Abstract
Objective: This study is to investigate the immunostimulatory activities of dendritic cells (DCs) transfected with HBcAg and/or HBsAg recombinant adenovirus (rAd). Methods: DCs were transfected with rAd (DC/Ad-C+Ad-S, DC/Ad-C, and DC/Ad-S), or pulsed with HBcAg antigen (DC/HBcAg). Flow cytometry was used to detect the phenotype of DCs and the cytokine production of T lymphocytes. Mice were vaccinated with DCs transfected with rAd or pulsed with antigen, and DNA vaccine. Mixed lymphocyte reaction (MLR) was used to evaluate the T-cell stimulatory capacity, and HBcAg-specific cytotoxic T lymphocyte (CTL) activity was assessed. Results: Phenotypic analysis showed that DCs transfected with rAd or pulsed with HBcAg antigen exhibited mature phenotypes. MLR indicated no significant differences in stimulating T-cell proliferation between the DC/rAd and DC/HBcAg groups. When mixed with DCs, Th and Tc cells mainly secreted IFN-γ, indicating type I immune responses. In vaccinated mice, DCs transduced with rAd and pulsed with HBcAg induced significantly more IFN-γ secretion from Th cells, compared with DNA vaccine, indicating stronger Th1 response. Moreover, DCs transduced with rAd stimulated Tc cells to produce more IFN-γ, indicating stronger Tc1 response. In vaccinated mice, HBcAg-specific CTL activities were decreased in the following order: the DC/Ad-C+Ad-S, DC/Ad-C, DC/Ad-S, DC/HBcAg, and DNA vaccine groups. Conclusion: DCs transfected with rAd induce stronger Th1/Tc1 (type I) cell immune responses and specific CTL response than HBcAg-pulsed DCs or DNA vaccine. Our findings suggest that DCs transfected with rAd-C/rAd-S might provide an effective approach in the treatment of persistent hepatitis B virus infection.
Keywords: Hepatitis B core antigen (HBcAg), hepatitis B surface antigen (HBsAg), dendritic cells (DCs), adenovirus, cytotoxic T lymphocytes
Introduction
Chronic hepatitis B virus (HBV) infection is associated with insufficient generation of specific CD8+ cytotoxic T lymphocyte (CTL) [1]. Accordingly, (therapeutic vaccination inducing HBV-specific CTL response represents promising therapeutic strategy to control the viral infection. At present, immunotherapeutic vaccines against HBV infection mainly include recombinant antigens (proteins and/or peptides) [2,3], DNA vaccines [4], and antigen-pulsed dendritic cells (DCs). DCs have been shown to be superior to recombinant antigens and DNA vaccines in causing CTL responses [5,6]. Moreover, in studies concerning cancer treatments and other anti-virus vaccines, DCs transfected with recombinant adenovirus carrying antigens could cause stronger CTL immune response than antigen-pulsed DCs [7].
Patients with persistent HBV infection are characterized by specific CTL responses with narrow spectrum, and the peripheral blood mononuclear cell-derived DCs are deficient in antigen-presenting function, which cannot deliver the signals of the viral antigens to the immune system, resulting in insufficient CTL response [8,9]. Therefore, immunotherapeutic treatment studies and vaccine development are mainly aiming at enhancing the antigen presentation and inducing effective immune responses. DCs are currently known as the strongest antigen-presenting cells (APCs), which can directly activate naive T cells in vitro and in vivo, playing a key role in the immune response [10]. Compared with hepatitis B virus surface antigen (HBsAg)-pulsed macrophages, HBsAg-pulsed DCs could initiate stronger MHC I-restricted CTL response [11], and HBV-specific CTL response is an important player in controlling the persistent viral infection and in the clearance of virus. It has been shown that immunization of DCs transfected with adenovirus vector carrying HBsAg (Ad-S) generates type I immune response in mice, and induces stronger Th1 and Tc1 responses, compared with HBsAg-pulsed DCs and DNA vaccines, indicating more effective cellular immunity against HBV infection [12]. Hepatitis B virus core antigen (HBcAg) is the strongest antigen produced by HBV, functioning in both T cell-dependent and -independent pathways. In self-limited HBV infection, HBcAg facilitates the proliferation of Th and CTL cells, which is involved in viral control [13-16].
In this study, the immunostimulatory activities of DCs transfected with adenovirus vector carrying HBcAg (DC/Ad-C) or HBsAg (DC/Ad-S) were investigated, both in vitro and in vivo, in comparison with HBcAg-pulsed DCs and DNA vaccine. Our findings provide theoretical and experimental evidence for the application of DC/Ad-C and/or DC/Ad-S in the treatment of persistent HBV infection.
Materials and methods
Animals, cell lines, and plasmids
Female 8-10-week old BALB/c (H-2d) mice, weighing 18-20 g, and female 8-10-week old C57BL/6 (H-2Kb) mice, weighing 20-22 g, were all provided by the Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). P815 cell line was kept by our laboratory
Recombinant adenovirus carrying HBcAg (Ad-C; a replication-deficient type 5 adenovirus with deletion in E1 and E3 genes, HBV genotype C, subtype ayw), recombinant adenovirus carrying HBsAg (Ad-S), as well as control adenovirus (Ad-lacZ) and pcDNA3.1 (+)-C were all constructed by our laboratory. The pcDNA3.1 (+) vector was purchased from Invitrogen (Carlsbad, CA, USA).
Dendritic cell culture and adenoviral transfection
Dendritic cell culture and adenoviral transfection were performed according to previously published protocols [5,17]. Briefly, bone marrow cells were harvested from the femur and tibia tissues of BALB/c mice, and the red blood cells were lysed with Tris-NH4Cl. Cells were re-suspended with cRPMI 1640 culture medium, containing 10 ng/ml granulocyte/macrophage colony-stimulating factor (GM-CSF; R&D, Minneapolis, MN, USA), 2 ng/ml interleukin (IL)-4 (R&D), 10% FCS, 100 U/ml penicillin, and 100 µg/ml streptomycin, and then planted onto 6-well plates. On Day 3, suspension cells were discarded, and the culture medium was replaced by fresh cRPMI 1640 medium containing GM-CSF and IL-4. On Day 6, three quarters of the medium was changed by cRPMI 1640 medium containing GM-CSF, IL-4, and 500 U/ml TNF-α (PeproTech, Rocky Hill, NJ, USA). On Day 8, non-adherent and loosely adherent cells were collected and planted onto culture plates. After 1 h, adherent mononuclear macrophages were discarded, and the remaining cells were DCs.
For the adenoviral transfection, DCs were washed with PBS, and re-suspended with RPMI 1640 medium containing 1% FCS. These cells were treated with LipofectamineTM 2000 (Invitrogen) and incubated with rAds (Ad-C, Ad-S, Ad-C+Ad-S, Ad-lacZ, and Ad-GFP) at 100 MOI, in the hybridization oven (60 rolls/min) at 37°C for 2 h. DCs without rAd transfection were used as the negative control. For the HBcAg-pulsed (DC/HBcAg) group, on Day 6, 1 µg/ml HBcAg (National Vaccine&Serum Institute, Beijing, China) was added into the culture medium. On Day 8, DCs were incubated with 100 µg/ml HBcAg at 37°C for 2 h. After treatments, DCs were subjected to the following assessments.
Flow cytometry
Flow cytometry was performed for the phenotypic analysis of DCs. Cells were washed with PBS, and then incubated with fluorescence-labeled anti-CD11c, I-Ad, H-2Kd, CD80, CD86, CD40, CD54, and DEC-205 monoclonal antibodies (all from Pharmingen, San Diego, CA, USA), respectively, in dark at room temperature for 20 min. The expression of DC surface molecules was analyzed by a flow cytometer (EPICS-XL; Beckman-Coulter, Fullerton, CA, USA). To block Fc receptor-mediated antibody binding, the cells were pre-treated with anti-CD16/32 antibody for 10 min (1 μg/106 cells).
Cytokine production
Cells were re-suspended with cRPMI 1640 medium at a density of 5×106 cells/ml, and incubated with 25 μg/ml PMA, 1 μg/ml ionomycin, and 1.7 μg/ml monensin, at 37°C for 6 h. The cells were subsequently treated with anti-mouse CD16/32, TC-CD3, and FITC-CD8 monoclonal antibodies (all from Caltag, Burlingame, CA, USA), rupture agent, and anti-mouse PE-IFNγ and PE-IL-4 monoclonal antibodies, in dark at room temperature. The cytokine expression was detected with flow cytometry.
Animal grouping and vaccination
For DC vaccination, 1×106 DCs were suspended in 100 µl PBS, and inoculated into BALB/c mice via tail vein injection. After 3 w, the same immunization procedure was repeated once. Mice were divided into the following groups: (1) the PBS control group (n = 4); (2) the DC group (n = 4); (3) the DC/HBcAg group (n = 10); (4) the DC/Ad-C group (n = 10); (5) the DC/Ad-S group (n = 10); (6) the DC/Ad-C+Ad-S group (n = 10); and (7) the DC/Ad-lacZ group (n = 4).
For DNA vaccination, 100 µg pcDNA3.1 (+)-C or pcDNA3.1 (+) was dissolved in 50 μl PBS with 25% sucrose, and then used to immunize BALB/c mice trough multi-point injection in bilateral hindlimb muscles. After 3 w, the same immunization procedure was repeated once. The mice were divided into the pcDNA3.1 (+)-C group (n = 8) and the pcDNA3.1 (+) control group (n = 4).
Mixed lymphocyte reaction (MLR)
Mouse splenocytes were collected from immunized C57BL/6 (H-2b) mice and BALB/c (H-2d) mice, respectively, and isolated by filtration through a nylon wool column. Allogeneic and autologous T lymphocytes were re-suspended with the cRPMI 1640 medium, and then reacted with DCs (treated with 25 μg/ml mitomycin C for 30 min) from the indicated groups, at different responder/stimulator (R/S) ratios, in a 96-well U-bottom dish for 4 d. 0.5 μCi [3H] thymidine was added into each well at 8 h before the assessment. Cells were smeared onto the filter paper, and the [3H] radioactivity (cpm) was detected. On the other hand, autologous T lymphocytes were mixed with CDs from the indicated groups at a R:S ratio of 5:1 on a 12-well plate for 5 d, and then the cytokines in proliferating T cells were detected with flow cytometry.
HBcAg-specific cytotoxic T lymphocyte (CTL) activity
After 2 w or 4 w after the last vaccination, splenocytes were collected from the immunized mice. After co-cultured with P815/c cells (treated with mitomycin C), these splenocytes were used as effectors. The P815 and P815/c cells were used as target cells. The target cells were planted in 96-well plates at a density of 1×105/ml cells/well. Effector cells were incubated with target cells at various effector/target (E/T) ratios (5:1, 10:1, 20:1, and 40:1, respectively) for 4 h. The HBcAg-specific CTL activity was assessed by the lactate dehydrogenase (LDH) release assay. Absorbance values from the supernatants were recorded at 490 nm. CTL activity was calculated according to the following equation: CTL activity (%) = [(Experimental release-Effector spontaneous release-Target spontaneous release)/(Target maximum release-Target spontaneous release)]×100%. The HBcAg-specific CTL activity was then calculated as follows: HBcAg-specific CTL activity (%) = CTLP815/C target cells-CTLP815 target cells.
Statistical analysis
Data were expressed as mean ± SD. SPSS 20.0 software was used for statistical analysis. F analysis and Student-Newman-Keuls (SNK) test were performed for pair-wise comparison. P < 0.05 was considered statistically significant.
Results
Culture and phenotypic analysis of transduced dendritic cells
Mouse bone marrow-derived DCs were cultured and induced by GM-CSF and IL-4. On Day 3, DC colonies showed up; on Day 5-6, DCs displayed typical morphology with elongated dendritic processes (Figure 1A-C). On Day 8, under fluorescence microscope, strong green fluorescence was observed in more than 90% DCs transfected with recombinant adenovirus at 100 MOI (Figure 1D). According to the flow cytometry analysis, the average proportion of DCs with fluorescence was as high as 93.72±3.01%, with the average fluorescence intensity of 246.18±43.81 MnX.
Figure 1.
Mouse dendritic cell (DC) culture and adenoviral transfection. A-C: Mouse bone marrow-derived DC culture on Day 3 (A, 100×; B, 200×) and Day 8 (C, 200×). D: DCs transfected with recombinant adenovirus carrying GFP (200×).
Phenotypic analysis of these DCs was performed with flow cytometry. DCs were pulsed with HBcAg antigen (DC/HBcAg), or transfected with rAds (DC/Ad-C, DC/Ad-S, DC/Ad-C+Ad-S, and DC/Ad-lacZ), for 2 h, and then cultured for another 24-48 h. Our results indicated that, when induced by cytokines, nearly 80% of the DCs expressed DEC-205, CD11c, MHC I and II molecules, and the expression levels of adhesion molecules (CD80, CD86, CD40, and CD54) were elevated, indicating phenotypic features of mature DCs. No significant differences were observed between these DC groups (Figure 2) (P > 0.05). These results suggest that the maturation of DCs would not be affected by HBcAg stimulation or rAd transfection.
Figure 2.
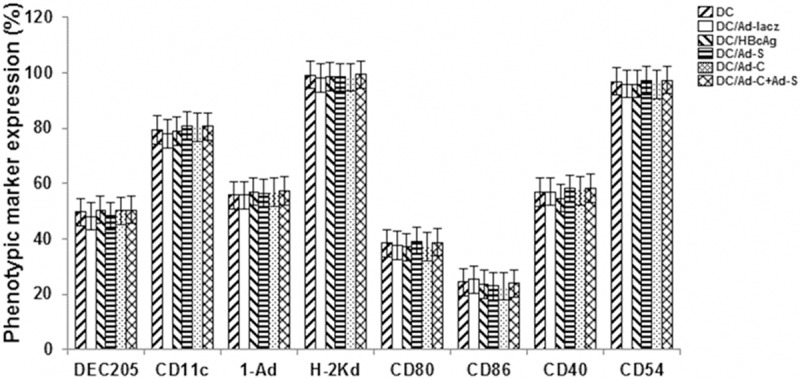
Phenotypic analysis of transduced DCs with flow cytometry. DCs were pulsed with HBcAg antigen (DC/HBcAg), or transfected with rAds (DC/Ad-C, DC/Ad-S, DC/Ad-C+Ad-S, and DC/Ad-lacZ), for 2 h, and then cultured for another 24-48 h. Then the expression patterns of DEC-205, CD11c, I-Ad, H-2KD, CD80, CD86, CD40, and CD 54 were determined with flow cytometry.
T-cell stimulatory capacity of transduced DCs
To investigate the T-cell stimulatory capacity of these transduced DCs, the mixed lymphocyte reaction (MLR) was performed. DCs obtained from BALB/c (H-2d) mice were treated with HBcAg antigen or rAds, and then subjected to allogeneic and autologous MLR involving T cells from C57BL/6 (H-2Kb) mice and BALB/c mice, respectively. After 4 d, T cell proliferation was assessed by the [3H] incorporation assay. Our results showed that in allogeneic or autologous MLR, no significant differences were observed in stimulating T-cell proliferation between these CD groups (Figure 3) (P > 0.05). These results suggest that neither HBcAg stimulation nor rAd transfection could affect the T-cell stimulatory capacity of DCs.
Figure 3.
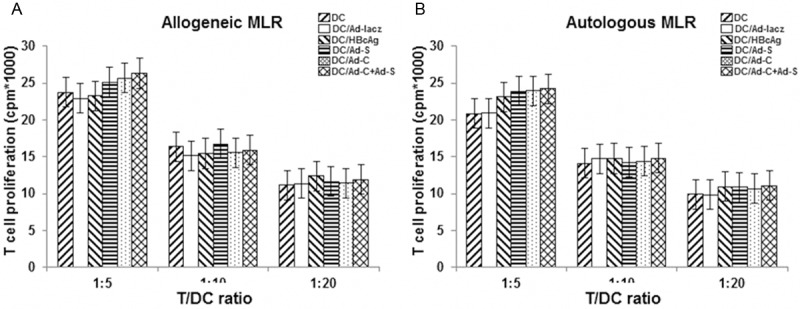
Mixed lymphocyte reaction (MLR). T-cell stimulatory capacity of transduced DCs was assess with MLR. DCs obtained from BALB/c (H-2d) mice were treated with HBcAg antigen (DC/HBcAg) or rAds (DC/Ad-C, DC/Ad-S, DC/Ad-C+Ad-S, and DC/Ad-lacZ), and then subjected to allogeneic (A) and autologous (B) MLR involving T cells from C57BL/6 (H-2Kb) mice and BALB/c mice, respectively, at various R/S ratios. After 4 d, T cell proliferation was assessed by the [3H] incorporation assay.
Cytokine production of T lymphocytes in vitro
The cytokine production of T lymphocytes in vitro was next evaluated in autologous MLR. DCs were mixed with autologous T cells at a responder/stimulator (R/S) ratio of 10:1 for 4 d, and then incubated with 25 µg/ml PMA, 1 µg/ml ionomycin, and 1.7 µg/ml monensin for 4 h. The production of IFN-γ and IL-4 was detected in CD3+CD8- Th cells and CD3+CD8+ Tc cells with flow cytometry. Our results showed that, when mixed with DCs, CD3+ CD8- Th cells mainly secret IFN-γ, exhibiting Th1 immune response; moreover, CD3+ CD8+ Tc cells also generally secret IFN-γ, indicating Tc1 immune response. However, no significant differences were observed in T cell responses between these DC groups (Figure 4A, 4B) (P > 0.05). Taken together, these results suggest that DCs could induce type I T cell response in vitro, which could not be influenced by either HBcAg stimulation or rAd transfection.
Figure 4.
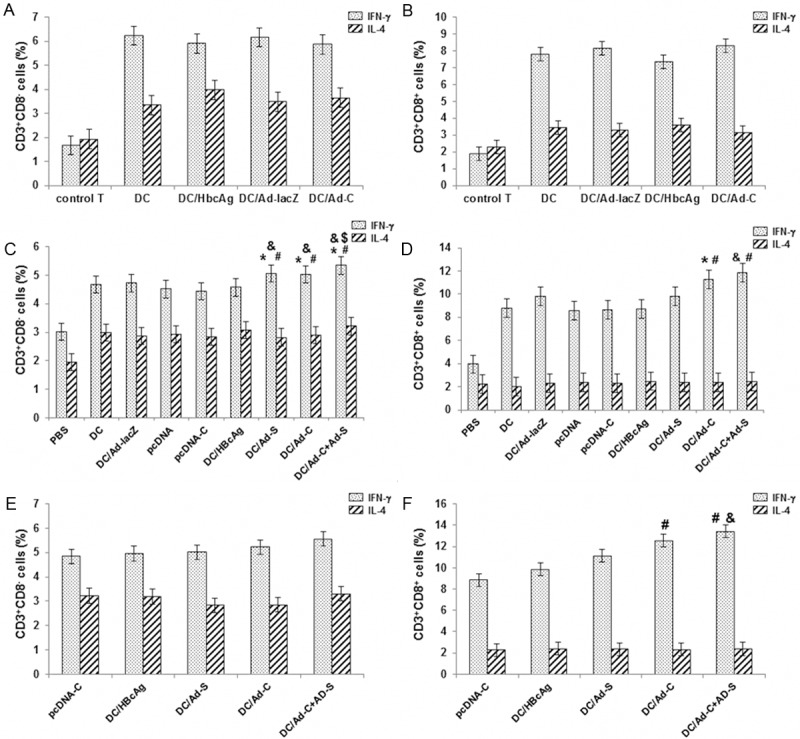
Cytokine production of T lymphocytes in vitro and in vivo. A, B: The cytokine production of T lymphocytes in vitro was evaluated in autologous MLR. DCs were mixed with autologous T cells at a R/S ratio of 10:1 for 4 d, and then incubated with 25 μg/ml PMA, 1 μg/ml ionomycin, and 1.7 μg/ml monensin for 4 h. The production of IFN-γ and IL-4 was detected in CD3+ CD8- Th cells (A) and CD3+ CD8+ Tc cells (B) with flow cytometry. C-F: Cytokine production by splenocytes from vaccinated mice was assessed. After vaccination, the production of IFN-γ and IL-4 was detected in CD3+ CD8- Th cells (2 w, C; 4 w, E) and CD3+ CD8+ Tc cells (2 w, D; 4 w, F). Compared with the DC/Ad-lacZ group, *P < 0.05; compared with the pcDNA-C group, #P < 0.05; compared with the DC/HBcAg group, &P < 0.05; compared with the DC/Ad-S and DC/Ad-C groups, $P < 0.05.
Cytokine production of T lymphocytes in vivo
Cytokine production by splenocytes from vaccinated mice was then assessed. At 2 w or 4 w after vaccination, the production of IFN-γ and IL-4 was detected in CD3+ CD8- Th and CD3+ CD8+ Tc cells, respectively. As shown in Figure 4C, 4D, at 2 w after vaccination, DCs transduced with rAds and pulsed with HBcAg induced significantly more IFN-γ secretion from Th cells, compared with the DNA vaccine, indicating stronger Th1 response (P < 0.05). Moreover, the strongest stimulating effect was observed in the DC/Ad-C+Ad-S group (P < 0.05). On the other hand, rAd-transduced DCs induced Tc cells to produce significantly more IFN-γ than DC/HBcAg and DNA vaccine, indicating stronger Tc1 response (P < 0.05). In addition, at 4 w after vaccination, compared with DC/HBcAg and DNA vaccine, rAd-transduced DCs induced increased secretion of IFN-γ in Th and Tc cells (Figure 4E, 4F) (P > 0.05 for Th cells, and P < 0.05 for Tc cells). These results suggest that, DC vaccination could stimulate the Th1 cell proliferation, while rAd-transduced DC enhances the Tc1 response.
HBcAg-specific CTL response
To evaluate the specific cytotoxicity of T lymphocytes in these vaccinated mice, HBcAg-specific CTL activities at various effector/target (E/T) ratios were measured. As shown in Figure 5, at 2 w after vaccination, for all the E/T ratios, HBcAg-specific CTL activities were decreased in the following order: the DC/Ad-C+Ad-S, DC/Ad-C, DC/Ad-S, DC/HBcAg, and pcDNA3.1 (+)-C groups; with statistical significance between groups (P < 0.05). Similar results were obtained for the time point at 4 w after vaccination. These results suggest that, compared with DC/HBcAg and DNA vaccine, mice immunized with rAd-transduced DCs develop stronger HBcAg-specific CTL responses.
Figure 5.
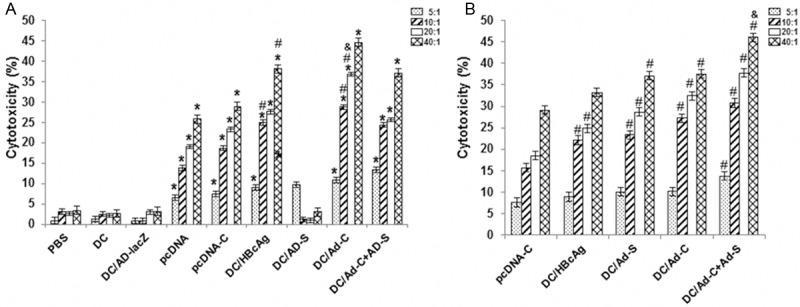
HBcAg-specific cytotoxic T lymphocyte (CTL) activity assessment. HBcAg-specific CTL activities were measured at 2 w (A) or 4 w (B) after vaccination, with various effector/target (E/T) ratios. Compared with the DC/Ad-lacZ group, *P < 0.05; compared with the pcDNA-C group, #P < 0.05; compared with the DC/HBcAg group, &P < 0.05; compared with the DC/Ad-S and DC/Ad-C groups, $P < 0.05.
Discussion
In recent years, DC vaccine attracts increasing attention in inducing cellular immune response in the treatment of HBV infection [5,6], including DCs pulsed with antigens and loaded with antigen genes. Studies concerning tumor vaccines [12] and HCV vaccines [7] have shown that DCs loaded with antigen genes induce stronger CTL response than antigen-pulsed DCs. In this study, mouse DCs were transfected with adenovirus carrying HBcAg and/or HBsAg, and the in vitro immune stimulating activity and in vivo immune regulating function were investigated, in comparison with HBcAg-pulsed DCs and DNA vaccine. Our results showed that, no significant differences were observed in the regulation of DC maturation, stimulation of autologous and allogeneic T-cell proliferation, and induction of Th1/Tc1 (type I) cellular responses, between DCs transfected with Ad-C and Ad-lacZ control vectors. In line with the previous reports, rAd-transfected DCs, no matter with or without target genes, could not influence DC phenotype, IL-12 production, or MLR [18-20].
We found that, the vaccination of DC/Ad-C induced stronger Th1 and Tc1 responses and resulted in enhanced HBcAg-specific CTL cytotoxicity, compared with DC/HBcAg and DNA vaccine. It has been accepted that, Th and Tc cells could further differentiate into type I and type II cells, respectively, depending on the pattern of cytokine production. Th1 and Tc1 cells produce IL-2 and IFN-γ, while Th2 and Tc2 cells secret IL-4 and IL-5. Type I cells are related to the cellular immunity, and type II cells are associated with the humoral immunity. Therefore, Th1/Tc1 cells play a key role in facilitating CTL in tumor and viral clearance [21]. Miquelena-Colina et al. [19] have reported that, mature DCs transfected with rAd carrying antigen gene stimulate CD8+ T-cell responses, more efficiently than antigen-pulsed DCs. Herein, we further found that rAd-transfected DCs mainly stimulated Tc1 cells that secreted IFN-γ. In addition, Chaput et al. [5] have found that, at 2 w after vaccination, HBcAg-pulsed DCs induce stronger CTL response than DNA vaccine, which is also consistent with our results. Based on these results, DC/Ad-C is superior to DC/HBcAg and DNA vaccine, in inducing type I immune response and stimulating HBcAg-specific CTL. On the other hand, Ad-C-transfected or HBcAg-pulsed DCs resulted in reduced anti-HBc responses, compared with DNA vaccine. In the latter case, all the immunized mice were positive for the antibody detection, and the average antibody level was higher than that in mice treated with DCs. This is probably due to the fact that DNA plasmids could express target proteins to cause humoral immune response.
APCs activate CD8+ T cells through two different pathways, i.e., the direct (endogenous) presentation and the cross-presentation (exogenous presentation). Endogenous presentation refers to the process in which APCs (especially DCs) deliver the endogenous antigens (from protein degradation) to MHC I molecules, presenting to CD8+ T cells. Exogenous presentation defines that, APCs capture exogenous virus or proteins produced by transduced cells, which are then presented to CD8+ T cells through MHC I molecules. Recent studies have shown that, the epitopes presented through the endogenous pathway could not be necessarily presented trough the exogenous pathway. Virus and tumor-associated antigens may be damaged during the cross-presentation, which could not be recognized by CD8+ T cells [22,23]. Therefore, vaccine design should focus on maximizing the generation of MHC I molecule-binding peptides to induce CD8+ T cell response. In this study, DC/Ad-S could function through the direct presentation pathway, inducing stronger Tc1 and CTL responses than DC/HBcAg (cross-presentation) or DNA vaccine (partial cross-presentation).
Our results showed that the rAd-transduced DCs induced potent Th1 and Th1 responses and specific CTL response, and the potencies were decreased in the following order: DC/Ad-C+Ad-S, DC/Ad-C, and then DC/Ad-S. HBcAg is the most immunogenic antigen produced by HBV, which could easily induce Th1 cellular immune response [13-16,24]. In chronic HBV infection, HBcAg is the only antigen that is able to trigger significant immune response [25]. The strong antigenicity of HBcAg relies on its special three-dimensional structure, consisting of classical antibody binding sites and interacting domains for the immunoglobulin. Moreover, HBcAg holds epitopes for CD4+ T cells and packaged nucleic acids.
In conclusion, our results showed that DCs transfected with rAd or pulsed with HBcAg antigen exhibited mature phenotypes. No significant differences were observed in stimulating T-cell proliferation between the DC/rAd and DC/HBcAg groups. Moreover, DCs could induce type I T-cell response, despite of HBcAg stimulation or rAd transfection. Furthermore, DC vaccination stimulated Th1 cell proliferation, while rAd-transduced DCs enhanced Tc1 response. In vaccinated mice, the HBcAg-specific CTL activity was decreased in the following order: the DC/Ad-C+Ad-S, DC/Ad-C, DC/Ad-S, DC/HBcAg, and DNA vaccine groups. Our findings suggest that DCs transfected with HBcAg/HBsAg might provide an effective approach in the treatment of persistent HBV infection.
Acknowledgements
This work was supported by the National Key Program for Infectious Diseases of China to YD Yang (No. 2013ZX10002001), the 12th Five-Year Significant New Drugs Creation Plan of the Ministry of Science and Technology of China to YD Yang (No. 2011ZX09302-003-03), the National Nature and Science Fund to HY Jia (No. 81100286), and the Fund of State Key Laboratory for Diagnosis and Treatment of Infectious Diseases to HY Jia.
Disclosure of conflict of interest
None.
References
- 1.Zhang Z, Zhang JY, Wang LF, Wang FS. Immunopathogenesis and prognostic immune markers of chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2012;27:223–230. doi: 10.1111/j.1440-1746.2011.06940.x. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Kosinska A, Lu M, Roggendorf M. New therapeutic vaccination strategies for the treatment of chronic hepatitis B. Virol Sin. 2014;29:10–16. doi: 10.1007/s12250-014-3410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Updated CDC recommendations for the management of hepatitis B virus-infected health-care providers and students. MMWR Recomm Rep. 2012;61:1–12. [PubMed] [Google Scholar]
- 4.McVicker BL, Thiele GM, Casey CA, Osna NA, Tuma DJ. Susceptibility to T cell-mediated liver injury is enhanced in asialoglycoprotein receptor-deficient mice. Int Immunopharmacol. 2013;16:17–26. doi: 10.1016/j.intimp.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Ménégaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J. Identification of CD8+ CD25+ Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520–529. doi: 10.1136/gut.2008.158824. [DOI] [PubMed] [Google Scholar]
- 6.Akbar SMF, Furukawa S, Hasebe A, Horiike N, Michitaka K, Onji M. Production and efficacy of a dendritic cell-based therapeutic vaccine for murine chronic hepatitis B virus carrierer. Int J Mol Med. 2004;14:295–299. [PubMed] [Google Scholar]
- 7.Chen W, Shi M, Shi F, Mao Y, Tang Z, Zhang B, Zhang H, Chen L, Chen L, Xin S, Wang FS. HBcAg-pulsed dendritic cell vaccine induces Th1 polarization and production of hepatitis B virus-specific cytotoxic T lymphocytes. Hepatol Res. 2009;39:355–365. doi: 10.1111/j.1872-034X.2008.00468.x. [DOI] [PubMed] [Google Scholar]
- 8.Beckebaum S, Cicinnati VR, Zhang X, Ferencik S, Frilling A, Grosse-Wilde H, Broelsch CE, Gerken G. Hepatitis B virus-induced defect of monocyte-derived dendritic cells leads to impaired T helper type 1 response in vitro: mechanisms for viral immune escape. Immunology. 2003;109:487–495. doi: 10.1046/j.1365-2567.2003.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, Kitamura H, Nishimura T. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 2010;70:2697–2706. doi: 10.1158/0008-5472.CAN-09-2982. [DOI] [PubMed] [Google Scholar]
- 10.Luo J, Li J, Chen RL, Nie L, Huang J, Liu ZW, Luo L, Yan XJ. Autologus dendritic cell vaccine for chronic hepatitis B carriers: a pilot, open label, clinical trial in human volunteers. Vaccine. 2010;28:2497–2504. doi: 10.1016/j.vaccine.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Nagarajan NA, Gonzalez F, Shastri N. Nonclassical MHC class Ib-restricted cytotoxic T cells monitor antigen processing in the endoplasmic reticulum. Nat Immunol. 2012;13:579–586. doi: 10.1038/ni.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Chen Z, Jia HY, Wu W, Cai LF, Zhou C. Immune response against hepatitis B virus induced by HBsAg recombinant adenovirus-transduced dendritic cells in BALB/c mice. Chinese J Microbiol Immunol. 2006;26:553–556. [Google Scholar]
- 13.Heathcote J, McHutchison J, Lee S, Tong M, Benner K, Minuk G, Wright T, Fikes J, Livingston B, Sette A, Chestnut R. A pilot study of the CY-1899 T-cell vaccine in subjects chronically infected with hepatitis B virus. The CY1899 T Cell Vaccine Study Group. Hepatology. 1999;30:531–536. doi: 10.1002/hep.510300208. [DOI] [PubMed] [Google Scholar]
- 14.Bertoletti A, Chisari FV, Penna A, Guilhot S, Galati L, Missale G, Fowler P, Schlicht HJ, Vitiello A, Chesnut RC. Definition of a minimal optimal cytotoxic T-cell epitope within the hepatitis B virus nucleocapsid protein. J Virol. 1993;67:2376–2380. doi: 10.1128/jvi.67.4.2376-2380.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin D, Liang W, Xing S, Gao Z, Zhang W, Guo Z, Gao S. Hepatitis B DNA vaccine-polycation nano-complexes enhancing immune response by percutaneous administration with microneedle. Biol Pharm Bull. 2013;36:1283–1291. doi: 10.1248/bpb.b13-00050. [DOI] [PubMed] [Google Scholar]
- 16.Ezzikouri S, Pineau P, Benjelloun S. Hepatitis B virus in the Maghreb region: from epidemiology to prospective research. Liver Int. 2013;33:811–819. doi: 10.1111/liv.12135. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ, Hong CY, Kim MH, Lee YK, Nguyen-Pham TN, Park BC, Yang DH, Chung IJ, Kim HJ, Lee JJ. In vitro induction of anterior gradient-2-specific cytotoxic T lymphocytes by dendritic cells transduced with recombinant adenoviruses as a potential therapy for colorectal cancer. Exp Mol Med. 2012;44:60–67. doi: 10.3858/emm.2012.44.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maccormac LP, Jacque JM, Chain B. The functional consequences of delivery of HIV-1 Nef to dendritic cells using an adenoviral vector. Vaccine. 2004;22:528–535. doi: 10.1016/j.vaccine.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Miquilena-Colina ME, Lozano-Rodríguez T, García-Pozo L, Sáez A, Rizza P, Capone I, Rapicetta M, Chionne P, Capobianchi M, Selleri M, Castilletti C, Belardelli F, Iacono OL, García-Monzón C. Recombinant interferon-alpha2b improves immune response to hepatitis B vaccination in haemodialysis patients: results of a randomised clinical trial. Vaccine. 2009;27:5654–5660. doi: 10.1016/j.vaccine.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura N, Nishioka Y, Shinohara T, Sone S. Enhanced efficiency by centrifugal manipulation of adenovirus-mediated interleukin 12 gene transduction into human monocyte-derived dendritic cells. Hum Gene Ther. 2001;12:333–346. doi: 10.1089/10430340150503966. [DOI] [PubMed] [Google Scholar]
- 21.Chamoto K, Kosaka A, Tsuji T, Matsuzaki J, Sato T, Takeshima T, Iwakabe K, Togashi Y, Koda T, Nishimura T. Critical role of the Th1/Tc1 circuit for the generation of tumor-specific CTL during tumor eradication in vivo by Th1-cell therapy. Cancer Sci. 2003;94:924–928. doi: 10.1111/j.1349-7006.2003.tb01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolkers MC, Brouwenstijn N, Bakker AH, Toebes M, Schumacher TNM. Antigen bias in T cell cross-priming. Science. 2004;304:1314–1317. doi: 10.1126/science.1096268. [DOI] [PubMed] [Google Scholar]
- 23.Zeng R, Li G, Ling S, Zhang H, Yao Z, Xiu B, He F, Huang R, Wei L. A novel combined vaccine candidate containing epitopes of HCV NS3, core and E1 proteins induces multi-specific immune responses in BALB/c mice. Antiviral Res. 2009;84:23–30. doi: 10.1016/j.antiviral.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Milich DR, Schödel F, Hughes JL, Jones JE, Peterson DL. The hepatitis B virus core and e antigens elicit different Th cell subsets: antigen structure can affect Th cell phenotype. J Virol. 1997;71:2192–2201. doi: 10.1128/jvi.71.3.2192-2201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menne S, Cote PJ. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J Gastroenterol. 2007;13:104–124. doi: 10.3748/wjg.v13.i1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]


