Abstract
Bufalin is used to treat many patients with solid malignant tumors clinically. Bufalin could induce gastric cancer cell apoptosis via BAX. microRNA (miRNA) plays important roles in gene regulation. However, miRNA involving in bufalin inducing apoptosis of gastric cancer cells remains to futher research. To study the regulatory role of miRNA in bufalin induced cancer cell apoptosis. Firstly, we verifed that bufalin could induce gastric cancer cell apoptosis by inducing BAX expression. miR-298 was predicted as a regulator of BAX and further study verified Bax was a target gene of miR-298 by luciferase reporter assay. miR-298 could down-regulate BAX on mRNA and protein level in gastric cancer cells. miR-298 promoted cell proliferation and inhibited apoptosis of gastric cancer cells. It was also found that bufalin inhibited cell proliferation and promoted cell apoptosis by down-regualtion of miR-298. In summary, bufalin-associated miR-298 may indirectly be involved in cell proliferation and apoptosis by targeting BAX, pointing to use as a potential molecular target in gastric cancer therapy.
Keywords: Bufalin, gastric cancer, BAX, apoptosis, miR-298
Introduction
Gastric cancer is one of the most common malignant tumors in the world and has high mortality [1]. It originates from the surface of epithelial cells in the stomach. However, the occurrence and progression of gastric cancer is a very complicated process that involves multiple factors, multiple steps, coding and non-coding genes [1]. Thus, it is of great value to investigate the precise molecular mechanism of gastric cancer. It is known that genetic and epigenetic alteration of oncogenes or tumour-suppressor genes in tumor is one of the most commen reason for carcinogenesis. Apart from conventional in the carcinogenesis of gastric cancer, nonprotein-coding RNAs, especially microRNAs (miRNAs), have emerged as a new player to shed light on the mechanism of GC development.
miRNAs are a class of non-coding small RNAs containing approximately 19-24 nt [2]. Most genes encoding miRNAs are single copy, multiple copies or gene clusters; other forms exist in the spacer region of protein coding genes or introns [2]. They are highly conserved, temporal and tissue-specific [3]. miRNAs do not code for proteins and regulate gene expression at the post-transcriptional level. Through complete or incomplete complementary binding to the 3’-untranslated regions (3’-UTRs) of target mRNAs, miRNAs promote targeted-mRNA degradation or translational suppression and negatively regulate the expression of target genes [3-5]. Growing evidence indicates that miRNAs are involved in various biological processes, such as development, differentiation, proliferation, apoptosis, invasion and metastasis [6-8]. It was also demonstrated that miRNAs can be used not only as biomarkers but also as potential therapeutic targets for cancer [9,10]. It has been found that miRNAs have been identified in gastric cancer as an oncogene or tumor suppressor [11].
Bufalin, one of Chinese medicine, inhibits cell proliferation and induces apoptosis in various tumor cell lines [12-15], including gastric cancer [16]. However, the precise molecular mechanisms of the bufalin induced apoptosis of gastric cancer cells are still unclear. At the present study, we will investigate mechanism of bufalin induced gastric cancer cell apoptosis. We found that bufalin could up-regualtion of miR-298 expression and miR-298 was a regulatory miRNA of BAX and it inhibited gastric cancer cell proliferation and induced apoptosis by targeting BAX.
Material and methods
Cell culture
Human gastric cancer cell line MKN45 was obtained from the American Type Culture Collection (Manassas, VA, USA). MGC803 and SGC7901 gastric cancer cell lines were from Shanghai Cancer Institute (Shanghai, China). The immortalized gastric mucosal epithelial cell line GES-1 was obtained from Beijing ComWin Biotech Co. Ltd. (Beijing, China). The cells were maintained in RPMI 1640 culture medium (Invitrogen, USA) supplemented with 10% fetal bovine serum (Invitrogen, USA) in a humidified cell incubator with an atmosphere of 5% CO2 at 37°C. HEK293T cells were maintained in DMEM (Invitrogen, USA) culture medium supplemented with 10% fetal bovine serum (FBS) in a humidified cell incubator an atmosphere of 5% CO2 at 37°C.
Tissue samples and ethics statement
Gastric cancer tissues were obtained from patients who underwent surgical resection at the Fourth Affiliated Hospital of Harbin Medical University (Harbin, China) from 2011 to 2013. None of the patients received preoperative treatment, such as radiation therapy or chemotherapy. Adjacent tissues more than 5cm away from tumors was randomly selected from 18 patients as controls. Specimens were typed histologically according to Lauren’s and the classifications of World Health Organization (IARC Press, Lyon, 2000), and categorized according to the UICC 2002 TNM classification. The study was approved by the Ethics Committee of the hospital. We obtained written informed consent from all participants involved in our study.
Antibodies and reagents
Bax, Bcl2, caspase 3 and PARP primary antibodies were ordered from Cell Signaling (Beverly, MA, USA). GAPDH primary antibody and secondary antibodies conjugated with HRP were purchased from Kang-Chen Biotech (Kangcheng, Shanghai, China). Bufalin was ordered from Sigma Adrich (Saint Louis, MO, USA).
miRNA extraction
miRNA was isolated from paraffin-embedded tissues according to the manufacturer’s instructions using a miRNeasy FFPE Kit (Bioteke, Beijing, China). Total RNA of cell lines was extracted using Trizol reagent (TaKaRa, Dalian, China) following the manufacturer’s protocol. The quality and quantity of the RNA samples were assessed by standard spectrophotometric methods (BioPhotometer plus; Eppendorf, Hamburg, Germany) and diluted to 2 ng/µl for RT-qPCR analysis.
Vector construction and lentivirus production miR-298 inhibitor BAX siRNA
An DNA fragment corresponding to pre-miR-298 and the flanking sequence was amplified from human genomic DNA and then cloned into pLVTHM lentiviral vector (Sinasun, Beijing, China). Another 2 lentiviral vectors for cDNA and siRNA delivery of BAX were described previously. The production, purification, and titration of lentivirus were performed as described in the protocol. The packaged lentiviruses were named as LV-miR-298 and LV-BAX respectively. The empty lentiviral vector LV-control was used as a control.
miRNA target validation
A fragment of BAX 3’UTR (untranslated regions) was amplified by PCR and cloned downstream of the firefly luciferase gene in pGL4 vector (Promega, Madision, WI, USA). The vector was named wild-type (wt) 3’UTR. Site-directed mutagenesis of the miR-298 binding site in BAX 3’UTR was performed using GeneTailor Site-Directed Mutagenesis System (Invitrogen, USA) and named mutant (mt) 3’UTR. For reporter assays, wt or mt 3’UTR vector and the control vector pRL-CMV ((cytomegalovirus) coding for Renilla luciferase (Promega, USA) were cotransfected. Luciferase activity was measured 36 hours after transfection using the Dual-Luciferase Reporter Assay System (Promega, USA).
RNA isolation and real-time RT-PCR
Total RNA was isolated from the cells using Trizol (Invitrogen, USA) following the manufacturer’s instructions. To measure mRNA expression, real-time RT-PCR was performed using a sequence detector (ABI-Prism, Applied Biosystems, USA). Primers were purchased from Invitrogen. The relative expression levels were calculated by comparing Ct values of the samples with those of the reference, all data normalized to the internal control GAPDH.
CCK-8 assay
The Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc. Beijing, China) was used to determine cell viability according to the manufacturer’s instructions. Cells were plated at a density of 3-5 × 103 cells per well in 96-well microtiter plates and cultured overnight at 37°C in a humidified incubator containing 5% CO2. After treatment with different reagents, the cells were incubated for 72 h. Next, at different time points, the culture medium was replaced with 100 ul of fresh medium followed by the addition of 10 ul of CCK-8 solution. The cells were further incubated for 2 h at 37°C, and the optical density at 450 nm was recorded. All experiments were performed in triplicate.
Detection of cell apoptosis
Cells were seeded at a density of 4 × 105 cells per well in 6-well plates, cultured with different reagents for 48 h, and then harvested. The cells were washed with PBS and then stained with 5 ul of Annexin V and 5 ul of propidium iodide (PI) for 15 min at room temperature in the dark according to the manufacturer’s instructions (BD Biosciences, San Jose, CA, USA). The apoptosis rate (%) of the stained cells was analyzed using a Beckman Coulter Epics Altra II cytometer (Beckman Coulter, CA, USA). The experiments were repeated three times.
Hoechst staining
In order to visualize nuclear morphology and the induction of apoptosis, Hoechst 33342 dye (Molecular Probes, Eugene, OR, USA) was used to stain the nuclear. Following treatment with either Green 1 or NSC 51046, cells were incubated with 10 µM of the Hoechst 33342 dye for 10 minutes at 37°C. Images were obtained using a Leica DM IRB inverted fluorescence microscope (Wetzlar, Germany) at 400 × magnification.
Western blot analysis
Western blots were performed as we described elsewhere. In brief, cells were lysed in RIPA buffer containing 1 × protease inhibitor cocktail, and protein concentrations were determined using the Bradford assay (Bio-Rad, Philadelphia, PA). Proteins were separated by SDS-PAGE and transferred to membranes (Millipore, Bedford, MA) at 80V for 2 h at 4°C. After blocking in 5% nonfat dry milk in TBS, the membranes were incubated with primary antibodies in TBS overnight at 4°C, washed three times with TBS-Tween 20, and then incubated with secondary antibodies conjugated with horseradish peroxidase in TBS for 1 hour at room temperature. Membranes were washed again in TBS-Tween 20 for three times at room temperature. Protein bands were visualized on X-ray film using an enhanced chemiluminescence detection system.
Statistical analysis
All the experiments were independently repeated three times. The results were given as means ± standard deviations (SDs). All statistical analyses were performed using SPSS 13.0 statistics software. Group comparisons were analyzed with one-way analysis of variance (ANOVA) with P < 0.05 as statistically significant difference.Gene expression was analyzed by Mann-Whitney U test. Differences with a P value of 0.05 or less were considered to be statistically significant.
Results
Bufalin suppresses the proliferation and promotes apoptosis in gastric cancer cells
To investigate the effect of bufalin on the proliferation of gastric cancer cells, MGC803 and SGC7901 cells were incubated with indicated doses of bufalin (0, 25, 50, 75, 100, 150, 200 nmol/l) or control for 48 h, and cell viability was measured using a CCK-8 kit. We found that bufalin significantly inhibited the proliferation of gastric cancer cells when the cells were treated with higher than 50 nmol/l bufalin compared with the control and the cell viability decreased significantly in a dose-dependent manner (Figure 1A and 1B). To investigate whether this treatment could also induce apoptosis in MGC803 and SGC7901 cells, we used flow cytometry to assess the percentage of apoptotic cells induced by the bufalin (100 nmol/l) treatment compared with the control cells (Figure 1C). These results revealed that bufalin inhibited the proliferation and induced apoptosis in gastric cancer cells.
Figure 1.

Bufalin inhibites cell viability and induces apoptosis in gastic cancer cells. A and B. Gastric cancer cells MGC803 and SGC 7901 were treated with 0, 25, 50, 75, 100, 150 and 200 nmol/l of bufalin or vehicle as control for 48 h. Cell viability was determined using a Cell Counting Kit-8 (CCK-8) assay to calculate the growth index. C. Bufalin induced gastric carcinoma cell apoptosis. The MGC803 and SGC 7901 cells treated with bufalin (100 nmol/l) were subjected to flow cytometry to measure cell apoptosis after stained with Annexin V/PI. Right panel was shown apoptosis rate from the left panel of pictures. D. Gastric cancer cells MGC803 and SGC 7901 were treated with 100 nmol/l of bufalin or vehicle as control for 48 h. Total protein was extracted from the cells and performed for Western Blotting. All the data were at least three independent experiments. *Significant difference from control, P < 0.05.
To elucidate the molecular mechanism of bufalin-induced apoptosis in gastric cancer cells, the expression levels of apoptosis associated proteins including caspases were examined by western blot analysis. The results demonstrated that caspase-3 cleaved and was significantly increased in cells after 48 h of exposure to bufalin. A similar tendency was observed for poly (ADP-Ribose) polymerase (PARP) cleavage, a typical feature of caspase dependent apoptosis. Meanwhile, bufalin also increased the ratio of Bax/Bcl-2 (Figure 1D). These results suggest that bufalin induced the apoptosis of gastric cancer cells at least partly by activating caspases and mitochondrial-mediated apoptotic pathway.
BAX is a new target gene of miR-298 in gastric cancer cells
BAX is involved in bufalin induced gastric cancer cell apoptosis and is predicted as a potential target gene of miR-298. Bioinformatic analysis showed that BAX was directly suppressed by miR-298 (Figure 2A). As shown in Figure 2B, the luciferase activity of pGL4-BAX-WT in HEK293T cells was much lower than in control cells. The luciferase activity of pGL4-BAX-Mut was rescued in the cells. Compared with control, endogenous BAX mRNA levels (Figure 2C) were down-regulated when MGC803 and SGC7901 cells were transfected with miR-298. BAX protein was down-regulated in the MGC803 and SGC7901 cells with miR-298 (Figure 2D).
Figure 2.
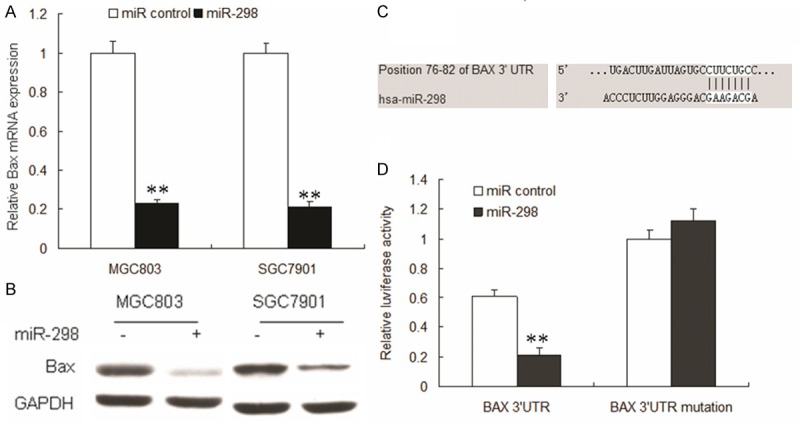
miR-298 targets BAX through interaction with BAX 3’-UTR. A. miR-298 reduces BAX mRNA levels. MGC803 and SGC7901 cells were transfected with miR-298 or control oligonucleotide for 48 h and then subjected to RT-PCR analyses. B. Knockdown of miR-298 induces BAX protein level. MGC803 and SGC7901 cells were transfected with miR-298 and control for 48 h and then examined for immunoblotting of BAX. C. Sequence alignment of the human miR-298 sequences with a region of the BAX 3’-UTR from indicated species. D. Luciferase reporter assay. The indicated cells were transfected with the indicated plasmids and oligonucleotide as well as β-galactosidase. After a 24 h incubation, cells were subjected to a luciferase assay. Luciferase activity was normalized with β-galactosidase. Relative luciferase activities are presented. Data represent three independent experiments in triplicate. **Significant difference from control, P < 0.01.
Increasing expression of miR-298 in human gastric cancer
To investigate the possible role of miR-298 in gastric cancer, we examined the expression of miR-298 in human gastric specimens by real time RT-PCR. We examined the expression of miR-298 in 20 tumor samples and 20 normal tissues. As shown in Figure 3A, the expression levels of miR-298 in tumor samples were higher than those in normal samples. Similarly, miR-298 was much more in human gastric cancer cells compared with normal gastric cells (Figure 3B). Together, these results provided us initial evidence that miR-298 may play a onco-miRNA in the development of human gastric cancer.
Figure 3.

The expression of miR-298 was increased in gastric cancer cell lines and clinical specimens. A. Average expression level of miR-298 in human gastric cancer specimens (n = 20) and normal gastric tissues (n = 20). B. Expression of miR-298 in 3 gastric cancer cell lines and 1 normal gastric cell. miRNA abundance was normalized to U6 RNA. Data represent three independent experiments in triplicate. Significant difference from control, **, P < 0.01.
miR-298 promotes cell proliferation and inhibits cell apoptosis by targeting BAX
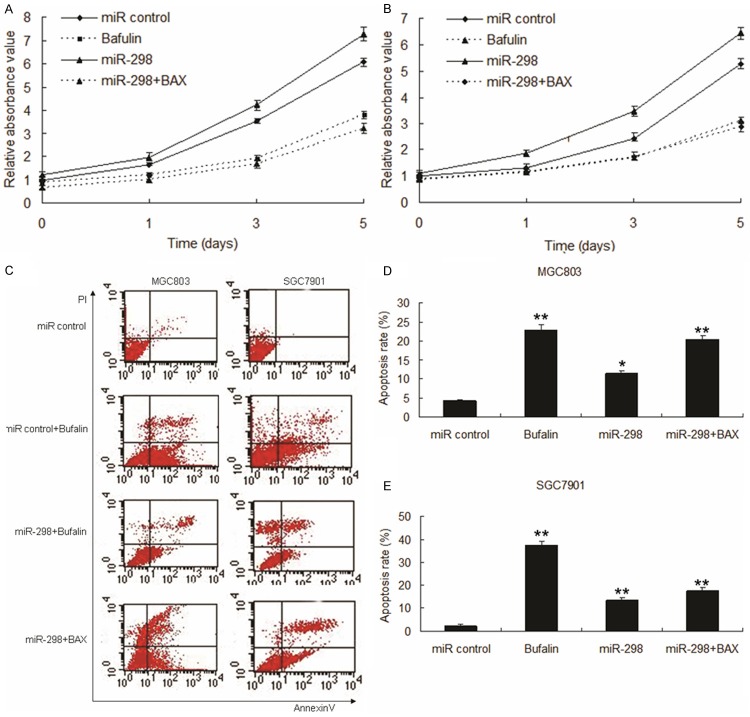
We next tested whether miR-298 promotes cell proliferation and inhibits apoptosis in gastric cancer cells, MGC803 and SGC7901 were infected with LV-miR-298 or LV-miR control with or without LV-BAX. The results of CCK8 assay displayed that miR-298 promoted cell proliferation in MGC803 (Figure 4A) and SGC7901 cells (Figure 4B). Data of flow cytometry assay showed that miR-298 suppressed apoptosis of gastric cancer cells stimulated by bufalin (Figure 4C-E).
Figure 4.
Enforced expression of miR-298 induced growth inhibition in gastric cancer cells by targeting BAX. A. Effect of miR-298 on cell proliferation was measured by MTT assay after miRNA or BAX transfection in MGC803 cells. B. Effect of miR-298 on cell proliferation was measured by MTT assay after miRNA or BAX transfection in SGC7901 cells. C. Representative pictures of apoptosis of LV-miR-298 or BAX infected MGC803 and SGC7901 cells, and apoptosis was detected by flow cytometry. D. Apoptosis rate of LV-miR-298 or BAX infected MGC803 cells from C. E. Apoptosis rate of LV-miR-298 or BAX infected SGC7901 cells from C. Data represent three independent experiments in triplicate. Significant difference from control, **, P < 0.01; *, P < 0.05 compared with control.
Knocking down of BAX protects gastric cancer cells from apoptosis
Above data showed that miR-298 prevented apoptosis via BAX and bufalin could induce BAX expression in gastric cancer cells. We want to know whether BAX could induce gastric cancer cells apoptosis. BAX protein was knocked down in MGC803 and SGC7901 cells transfected with BAX siRNA (Figure 5A). Knocking down of BAX could protect gastric cancer cells from bufalin induced apoptosis (Figure 5B). Protection of -induced apoptosis in MGC803 and SGC7901 cells was morphologically identified using fluorescence staining with Hoechst 33342, as shown in Figure 5C, chromosomal condensation and nuclear fragmentation were observed in colchicine treated cells.
Figure 5.

Knocking down of BAX protects gastric cancer cells from apoptosis. A. The MGC803 and SGC7901 cells were tranfected with BAX siRNA or control siRNA and then protein was extracted for Western blot to verify BAX protein down-regulation. B. MGC803 and SGC7901 cells were treated with bufalin and subjected to flow cytometry to measure cell apoptosis after stained with Annexin V/PI. Data from cell apoptosis shown was a representative of at least three independent experiments and histograms were shown for analyzed cells. C. Morphology of apoptotic cells. MGC803 and SGC7901 cells were exposed to bufalin for 24 h and cell apoptosis was assayed by Hoechst 33342 fluorescence staining to detect chromosomal cendensation and nuclear fragmentation.
Discussion
Bufalin has been used in clinical trials for the treatment of advanced tumors for many years. Bufalin was considered to have great therapeutic effects including inhibition of proliferation, angiogenesis, induction of differentiation and apoptosis, disruption of cell cycle, reversal of multi-drug resistance, and regulation of immune response in many types of tumor [17,18]. Treatment with bufalin has been reported to induce apoptosis in several identified cancer cells [12-16]. Previous report shows that when the liver cancer cells were treated with bufalin and cell apoptosis was increased companied with BAX expression [19]. In this study, firstly, we verified that bufalin induces cell apoptosis and inhibits cell proliferation of gastric cancer and BAX increased in the gastric cancer cells exposed to bufalin.
Many miRNAs regulate various processes in tumorigenesis, including apoptosis and metastasis, and have received increasing attention in cancer research. MiR-298 was a predicted regulatory miRNA of BAX gene and observed miR-298 expression was decreased significantly in a dose-dependent manner with bufalin treatment in gastric cancer cells. We also showed inhibition of miR-298 to induce significant apoptosis through down-regulation of BAX protein. Furthermore, miR-298 inhibitor largely promotes bufalin-induced apoptosis. Our results indicate that miR-298 mediates a downstream, bufalin-induced apoptosis pathway, and suggest a more detailed model for bufalin-induced apoptosis in which bufalin induces loss of expression of miR-298, which in turn inhibits BAX protein, resulting in apoptosis.
Although various studies present the mechanism by which bufalin induces apoptosis in cancer cells, the anti-tumor activity of bufalin and miRNAs in inducing miR-298 expression had not been shown before this study. Our study also showed that apopotosis and its associated protein including BAX was increased when gastric cancer cells MGC803 and SGC7901 were exposed to bufalin. Bufalin induces cancer cell apoptosis was verifed in gastric cancer.
Based on our result, we presented here a more detailed model for bufalin-induced apoptosis. BAX is an apoptosis protein and its increase usually triggers mitochondrion mediated apoptosis pathway by caspase-3 proteins activation. Therefore, we also assayed caspase-3 activity by cleavage of aminoluciferin-coupled caspase-3 substrate in lysate of gastric cancer cells treated with or without bufalin. After bufalin treatment, miR-298 inhibitor transfected cell lysate, showed reduction of caspase-3 activity when compared with untransfected lysate; whereas transfection with a negative control inhibitor did not reduce bufalin-induced caspase-3 activity. The cell lysates were further subjected to western blot analysis with a caspase-3 antibody that recognizes both pro- and cleaved caspase-3. After bufalin treatment, pro-caspase-3 was cleaved to a smaller active form that can lead to apoptosis. miR-298, however, largely reduced the activating cleavage of pro-caspase-3 and the level of the active form of caspase-3.
In a conclusion, the study for the first time showed that bufalin treatment could decrease miR-298 expression. We also show that deletion of miR-298 contributes to bufalin-induced apoptosis in gastric cancer cells by targeting BAX, an apoptosis protein. Thus, our study illustrated a new pharmacological mechanism for bufalin in anti-tumor therapy.
Disclosure of conflict of interest
None.
References
- 1.Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979–2990. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim VN, Nam JW. Genomics of microRNA. Trends Genet. 2006;22:165–173. doi: 10.1016/j.tig.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Singh TR, Gupta A, Suravajhala P. Challenges in the miRNA research. Int J Bioinform Res Appl. 2013;9:576–583. doi: 10.1504/IJBRA.2013.056620. [DOI] [PubMed] [Google Scholar]
- 4.Wang C, Leng X, Zhang Y, Kayesh E, Zhang Y, Sun X, Fang J. Transcriptome-wide analysis of dynamic variations in regulation modes of grapevine microRNAs on their target genes during grapevine development. Plant Mol Biol. 2014;84:269–285. doi: 10.1007/s11103-013-0132-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release. 2013;172:962–974. doi: 10.1016/j.jconrel.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhuo Y, Gao G, Shi JA, Zhou X, Wang X. miRNAs: biogenesis, origin and evolutio n, functions on virus-host interaction. Cell Physiol Biochem. 2013;32:499–510. doi: 10.1159/000354455. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X, Li X, Yuan H. microRNAs in gastric cancer invasion and metastasis. Front Biosci (Landmark Ed) 2013;18:803–810. doi: 10.2741/4144. [DOI] [PubMed] [Google Scholar]
- 8.Gaál Z, Oláh E. MicroRNA-s and their role in malignant hematologic diseases. Orv Hetil. 2012;153:2051–2059. doi: 10.1556/OH.2012.29511. [DOI] [PubMed] [Google Scholar]
- 9.Petri A, Lindow M, Kauppinen S. MicroRNA silencing in primates: towards development of novel therapeutics. Cancer Res. 2009;69:393–395. doi: 10.1158/0008-5472.CAN-08-2749. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Crawford M, Yu B, Mao Y, Nana-Sinkam SP, Lee LJ. MicroRNA delivery by cationic lipoplexes for lung cancer therapy. Mol Pharm. 2011;8:1381–1389. doi: 10.1021/mp2002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li PF, Chen SC, Xia T, Jiang XM, Shao YF, Xiao BX, Guo JM. Non-coding RNAs and gastric cancer. World J Gastroenterol. 2014;20:5411–5419. doi: 10.3748/wjg.v20.i18.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang XH, Xu ZY, Gong YB, Wang LF, Wang ZQ, Xu L, Cao F, Liao MJ. Bufalin reverses HGF-induced resistance to EGFR-TKIs in EGFR mutant lung cancer cells via blockage of Met/PI3k/Akt pathway and induction of apoptosis. Evid Based Complement Alternat Med. 2013;2013:243859. doi: 10.1155/2013/243859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsiao YP, Yu CS, Yu CC, Yang JS, Chiang JH, Lu CC, Huang HY, Tang NY, Yang JH, Huang AC, Chung JG. Triggering apoptotic death of human malignant melanoma a375. s2 cells by bufalin: involvement of caspase cascade-dependent and independent mitochondrial signaling pathways. Evid Based Complement Alternat Med. 2012;2012:591241. doi: 10.1155/2012/591241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan S, Qu X, Xu C, Zhu Z, Zhang L, Xu L, Song N, Teng Y, Liu Y. Down-regulation of Cbl-b by bufalin results in upregulation of DR4/DR5 and sensitization of TRAIL-induced apoptosis in breast cancer cells. J Cancer Res Clin Oncol. 2012;138:1279–1289. doi: 10.1007/s00432-012-1204-4. [DOI] [PubMed] [Google Scholar]
- 15.Tsai SC, Yang JS, Peng SF, Lu CC, Chiang JH, Chung JG, Lin MW, Lin JK, Amagaya S, Wai-Shan Chung C, Tung TT, Huang WW, Tseng MT. Bufalin increases sensitivity to AKT/mTOR-induced autophagic cell death in SK-HEP-1 human hepatocellular carcinoma cells. Int J Oncol. 2012;41:1431–1442. doi: 10.3892/ijo.2012.1579. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Qu X, Hou K, Zhang Y, Dong Q, Teng Y, Zhang J, Liu Y. PI3K/Akt is involved in bufalin-induced apoptosis in gastric cancer cells. Anticancer Drugs. 2009;20:59–64. doi: 10.1097/CAD.0b013e3283160fd6. [DOI] [PubMed] [Google Scholar]
- 17.Yang Z, Luo H, Wang H, Hou H. Preparative isolation of bufalin and cinobufagin from Chinese traditional medicine ChanSu. J Chromatogr Sci. 2008;46:81–85. doi: 10.1093/chromsci/46.1.81. [DOI] [PubMed] [Google Scholar]
- 18.Zhai XF, Fang FF, Liu Q, Meng YB, Guo YY, Chen Z. MiR-181a contributes to bufalin-induced apoptosis in PC-3 prostate cancer cells. BMC Complement Altern Med. 2013;13:325. doi: 10.1186/1472-6882-13-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu F, Han J, Zhai B, Ming X, Zhuang L, Liu Y, Pan S, Liu T. Blocking autophagy enhances the apoptosis effect of bufalin on human hepatocellular carcinoma cells through endoplasmic reticulum stress and JNK activation. Apoptosis. 2014;19:210–223. doi: 10.1007/s10495-013-0914-7. [DOI] [PubMed] [Google Scholar]