic caspase-3 substrate acetyl-Asp-Glu-Val-Asp-p-nitroanilide was added to the reaction mixture, with a control reaction prepared in parallel to exclude nonspecific hydrolysis of the substrate. Both mixtures were incubated at 37°C for 1-2 h and the absorbance was read at 405 nm; caspase-3 activity is expressed as an optical density value at 405 nm.
Statistical analysis
Quantitative data are expressed as mean ± standard deviation. One-way analysis of variance was used to compare experimental groups. P values < 0.05 were considered statistically significant. Analyses were performed using SPSS v.15.0 (SPSS Inc., Chicago, IL, USA).
Results
SCI model
Rats in the SCI and SCI + HBO groups showed complete paralysis of both lower extremities (BBB scores of 0 or 1) on the right postoperative day, whereas those in the sham group were able to stand and walk on their hind limbs (BBB scores of 20 or 21 points), demonstrating that a reliable model of SC impact injury was established (Figure 1).
Figure 1.
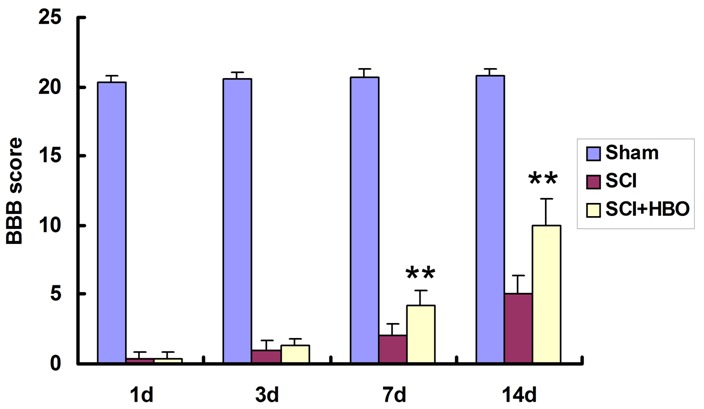
BBB scores for hind limb motor function. Rats in the SCI groups were subjected to SC contusion using a 10-g weight dropped from a height of 25 mm. Sham-operated control animals underwent surgery without injury. The SCI + HBO group received HBO treatment (100% O2) after the surgery, while those in the Sham and SCI groups were exposed to normobaric air (21% O2) for the same amount of time. Motor function was evaluated 1, 3, 7, and 14 days post-surgery. Data represent mean ± SD. **P < 0.01 vs. SCI.
HBO promotes the recovery of motor function after SCI
The BBB locomotor rating scale was used to evaluate the extent recovery of motor function following SCI. Scores in the SCI and SCI + HBO groups were significantly lower than in the sham group (P < 0.01; Figure 1). Compared to the SCI group, injured rats that received HBO treatment had higher scores on post-operative days 7 and 14 (P < 0.01), indicating that the treatment improved motor function in these animals.
HBO attenuates neuronal damage following SCI
In sham-operated rats, SC neurons were intact (Figure 2A). SC contusion caused tissue damage resulting in neurons that were shrunken or had pale, homogeneous cytoplasm (Figure 2B). The occurrence of these features was markedly attenuated in the SC of rats that received HBO treatment (Figure 2C). The differences between SCI + HBO and SCI groups were observed on days 3 and 7 post-injury (Figure 2D).
Figure 2.
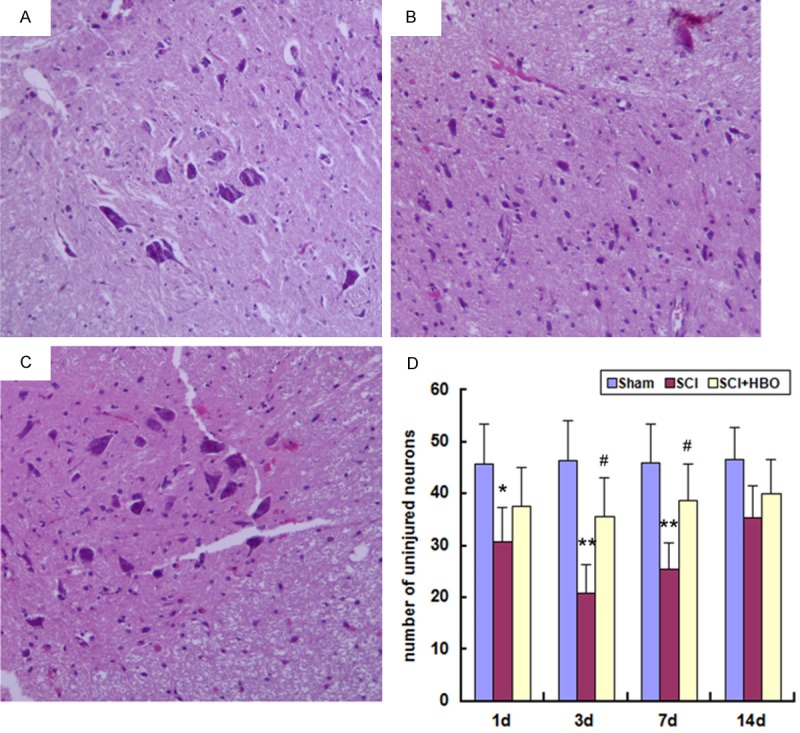
Histological analysis of the SC on day 3 after surgery. Experimental groups are as described in the Figure 1 legend. Tissue sections were stained with hematoxylin and eosin and visualized by light microscopy. The number of uninjured neurons was quantified at 200 × magnification. Data represent mean ± SD. *P < 0.05, **P < 0.01 vs. Sham; #P < 0.05 vs. SCI.
HBO inhibits the ERS response via upregulation of GRP78 and downregulation of p-JNK
The mechanism underlying the effects of HBO treatment was investigated by immunohistochemical detection of GRP78 and p-JNK, two proteins that are implicated in the ERS response. In the control group, GRP78 and p-JNK immunoreactivity was observed at low levels in gray and white matter (Figure 3A, 3D). GRP78 and p-JNK expression was increased in animals subjected to SCI (Figure 3B, 3E); however, in those that received HBO treatment, the expression of GRP78 was higher (Figure 3C) and that of p-JNK was lower (Figure 3F) than in untreated SCI animals. Between-groups comparisons of GRP78- and p-JNK-positive cells at different time points are shown in Tables 2 and 3.
Figure 3.
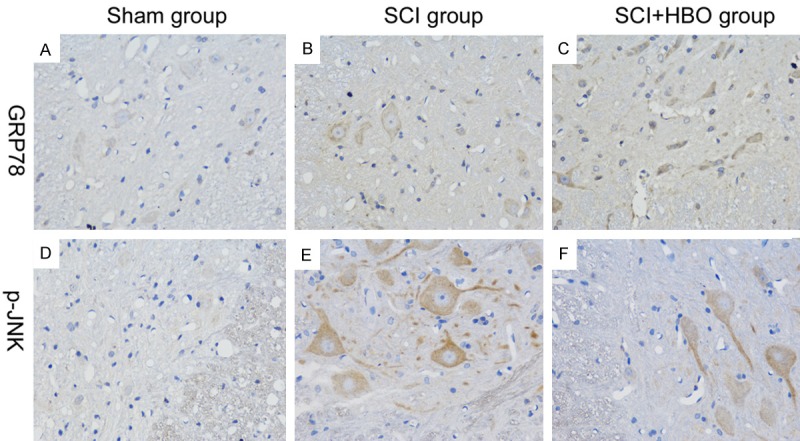
GRP78 and p-JNK expression in the SC 1 day after surgery. Experimental groups are as described in the Figure 1 legend. GRP78- and p-JNK-positive cells were identified by immunohistochemistry and analyzed at 400 × magnification.
Table 2.
Number of GRP78 positive cells
| Group | 1d | 3d | 7d | 14d |
|---|---|---|---|---|
| Sham | 10.3 ± 2.2 | 10.5 ± 1.8 | 10.5 ± 2.0 | 10.1 ± 1.9 |
| SCI | 55.3 ± 7.7** | 33.6 ± 4.4** | 12.5 ± 1.9 | 12 ± 2.4 |
| SCI + HBO | 65.4 ± 8.7** | 46.2 ± 6.2**,# | 15.3 ± 2.7* | 13 ± 2.1 |
Data are presented as mean ± standard deviation.
P < 0.05 vs. Sham;
P < 0.01 vs. Sham.
P < 0.05 vs. SCI.
Table 3.
Number of p-JNK positive cells
| Group | 1d | 3d | 7d | 14d |
|---|---|---|---|---|
| Sham | 9.5 ± 1.5 | 9.5 ± 1.3 | 9.3 ± 1.4 | 9.6 ± 1.6 |
| SCI | 62.9 ± 6.7** | 48.9 ± 5.1** | 31.9 ± 4.2** | 10.3 ± 2.0 |
| SCI + HBO | 72.7 ± 8.9** | 61.7 ± 6.2**,# | 43.7 ± 4.9**,## | 12.4 ± 1.7 |
Data are presented as mean ± standard deviation.
P < 0.01 vs Sham.
P < 0.05 vs SCI;
P < 0.01 vs SCI.
GRP78 and p-JNK mRNA and protein levels were low in sham-operated animals, and were upregulated following SCI, reaching a peak on day 1 (P < 0.01) (Figures 4 and 5); the elevated expression levels of GRP78 and p-JNK persisted on days 3 and 7, respectively, before declining to baseline on days 7 (for GRP78) and 14. The mRNA and protein levels of GRP78 were upregulated, while those of p-JNK were downregulated, in the SCI + HBO relative to the SCI group on day 3 (for GRP78) and on day 3 and 7 (for p-JNK) (P < 0.05).
Figure 4.
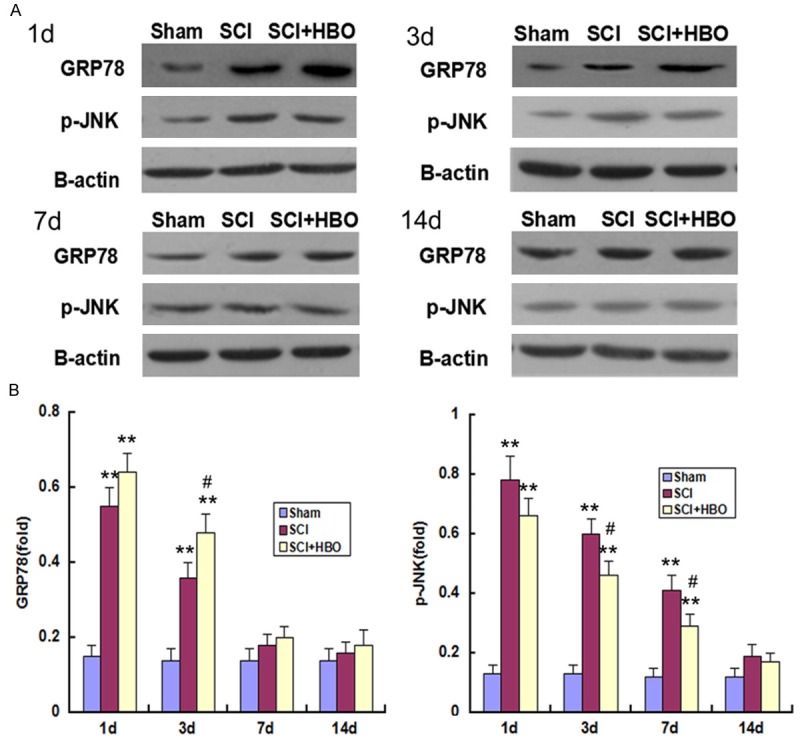
GRP78 and p-JNK protein expression in the SC following SCI and HBO treatment. Experimental groups are as described in the Figure 1 legend. A: Representative immunoblots of GRP78 and p-JNK expression at indicated time points are shown. B: Quantitative analysis of GRP78 and p-JNK levels. Data represent mean ± SD. **P < 0.01 vs. Sham; #P < 0.05 vs. SCI.
Figure 5.
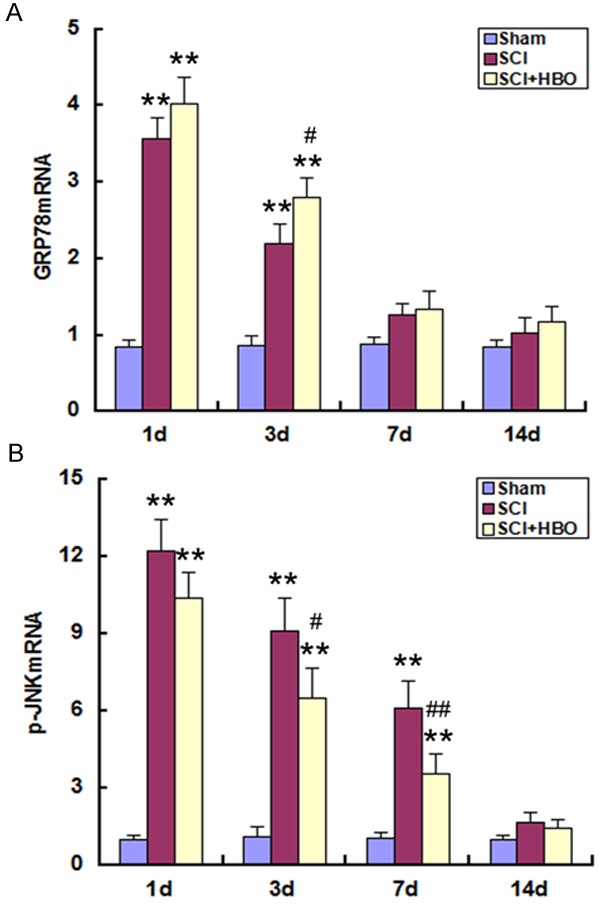
GRP78 and p-JNK mRNA expression in the SC following SCI and HBO treatment. Experimental groups are as described in the Figure 1 legend. (A) GRP78 and (B) p-JNK mRNA expression was determined by real-time PCR at indicated time points. Data represent mean ± standard deviation. **P < 0.01 vs. Sham; #P < 0.05, ##P < 0.01 vs. SCI.
HBO suppresses apoptosis by inhibiting caspase-3 activation
To investigate the molecular basis for the functional recovery observed in HBO-treated animals, the expression of activated caspase-3 was examined. SCI increased caspase-3 activation in SC tissue relative to control animals on days 1, 3, and 7 post-injury (P < 0.01) (Figure 6). In HBO-treated animals, caspase-3 activation was decreased on days 3 and 7 as compared to the SCI group (P < 0.05), indicating that HBO mitigates ERS-induced apoptosis resulting from injury to the SC.
Figure 6.
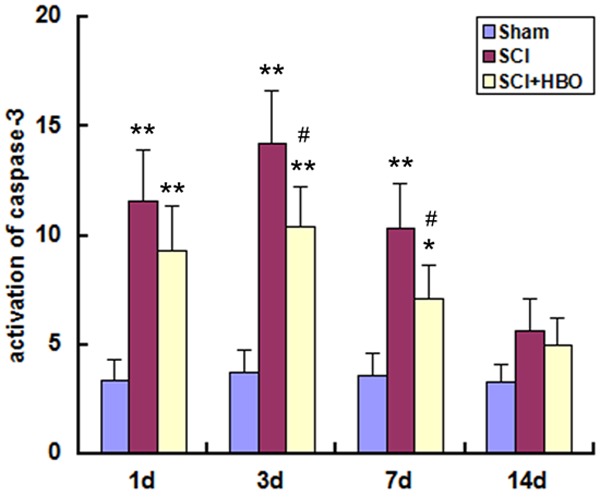
Activation of caspase-3 in the SC following SCI and HBO treatment. Experimental groups are as described in the Figure 1 legend. Activation of caspase-3 was determined by enzyme-linked immunosorbent assay at indicated time points. Data represent mean ± standard deviation. *P < 0.05, **P < 0.01 vs. Sham; #P < 0.05 vs. SCI.
Discussion
The results of this study demonstrated that HBO treatment in rats following SCI inhibited the ERS response by inducing the upregulation of GRP78 and suppressing p-JNK expression levels, thereby inhibiting caspase-3 activation. This led to a greater recovery of hind limb motor function in these animals as compared to those that were injured but untreated.
ER function is dysregulated when cells are exposed to stress. Since the folding and quality control of membrane and secretory proteins occur in the ER, cellular stress results in the accumulation of unfolded proteins in the ER lumen, which can trigger an apoptotic program via the UPR [13]. A major aspect of this response is the upregulation of ER-localized molecular chaperones such as GRP78 and GRP94, which are involved in the repair of unfolded proteins [14]. Suppressing GRP78 expression enhances apoptosis and disrupts cellular calcium homeostasis in hippocampal neurons exposed to excitotoxic and oxidative stress [15], and its expression was induced in motor neurons after SC ischemia-reperfusion injury [16]. In this study, GRP78 expression was upregulated 1 day after SCI and declined thereafter, indicating that the ERS response was immediately activated after injury.
The JNK signaling pathway is implicated in ERS-mediated cell death [17]. Genomic profiling analyses have shown an up regulation of c-Jun transcription 1, 24, and 48 h after SCI [18]. JNK activation was demonstrated starting 1 h after SCI and persisted for 3 days [19]; a similar time course for c-Jun expression has also been shown [20]. In the present study, the expression of p-JNK-the activated form of the protein-increased after SCI and persisted for 7 days. JNK activation induces apoptosis of neurons after SCI, and its suppression may have neuroprotective effects [19,21,22].
HBO treatment has been shown to improve limb mobility and suppress various pathological changes following SCI by increasing the partial pressure of oxygen in damaged nerve tissue, inhibiting a secondary inflammatory response and apoptosis triggered by injury, and promoting the regeneration of nerve tissue [7-10,23,24]. Thus, HBO is the major treatment approach recommended in cases of SCI, although the effects of HBO treatment on the ERS response have not yet been reported. We addressed this question by evaluating changes in GRP78 expression level and JNK activation in response to HBO in rats with SCI. Compared to untreated, injured animals, HBO treatment induced an upregulation in GRP78 and a downregulation in p-JNK expression post-injury, suggesting that HBO inhibits ERS in the early stages following SCI.
The signaling pathways that are activated during ERS-induced apoptosis converge on caspase-3 activation, which results in extensive neuronal death [25]. In this study, in accordance with changes in p-JNK expression, SCI induced an increase in activated caspase-3 level, which was suppressed by HBO treatment. These results, combined with the observation that HBO treatment improved motor function scores in injured animals, suggest that HBO treatment suppresses caspase-3 activation and neuronal apoptosis via inhibition of JNK signaling, leading to greater recovery of hind limb motor function.
In summary, the results of this study demonstrate that the ERS response causes secondary injury to the SC; this can be mitigated to a large extent by exposure to HBO following injury, which inhibits the induction of neuronal apoptosis and promotes the recovery of neurological function. These findings elucidate the molecular basis of HBO treatment and provide evidence that it can be used for clinical applications to improve the outcome of patients who experience SCI.
Acknowledgements
This study was supported by the grant from International Science and Technology Cooperation Program of Ministry of Science and Technology of China (2012DFA31240).
Disclosure of conflict of interest
None.
References
- 1.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 2.Penas C, Guzmán MS, Verdu E, Forés J, Navarro X, Casas C. Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J Neurochem. 2007;102:1242–1255. doi: 10.1111/j.1471-4159.2007.04671.x. [DOI] [PubMed] [Google Scholar]
- 3.Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman M, Whittemore SR. Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia. 2011;59:1489–1502. doi: 10.1002/glia.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Chan PH. Induction of GRP78 by ischemic preconditioning reduces endoplasmic reticulum stress and prevents delayed neuronal cell death. J Cereb Blood Flow Metab. 2003;23:949–961. doi: 10.1097/01.WCB.0000077641.41248.EA. [DOI] [PubMed] [Google Scholar]
- 5.Tabas I, Ron D. Integrating mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aouadi M, Laurent K, Prot M, Le Marchand-Brustel Y, Binétruy B, Bost F. Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages. Diabetes. 2006;55:281–289. doi: 10.2337/diabetes.55.02.06.db05-0963. [DOI] [PubMed] [Google Scholar]
- 7.Dayan K, Keser A, Konyalioglu S, Erturk M, Aydin F, Sengul G, Dagci T. The effect of hyperbaric oxygen on neuroregeneration following acute thoracic spinal cord injury. Life Sci. 2012;90:360–364. doi: 10.1016/j.lfs.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Lu PG, Feng H, Yuan SJ, Zhang RW, Li M, Hu R, Liu ZS, Yin J. Effect of preconditioning with hyperbaric oxygen on cell apoptosis after spinal cord injury in rats. J Neurosurg Sci. 2013;57:253–258. [PubMed] [Google Scholar]
- 9.Yang J, Liu X, Zhou Y, Wang G, Gao C, Su Q. Hyperbaric oxygen alleviates experimental (spinal cord )injury by downregulating HMGB1/NF-κB expression. Spine (Phila Pa 1976) 2013;38:E1641–1648. doi: 10.1097/BRS.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Zhou Y, Wang Z, Yang J, Gao C, Su Q. Effect of VEGF and CX43 on the promotion of neurological recovery by hyperbaric oxygen treatment in spinal cord-injured rats. Spine J. 2014;14:119–127. doi: 10.1016/j.spinee.2013.06.084. [DOI] [PubMed] [Google Scholar]
- 11.Allen EA, Erhardt EB, Calhoun VD. Data visualization in the neurosciences: overcoming the curse of dimensionality. Neuron. 2012;74:603–608. doi: 10.1016/j.neuron.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang JW, Lee JK, Kim SH. Activation of matrix metalloproteinases-9 after photothrombotic spinal cord injury model in rats. J Korean Neurosurg Soc. 2011;50:288–292. doi: 10.3340/jkns.2011.50.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002;3:411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- 14.Luo S, Baumeister P, Yang S, Abcouwer SF, Lee AS. Induction of Grp78/BiP by translational block: activation of the Grp78 promoter by ATF4 through and upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J Biol Chem. 2003;278:37375–37385. doi: 10.1074/jbc.M303619200. [DOI] [PubMed] [Google Scholar]
- 15.Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999;155:302–314. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- 16.Sakurai M, Takahashi G, Abe K, Horinouchi T, Itoyama Y. Endoplasmic reticulum stress induced in motor neurons by transient spinal cord ischemia in rabbits. J Thorac Cardiovasc Surg. 2005;130:640–645. doi: 10.1016/j.jtcvs.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bareyre FM, Schwab ME. Inflammation, degeneration and regeneration in the injured spinal cord: insights from DNA microarrays. Trends Neurosci. 2003;26:555–563. doi: 10.1016/j.tins.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Li QM, Tep C, Yune TY, Zhou XZ, Uchida T, Lu KP, Yoon So. Opposite regulation of oligodendrocyte apoptosis by JNK3 and Pin1 after spinal cord injury. J Neurosci. 2007;27:8395–8404. doi: 10.1523/JNEUROSCI.2478-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin KJ, Kim GM, Lee JM, He YY, Xu J, Hsu CY. JNK activation contributes to DP5 induction and apoptosis following traumatic spinal cord injury. Neurobiol Dis. 2005;20:881–889. doi: 10.1016/j.nbd.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Paterniti I, Melani A, Cipriani S, Corti F, Mello T, Mazzon E, Esposito E, Bramanti P, Cuzzocrea S, Pedata F. Selective adenosine A2A receptor agonists and antagonists protect against spinal cord injury through peripheral and central effects. J Neuroinflammation. 2011;8:31. doi: 10.1186/1742-2094-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Repici M, Chen X, Morel MP, Doulazmi M, Sclip A, Cannaya V, Veglianese P, Kraftsik R, Mariani J, Borsello T, Dusart I. Specific inhibition of the JNK pathway promotes locomotor recovery and neuroprotection after mouse spinal cord injury. Neurobiol Dis. 2012;46:710–721. doi: 10.1016/j.nbd.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Tai PA, Chang CK, Niu KC, Lin MT, Chiu WT, Lin CM. Attenuating experimental spinal cord injury by hyperbaric oxygen: stimulating production of vasculoendothelial and glial cell line-derived neurotrophic growth factors and interleukin-10. J Neurotrauma. 2010;27:1121–1127. doi: 10.1089/neu.2009.1162. [DOI] [PubMed] [Google Scholar]
- 24.Rinaldi B, Cuzzocrea S, Donniacuo M, Capuano A, Di Palma D, Imperatore F, Mazzon E, Di Paola R, Sodano L, Rossi F. Hyperbaric oxygen therapy reduces the toll-like receptor signaling pathway in multiple organ failures. Intensive Care Med. 2011;37:1110–1119. doi: 10.1007/s00134-011-2241-1. [DOI] [PubMed] [Google Scholar]
- 25.Hitomi J, Katayama T, Taniguchi M, Honda A, Imaizumi K, Tohyama M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci Lett. 2004;357:127–130. doi: 10.1016/j.neulet.2003.12.080. [DOI] [PubMed] [Google Scholar]


