Abstract
Background: Parathyroid hormone (PTH) increases both bone formation (BMD) and bone resorption, whereas alendronate reduces bone resorption. It is possible that the combination therapy of PTH with alendronate will enhance their effects on BMD. Therefore, we conducted this meta-analysis to evaluate the efficacy of the combination therapy of PTH with alendronate in the treatment of patients with osteoporosis. Methods: A comprehensive literature search of PubMed, Embase, and Web of Science was conducted to identify relative studies. Eligible studies were randomized controlled trials (RCT), which assessed the efficacy of combination therapy in patients with osteoporosis. The outcomes included the mean percent increases in BMD of lumbar spine, femoral neck, total hip, and distal radius. Weighted mean difference (WMD) with 95% confidence intervals (CIs) were calculated using of random-effects or fixed-effects model, depending on the heterogeneity between the included studies. Results: Six RCTs with a total number of 833 patients were included in this meta-analysis. The pooled estimates showed that, the combination therapy of PTH with alendronate resulted in a higher mean percent change of increased BMD in distal radius (WMD = 2.45, 95% CI: 1.58, 3.31; P = 0.000), but not in lumbar spine (WMD = -0.83, 95% CI: -3.48, 1.81; P = 0.538), femoral neck (WMD = -0.99, 95% CI: -2.04, 0.07; P = 0.068), and total hip (WMD = -0.06, 95% CI: -0.93, 0.81; P = 0.892). The subgroup analysis based on the dosage and schedule of PTH, study duration, gender of patients, and anabolic agents, were conducted. And results revealed that among the patients in the combination therapy group, greater increases in the spine BMD were observed when the PTH was administered with a dosage of 20 μg (WMD = 2.33, 95% CI: 1.24, 3.43; P = 0.000), or the treatment duration lasted more than 12 months (WMD = 2.23, 95% CI: 1.00, 3.47; P = 0.000), or the combination therapy was used in osteoporosis women (WMD = 1.58, 95% CI: 0.63, 2.53; P = 0.001). However, the combination of PTH of 40 μg with alendronate produced a decrease in the BMD at spine (WMD = -4.56, 95% CI: -7.56, -1.56; P = 0.003) and femoral neck (WMD = -5.82, 95% CI: -9.91, -1.72; P = 0.005). Conclusion: Our findings indicated that the addition of alendronate to PTH in the treatment of osteoporosis, reduced the ability of PTH therapy to increase the BMD at the lumbar spine, femoral neck, and total hip.
Keywords: Osteoporosis, parathyroid hormone, alendronate, meta-analysis
Introduction
Osteoporosis is a disease characterized by loss of bone mass and deterioration of microarchitecture, which lead to bone fragility and increases the risk of fracture [1]. The ideally therapeutic approaches for patients with osteoporosis should increase bone mass, improve bone architecture, and thereby reduce the risk of fracture [2-6].
Alendronate, a potent nitrogen-containing bisphosphonate, is the most widely available treatment for osteoporosis [7]. As an antiresorptive drug, alendronate could directly reduce the number and activity of osteoclasts, and thereby suppressing the bone formation [8]. The results from double-blind, randomized, placebo-controlled trials have demonstrated that through inhibiting the bone resportion, alendronate significantly increased the bone mineral density (BMD) and reduced the risk of fracture [9-11].
Unlike the alendronate, parathyroid hormone (PTH), is a potent bone formation agent [12-14]. It acts by increasing the osteoblast birth rate and improving the new bone formation [15,16]. The previous studies [17-19] suggest that, both PTH (1-34) (teriparatide) and PTH (1-84) significantly increased the BMD by stimulating bone formation rather than suppressing bone resportion.
PTH has been used in the combination with alendronate in the treatment of patients with osteoporosis or have a high risk of fracture, however, the effect remains controversial. In postmenopausal women, the combined therapy of PTH (1-84) with alendronate for 1 year reduced the ability of PTH (1-84) to increase trabecular BMD of the spine, and the BMD of spine and femoral neck [20]. In osteoporosis men, the combined therapy of PTH (1-34) with daily alendronate for 2 years reduced the ability of PTH (1-34) to increase BMD of the spine and hip [21]. The differences between the effects of combining intact PTH or teriparatide with alendronate in these two studies, may be attributable to the differences in gender (female VS male), treatment duration (2 years VS 1 year), anabolic agents (intact PTH VS teriparatide). Therefore, we conducted this meta-analysis to assess the efficacy of PTH and alendronate on BMD at various skeletal sites (lumbar spine, total hip, femoral neck and distal radius), as well as to evaluate the potential factors (gender, dosage of PTH, study duration, and anabolic agents) which may have an impact on the treatment outcomes.
Methods and materials
Search strategy
We conducted a comprehensive literature search in multiple electronic databases, including PubMed, Embase and Web of Science, from their inception through July 7, 2014. The search algorithm was generated as following: ((“osteoporosis, postmenopausal” [MeSH Terms] OR (“osteoporosis” [All Fields] AND “postmenopausal” [All Fields]) OR “postmenopausal osteoporosis” [All Fields] OR “osteoporosis” [All Fields] OR “osteoporosis” [MeSH Terms]) AND (“parathyroid hormone” [MeSH Terms] OR (“parathyroid”[All Fields] AND “hormone” [All Fields]) OR “parathyroid hormone” [All Fields]) AND (“alendronate” [MeSH Terms] OR “alendronate” [All Fields])) AND Clinical Trial [ptyp]. The search was limited to human subjects and randomized controlled trials (RCTs). No language limitation was imposed. Furthermore, we also manually searched the reference lists of those included studies to identify potential articles which may not be indexed in the common databases.
Study inclusion and exclusion criteria
Studies were included in this meta-analysis if they met the following inclusion criteria: (1) study design: RCT with a duration of at least 6 months; (2) study subjects: patients had a T score of at least 2SD for bone mineral density(BMD) below the mean of young normal people at the femoral neck, total hip, or spine; (3) study intervention: patients in the treatment group received the combined therapy of PTH with alendronate, whereas patients in the control group received other treatment; (4) outcome measure: the outcome measurement included the mean percent increases in BMD of lumbar spine, femoral neck, total hip, and distal radius. Exclusion criteria included non-RCTs or studies published as the following article type: abstracts, review articles, editorials and letters.
Data extraction and quality assessment
A standardized data collection form was used to extract the following information: first author, year of publication, number of patients (intervention/control), the mean percent changes in BMD. In case of multiple publications from the same clinical trial with identical population, only the latest or most information article was included. The disagreements between two investigators were resolved by discussion and consensus. The methodological quality of each study was evaluated by Jadad scale [22]. The scale consists of three items, including randomization (0-2 points), blinding (0-2 points), and dropouts and withdraws (0-1 point), to report the quality of a RCT. The quality scale ranges from 0 to 5 points. Any studies with a score ≥ 3 points are said to be of high quality [23].
Statistical analysis
We assessed the effect of PTH plus alendronate on osteoporosis based on data from the included studies. The mean percent change in the BMD was treated as continuous variables, thus they were expressed as weighted mean difference (WMD) with 95% confidence intervals (CIs). We used the Cochrane Q chi-square test to detect the heterogeneity between the studies, in which significant heterogeneity was defined as a P value less than 0.1. A fixed-effects model (Mantel-Haenszel method) [24] or random-effects model (DerSimonian-Laird method) [25] was used to pool the estimates, depending on the absence or presence of heterogeneity. I2 statistic was estimated to describe the percentage of variability owning to heterogeneity between the studies. Studies with an I2 statistic of < 25%, ~50%, ~75%, ~100% were considered to have no, low, moderate, and high heterogeneity, respectively [26]. Whenever heterogeneity was present, subgroup analysis was conducted to identify the potential sources. Publication bias was assessed by using Begg [27] and Egger tests [28]. A P value less than 0.05 was judged as statistically significant. All analyses were performed by using STATA version 12.0 (Stata Corporation, College Station, TX, USA).
Results
Identification of eligible studies
A total of 347 potential studies were identified by the search strategy defined above, of which 95 articles were excluded for the duplicate records (Figure 1). After manually reviewing the title and abstracts, 201 were excluded based on various reasons (reviews, non-RCTs, animal experiments, or unrelated with our topics). Then the remaining 51 articles were screened for the full-text review, and 45 of them were removed due to lack of sufficient data, or examine other treatment but not the combination therapy of PTH with alendronate. Finally, 6 RCTs met the inclusion criteria and were included in the meta-analysis [20,21,29-32].
Figure 1.
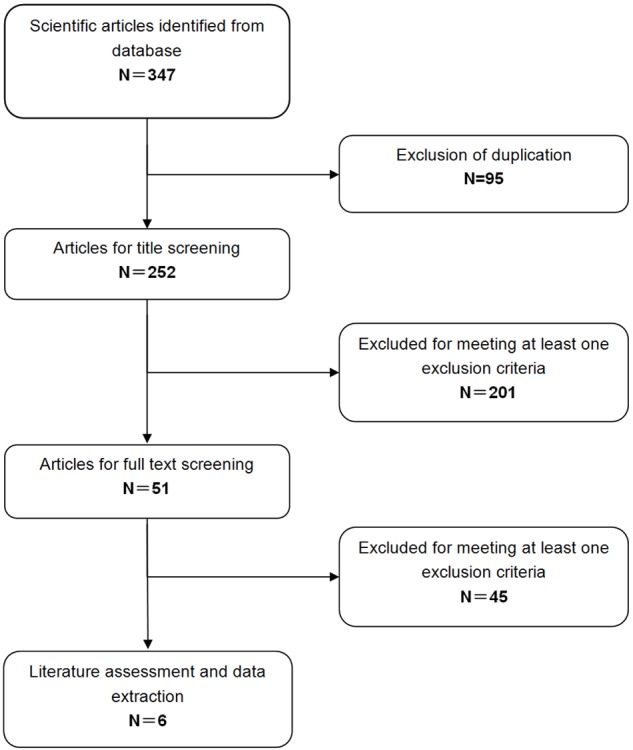
Identification of eligible randomized-controlled trials.
Study characteristics
The main characteristics of the seven RCTs in this meta-analysis were presented in Table 1. The total number of included patients was 833, ranging from 40 to 292 patients per study. These studies were published between 2003 and 2010. Among the 6 studies included here, 5 involved postmenopausal women with osteoporosis [20,29-32], and one study involved osteoporotic men [21]. The follow-up ranged from 12 [20,31] to 30 months [21,29]. The dosage and schedule of PTH was 20 μg daily in three studies [30-32], 40 μg daily in two studies [21,29], and 100 μg daily in one study [20]. The median Jadad score of the include studies was 3 (range from 3 to 4).
Table 1.
Baseline characteristics of the trials included in the meta-analysis
| Author | Treatment regimen | No. of patients | Median age (year, range) | Gender (F/M) | Duration (months) | outcomes | Jaded score |
|---|---|---|---|---|---|---|---|
| Black DM [20] | PTH (1-84) 100 μg daily+ alendronate 10 mg daily | 59 | 70.2 ± 6.8 | F | 12 | Areal BMD: lumbar spine, total hip, femoral neck, distal radius. Volumetric BMD: lumbar spine, total hip, femoral neck, distal radius. Markers of bone turner | 4 |
| PTH (1-84) 100 μg daily | 119 | 69.4 ± 7.3 | F | 12 | |||
| Finkelstein JS [21] | Teriparatide 40 μg subcutaneously daily+ alendronate 10 mg orally once daily | 25 | 58 ± 8 | M | 30 | Areal BMD: Posteroanterior spine, Lateral spine, total hip, femoral neck, distal radius, total body. | 3 |
| Teriparatide 40 μg sc daily | 20 | 57 ± 9 | M | 30 | |||
| Finkelstein JS [29] | Teriparatide 40 μg sc daily+ alendronate 10 mg daily | 20 | 62 ± 7 | F | 30 | Areal BMD: Posterior-anterior spine, total hip, femoral neck, distal radius. Bone turner markers. | 3 |
| Teriparatide 40 μg sc daily | 20 | 65 ± 7 | F | 30 | |||
| Boonen S [30] | Teriparatide 20 μg/d and daily supplements of 500 mg elemental calcium and 400-800 IU vitamin D + alendronate | 107 | 70.3 | F | 24 | Areal BMD: total hip, femoral neck, lumbar spine. | 4 |
| Teriparatide 20 μg/d and daily supplements of 500 mg elemental calcium and 400-800 IU vitaminD + non- bisphosphonate | 49 | 67.9 | F | 24 | |||
| Miller PD [31] | Teriparatide 20 μg/d+ alendronate 10 mg daily or 70 mg weekly | 146 | 67.7 ± 7.8 | F | 12 | Areal BMD: total hip, femoral neck. Volumetric BMD: lumbar spine, total hip. Bone turner markers. | 3 |
| Teriparatide 20 μg/d | 146 | 69.3 ± 7.4 | F | 12 | |||
| Cosman F [32] | Teriparatide 20 μg/d and daily supplements of 500 mg elemental calcium and 400-800 IU vitamin D + alendronate | 52 | 67.8 ± 1.4 | F | 18 | Areal BMD: lumbar spine, total hip, femoral neck. Biochemical markers of bone metabolism. | 3 |
| Teriparatide 20 μg/d and daily supplements of 500 mg elemental calcium and 400-800 IU vitamin D | 50 | 69.1 ± 1.4 | F | 18 |
Effect of PTH plus alendronate on BMD of lumbar spine
Five studies reported the data of lumbar spine areal BMD [20-32]. The aggregated results showed that the combination therapy with PTH and alendronate was not associated with a significant increase in lumbar spine BMD from baseline compared with monotherapy with PTH or alendronate (WMD = -0.83, 95% CI: -3.48, 1.81; P = 0.538) (Figure 2). The test for heterogeneity was significant (P = 0.000, I2 = 84.0%). Therefore, we performed subgroup analysis based on the dosage and schedule of PTH, study duration and anabolic agents, to explore potential sources of heterogeneity. The results suggested that, the combined therapy of 20 μg PTH with alendronate significantly increased lumbar spine BMD (WMD = 2.33, 95% CI: 1.24, 3.43; P = 0.000), whereas the 40 μg PTH significantly decreased the lumbar spine BMD (WMD = -4.56, 95% CI: -7.56, -1.56; P = 0.003). Subgroup analysis on the basis of study duration showed that, the lumbar spine BMD with combination therapy significantly decreased at the 12-month period (WMD = -4.56, 95% CI: -7.56, -1.56; P = 0.000), but increased over the 12-month period (WMD = 2.23, 95% CI: 1.00, 3.47; P = 0.000).
Figure 2.
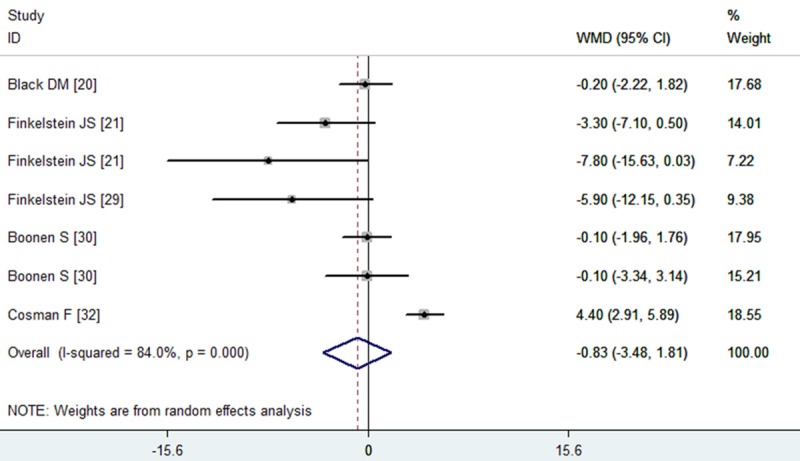
Assessment of the effects of PTH plus alendronate on BMD of the lumbar spine.
In addition, we also conducted the subgroup analysis based on the gender of patients and anabolic agents. For postmenopausal women, the pooled results from 5 studies indicated that, increases in BMD was higher in the combination therapy with PTH and alendronate (WMD = 1.58, 95% CI: 0.63, 2.53; P = 0.001). According to subgroup analysis on the basis of anabolic agents, neither the intact PTH (1-84) (WMD = -0.20, 95% CI: -2.22, 1.82; P = 0.846) nor teriparatide (WMD = -1.16, 95% CI:-4.45, 2.12; P = 0.488) yielded significant increments in lumbar spine BMD, when they were used in the combination therapy with alendronate.
The Begg’s test indicated no existence of publication bias (Z = 1.5, P = 0.133), but the Egger’s test suggested the existence of publication bias (t = -2.94, P = 0.032).
Effect of PTH plus alendronate on BMD of femoral neck
Five studies reported the data of femoral neck BMD [21,29-32], of which all patients were administered with teriparatide rather than the PTH (1-84). The aggregated results showed that the increases in the femoral neck BMD were not significant between the combination therapy and monotherapy (WMD = -0.99, 95% CI: -2.04, 0.07; P = 0.068) (Figure 3). The test for heterogeneity was significant (P = 0.000, I2 = 88.7%). Therefore, we performed subgroup analysis based on the dosage and schedule of PTH, and study duration, to explore potential sources of heterogeneity. The results suggested that, the 40 μg of teriparatide in combination with alendronate lead to a lower BMD at femoral neck (WMD = -5.82, 95% CI: -9.91, -1.72; P = 0.005), when compared to the monotherapy. Subgroup analysis on the basis of study duration showed that, the combination therapy did not significantly increase the femoral neck BMD at 12 months (WMD = -0.75, 95% CI: -2.12, 0.61; P = 0.280) or over 12 months (WMD = -2.01, 95% CI: -4.50, 0.48; P = 0.114).
Figure 3.
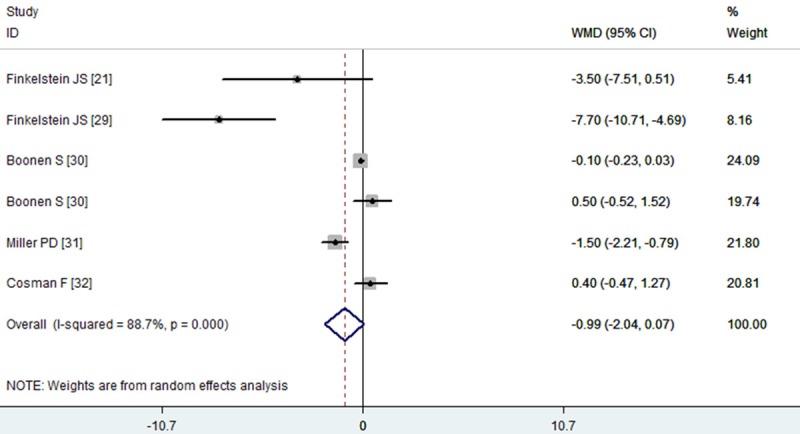
Assessment of the effects of PTH plus alendronate on BMD of the femoral neck.
In addition, we also conducted the subgroup analysis based on the gender of patients and anabolic agents. For postmenopausal women, the pooled results from 4 studies indicated that, the combination therapy did not result in increased femoral neck BMD (WMD = -0.83, 95% CI: -1.91, 0.24; P = 0.128). For men, since there was only one trial assessed the effect of the combination therapy, the pooled effect was not performed.
The Begg’s test and Egger’s test indicated no existence of publication bias (for Begg’s test, Z = 1.13, P = 0.260; for Egger’s test, t = -1.64, P = 0.175).
Effect of PTH plus alendronate on BMD of total hip
All the studies reported the data of total hip BMD [20,21,29-32]. The aggregated results showed that the combination therapy with PTH and alendronate was not associated with a significant increase in total hip BMD from baseline compared with monotherapy with PTH or alendronate (WMD = -0.06, 95% CI: -0.93, 0.81; P = 0.892) (Figure 4). The test for heterogeneity was significant (P = 0.000, I2 = 87.3.0%). Therefore, we performed subgroup analysis based on the dosage and schedule of PTH, study duration and anabolic agents, to explore potential sources of heterogeneity. The results suggested that, neither 20 μg nor 40 μg of PTH in combination with alendronate significantly improved the total hip BMD (for 20 μg PTH: WMD = 0.03, 95% CI: -0.86, 0.92, P = 0.950; for 40 μg PTH: WMD = -3.09, 95% CI: -7.11, 0.93, P = 0.131). Subgroup analysis on the basis of study duration showed that, the increased total hip BMD with the combination therapy was not observed at the 12 months or over 12 months (for 12 months: WMD = -0.10, 95% CI: -1.27, 1.07, P = 0.866; for over 12 months: WMD = -0.51, 95% CI: -2.68, 1.67, P = 0.649).
Figure 4.
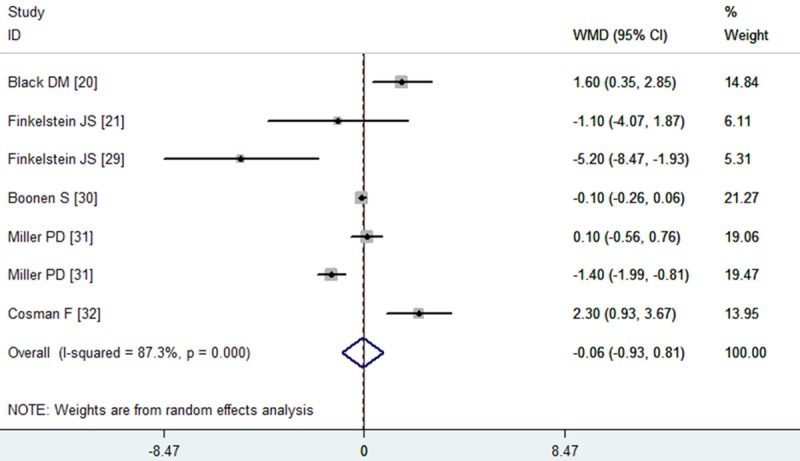
Assessment of the effects of PTH plus alendronate on BMD of the total hip.
In addition, we also conducted the subgroup analysis based on the gender of patients and anabolic agents. For postmenopausal women, the pooled results from 5 studies indicated that, increases in BMD were not higher in the combination therapy with PTH and alendronate (WMD = 0.01, 95% CI: -0.90, 0.91; P = 0.989), when compared to monotherapy. According to subgroup analysis on the basis of anabolic agents, the teriparatide did not yield significant increments in total hip BMD, when used in the combination with alendronate (WMD = -0.34, 95% CI:-1.25, 0.57; P = 0.465).
The Begg’s test and Egger’s test indicated no existence of publication bias (for Begg’s test, Z = 0.00, P = 1.000; for Egger’s test, t = -0.09, P = 0.928).
Effect of PTH plus alendronate on BMD of distal radius
Three studies reported the data of distal radius BMD [20,21,29]. The aggregated results showed that the combination therapy with PTH and alendronate was associated with a significant increase in distal radius BMD from baseline compared with monotherapy (WMD = 2.45, 95% CI: 1.58, 3.31; P = 0.000) (Figure 5). The test for heterogeneity was not significant (P = 0.179, I2 = 41.9%). Due to the number of available studies for analysis was less than 5, the publication bias was not performed.
Figure 5.
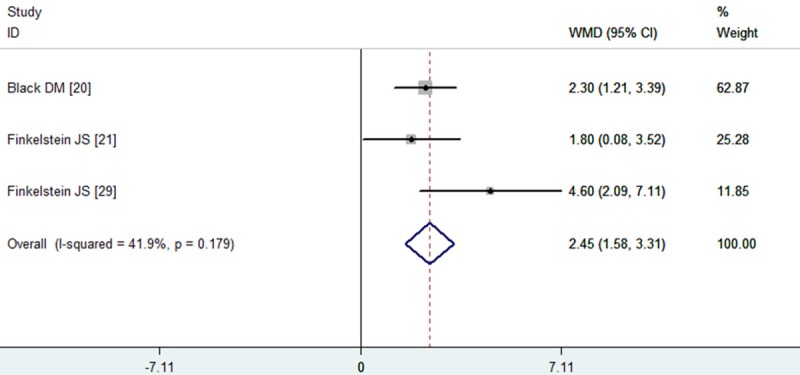
Assessment of the effects of PTH plus alendronate on BMD of the distal radius.
Discussion
The purpose of this meta-analysis was to assess the efficacy of the combined therapy of PTH with alendronate in osteoporosis. Our study indicated that the combination therapy with PTH and alendronate significantly increased the BMD at the distal radius (WMD = 2.45, 95% CI: 1.58, 3.31; P = 0.000), but not at the spine(WMD = -0.06, 95% CI: -0.93, 0.81; P = 0.892), femoral neck (WMD = -0.99, 95% CI: -2.04, 0.07; P = 0.068), and total hip (WMD = -0.06, 95% CI: -0.93, 0.81; P = 0.892). Moreover, among the patients in the combination therapy group, greater increases in the spine BMD were observed when the PTH was administered with a dosage of 20 μg (WMD = 2.33, 95% CI: 1.24, 3.43; P = 0.000), or the treatment duration lasted more than 12 months (WMD = 2.23, 95% CI: 1.00, 3.47; P = 0.000), or the combination therapy was used in osteoporosis women (WMD = 1.58, 95% CI: 0.63, 2.53; P = 0.001). However, the combination of PTH of 40 μg with alendronate produced a decrease in the BMD at spine (WMD = -4.56, 95% CI: -7.56, -1.56; P = 0.003) and femoral neck (WMD = -5.82, 95% CI: -9.91, -1.72; P = 0.005).Our findings indicated that the addition of alendronate to PTH in the treatment of osteoporosis, reduced the ability of PTH therapy to increase the BMD at the lumbar spine, femoral neck, and total hip.
To the best of our knowledge, this is the first comprehensive meta-analysis to assess the efficacy of combined therapy of PTH with alendronate in patients with osteoporosis. According to previous studies, the daily injection of PTH (1-34) or PTH (1-84) have been shown to increase the BMD [16,33], and reduce the risk of fracture [17]. PTH exerts its anabolic effect on bone through increasing the both bone formation and bone resorption, in which bone formation is preferentially increased over bone resorption. Similarly, alendronate has the effect of increasing the BMD and reducing the risk of fracture, however, its mechanism of action is different from that of PTH. In the process of alendronate’s action, the bone resorption is preferentially inhibited over the bone formation [9]. Therefore, the combined therapy of PTH with alendronate, in which the stimulation of bone formation and inhibiting of bone resorption are acted simultaneously, may result in more effective outcomes than the treatment with PTH or alendronate alone. However, in this meta-analysis, we found that the combination therapy of PTH with alendronate did not produce a significant increase in the areal BMD.
Additionally, in the randomized, double-blind clinical study conducted by Black DM et al. [20], patients in the PTH group had an early increase in the levels of marker of bone formation, and a delayed but substantial increase in the levels of marker of resorption. Thus, it was postulated that patients in the combined therapy of PTH with alendronate should maintain the similar outcomes as the patients treated with PTH. However, the dampening increases in bone resorption were not observed in the patients with combination therapy. Interestingly, among the patients in the combination therapy group, despite the level of markers of bone formation kept relatively constant and the level of markers of bone resorption significantly decreased over the 12-month period, the sustained increases in bone formation decreased after 1 month. Since the increases in bone formation represent the effect of PTH on bone, the results from this RCT indicates that the anabolic actions of PTH might be blocked with the combination therapy.
Of the studies included in this meta-analysis, we noticed that the schedule of the PTH varied greatly. In the study conducted by Black DM et al. [20], the PTH (1-84) and alendronate were concurrently administered in the women who were not already taking medication for osteoporosis; in the two studies, both of which were conducted by Finkelstein JS et al. [21,29], the PTH (1-34) therapy was initiated after 6 months of alendronate monotherapy; in the study conducted by Boonen S et al. [30], the 2 year of PTH (1-34) therapy was used for patients who previous were treated with alendronate. Despite all of the PTH therapy in the latter three studies [21,29,30] were administered after alendronate therapy, the combination therapy of PTH with alendronate resulted in different outcomes. In both of the two studies conducted by Finkelstein JS et al. [21,29], the increase in BMD achieved with the combination therapy were similar to those observed with the monotherapy. However, in the study conducted by Boonen S et al. [30], the combination therapy produced positive effects on BMD. The reason for these opposite outcomes remained unknown. Moreover, no studies of alendronate therapy given after PTH have been reported, thus it is not unclear whether the alendronate therapy should be used after the PTH therapy.
According to this study, we found that the increase in femoral neck BMD was higher when the combination therapy with PTH and alendronate was administered over 12 months. Thus, the PTH should be administered in combination with alendronate for more than 12 months in order to attain the optimal benefits at this site.
There were certain limitations in this meta-analysis which should be considered when interpreting our results. First, substantial heterogeneity was observed among the included studies, which was not surprising given the differences in characteristics of populations (gender and age), and the treatment regimen (dosage and schedule, duration of administration, and anabolic agents). These factors may result in potential impact on the consistency of the results. Second, our analysis was based on six RCTs, and most of them had a modest sample size. This may lead to an overestimation of the treatment effect, when compared with larger trials. Third, some of the subgroup analysis were based on 3 to 4 RCTs. Therefore, applying these findings into the clinical practice should be with caution. Finally, due to the insufficiency data, we did not assess the effect of alendronate administered before or after a course of PTH therapy.
In conclusion, this study indicated that the use of alendronate impairs the ability of PTH to increase BMD at the lumbar spine, femoral neck, and total hip in patients with osteoporosis. The combination therapy of PTH with alendronate was not recommended for the treatment of patients with osteoporosis. However, considering the relatively small number of included studies, more prospective well-designed, randomized-controlled trials are needed to identify our findings.
Disclosure of conflict of interest
None.
References
- 1.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.Rosen CJ, Bilezikian JP. Clinical review 123: anabolic therapy for osteoporosis. J Clin Endocrinol Metab. 2001;86:957–964. doi: 10.1210/jcem.86.3.7366. [DOI] [PubMed] [Google Scholar]
- 3.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 4.Einhorn TA. Bone strength: the bottom line. Calcif Tissue Int. 1992;51:333–339. doi: 10.1007/BF00316875. [DOI] [PubMed] [Google Scholar]
- 5.Genant HK, Cooper C, Poor G, Reid I, Ehrlich G, Kanis J, Nordin BE, Barrett-Connor E, Black D, Bonjour JP, Dawson-Hughes B, Delmas PD, Dequeker J, Ragi Eis S, Gennari C, Johnell O, Johnston CC Jr, Lau EM, Liberman UA, Lindsay R, Martin TJ, Masri B, Mautalen CA, Meunier PJ, Khaltaev N, et al. Interim report and recommendations of the World Health Organization Task Force for Osteoporosis. Osteoporos Int. 1999;10:259–264. doi: 10.1007/s001980050224. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson SF, Watts NB, Bilezikian JP, Clarke BL, Gray TK, Harris DW, Johnston CC, et al. American Association of Clinical Endocrinologists 2001 Medical Guidelines for Clinical Practice for the Prevention and Management of Postmenopausal Osteoporosis. Endocr Pract. 2001;7:293–312. [PubMed] [Google Scholar]
- 7.Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106. doi: 10.1016/s8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 8.Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997;100:1475–1480. doi: 10.1172/JCI119668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 10.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–82. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 11.Cranney A, Wells G, Willan A, Griffith L, Zytaruk N, Robinson V, Black D, Adachi J, Shea B, Tugwell P, Guyatt G Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev. 2002;23:508–16. doi: 10.1210/er.2001-2002. [DOI] [PubMed] [Google Scholar]
- 12.Reeve J, Meunier PJ, Parsons JA, Bernat M, Bijvoet OL, Courpron P, Edouard C, Klenerman L, Neer RM, Renier JC, Slovik D, Vismans FJ, Potts JT Jr. Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. Br Med J. 1980;280:1340–1344. doi: 10.1136/bmj.280.6228.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam CS, Heersche JN, Murray TM, Parsons JA. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982;10:506–512. doi: 10.1210/endo-110-2-506. [DOI] [PubMed] [Google Scholar]
- 14.Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136:3632–3638. doi: 10.1210/endo.136.8.7628403. [DOI] [PubMed] [Google Scholar]
- 15.Hodsman AB, Steer BM. Early histomorphometric changes in response to parathyroid hormone therapy in osteoporosis: evidence for de novo bone formation on quiescent cancellous surfaces. Bone. 1993;14:523–527. doi: 10.1016/8756-3282(93)90190-l. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350:550–555. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 17.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in post postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 18.Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9–17. doi: 10.1359/jbmr.2003.18.1.9. [DOI] [PubMed] [Google Scholar]
- 19.Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000;85:3069–76. doi: 10.1210/jcem.85.9.6818. [DOI] [PubMed] [Google Scholar]
- 20.Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ PaTH Study Investigators. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 21.Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349:1216–1226. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- 22.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 23.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135:982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 24.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95:1838–45. doi: 10.1210/jc.2009-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boonen S, Marin F, Obermayer-Pietsch B, Simões ME, Barker C, Glass EV, Hadji P, Lyritis G, Oertel H, Nickelsen T, McCloskey EV EUROFORS Investigators. Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2008;93:852–60. doi: 10.1210/jc.2007-0711. [DOI] [PubMed] [Google Scholar]
- 31.Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, Saag K, Greenspan SL, Seeman E, Boonen S, Meeves S, Lang TF, Bilezikian JP Open-label Study to Determine How Prior Therapy with Alendronate or Risedronate in Postmenopausal Women with Osteoporosis Influences the Clinical Effectiveness of Teriparatide Investigators. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab. 2008;93:3785–93. doi: 10.1210/jc.2008-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosman F, Wermers RA, Recknor C, Mauck KF, Xie L, Glass EV, Krege JH. Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab. 2009;94:3772–80. doi: 10.1210/jc.2008-2719. [DOI] [PubMed] [Google Scholar]
- 33.Rittmaster RS, Bolognese M, Ettinger MP, Hanley DA, Hodsman AB, Kendler DL, Rosen CJ. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab. 2000;85:2129–34. doi: 10.1210/jcem.85.6.6614. [DOI] [PubMed] [Google Scholar]


