Abstract
Prostate cancer remains the second leading cause of cancer death in men due to inefficiency of androgen deprivation therapy or androgen blockade. Endothelins (ETs) and the two endothelin receptor family members A and B (ETA and ETB) are known to play important roles in the progression of many malignancies, including prostate cancer. However, phase III clinical studies did not reach a unanimous conclusion regarding ETA receptor antagonists in prostate cancer treatment. Here, we provide a meta-analysis of clinical studies using ETA receptor antagonists to treat prostate cancer, especially the hormone refractory prostate cancer (HRPC). Data were extracted from nine studies that used Zibotentan or Atrasentan, two selective ETA receptor antagonists, to treat prostate cancer and meet the selection criteria. The results indicated that the overall survival (OS) and the progression-free survival (PFS) of patients treated with Zibotentan did not show significant difference with the patients treated with placebo (pooled hazard ratio (HR) for OS, 0.86, 95% CI 0.70-1.06; pooled HR for PFS, 0.98, 95% CI 0.91-1.06). No statistically significant difference was detected either as to the OS and PFS of patients between the Atrasentan treated group and the group treated with placebo (pooled HR for OS, 0.99, 95% CI 0.90-1.08; pooled HR for PFS, 0.94, 95% CI 0.86-1.02). Notably, the level of prostate-specific antigen (PSA) and the incidence of bone pain were significantly lower in the Atrasentan treated patients compared to the controls (pooled HR for time of PSA progression, 0.87, 95% CI 0.78-0.97; and pooled relative risk (RR) for bone pain, 0.68, 95% CI 0.48-0.97). In addition, increasing of PSA and bone alkaline phosphatase (BALP) were significantly delayed with Atrasentan treatment (P<0.05). Together, these data suggest that Atrasentan has an effect on cancer-related bone pain and skeletal-events in patients with prostate cancer.
Keywords: ETA receptor antagonist, Atrasentan, Zibotentan, prostate cancer, meta-analysis
Introduction
Prostate cancer is the most commonly diagnosed non-skin cancer and is also one of the leading causes of cancer death in men in the United States (second to lung cancer). The standard treatment for advanced prostate cancer includes androgen-deprivation combined with androgen blockade. However, due to unresponsiveness to androgen blockade, the prognosis of hormone refractory prostate cancer (HRPC) remains poor. Advanced HRPC is characterized by development of painful bone metastasis. The docetaxel-based chemotherapy was reportedly to be able to improve its survival, but affects only half of the patients with metastatic HRPC [1-3]. New therapies are needed to improve survival as well as to maintain or improve the quality of life for patients diagnosed with HRPC [4].
Endothelins (ETs) are short peptides that are known to be involved in the pathogenesis of multiple diseases, including cancer (majorly prostate cancer) and cancer metastasis [5]. There are two endothelin receptor family members A and B (ETA and ETB) that belong to the G protein-coupled receptor (GPCR) superfamily [6,7]. ETA interacts with a pertussis-insensitive G protein and activates multiple signaling pathways, including PKC and MAPK etc [8]. These pathways work synergistically to promote cell proliferation, which are mediated primarily by endothelin-1 (ET-1) through the endothelin-A (ETA) receptor [9,10]. As a weak mitogen for prostate cancer cell lines, ET-1 strongly inhibits chemotherapy-induced apoptosis both in vitro and in vivo [3]. ET-1 has also been implicated multiple functions during angiogenesis, cell invasion, tumor cell proliferation, and apoptosis that affect tumor progression [11-22]. Inhibition of ETA receptor in prostate cancer may restore its sensitivity to docetaxel-based chemotherapy. To evaluate the clinical effectiveness and to guide clinical practice, we performed a meta-analysis to determine the efficacy of ETA receptor antagonists, herein Atrasentan or Zibotentan, in treatment of HRPC.
Methods
Search strategy
The MEDLINE bibliographical database was searched for eligible articles up to the end of 2014. Following keywords were used for the searching: “endothelin”, “Atrasentan”, “ZD4054”, “Aibotentan” and “prostate cancer”. Google academic searching was also performed to obtain additional information that may be missed by above searching methods.
Inclusion criteria
Randomized clinical trials that evaluated the therapeutic efficacy of the endothelin-A receptor antagonist Atrasentan and Zibotentan with placebo control for treatment of prostate cancer were included for analysis. Conference abstracts providing sufficient data to assess the quality of the studies or the results were described in detail were also included. No pharmacokinetic studies, animal studies, laboratory studies, and non-randomized trials were includ-ed for this study. All the studies provided appro-val from institutional review board with appropriate consent [22].
Data extraction
Data were collected by two reviewers who independently examined the titles and abstracts of the studies using above searching criteria. Obviously ineligible studies were excluded by initial screening of the abstracts. Full texts of all relevant studies were reviewed and disagreement was determined by a third reviewer [22,23]. The following information was extracted from each paper which includes: the trial’s name, first author, year of publication, journal, number of patients in both groups, age, hazard ratios (HR) for OS, PFS and time to first 50% increase in PSA and their 95 % confidence intervals (CI), and the incidence of bone pain.
Quality assessment
Assessment of the trials was conducted using the methods as previously described [24]. Briefly, following aspects were considered for quality assessment: 1) Randomization (the trial was described as randomized); 2) Generation of random numbers using random number table, computer, tossed coins or shuffled cards etc.; 3) Double-blind study-design; 4) Using a proper allocation concealment; and, 5) Clearly reporting the number and reasons for dropouts. The assessment were recorded and scored ranging from 0 to 5 with 5 as the highest quality [25].
Statistical analysis
Hazard ratios (HR) for OS, PFS and time to first 50% increase in PSA, relative risk (RR) for incidence of bone pain were calculated and compared between the two groups: endothelin-A receptor antagonist treated patients or placebo controls. A statistical test with a P value <0.05 was considered to be significant. The values of HR and RR>1 reflect more progression or deaths and more toxicities in endothelin-A receptor antagonist treated patients. We estimated the degree of heterogeneity among the trials using the χ2 statistics (with a P-value <0.10 considered significant) and the I2 test (25%, 50%, and 75% represent low, moderate and high heterogeneity respectively). When significant heterogeneity (P<0.1 or I2>50%) was achieved, we used the random effect model to combine the effect sizes of the included studies. If no significant heterogeneity was found, we selected a fixed effect to pool the data [26]. All CI had two-sided probability coverage of 95%. Potential publication bias was estimated using the Begg’s test. We used a forest plot to analyze and to display the results. All calculations were accomplished using the STATA (version 11.0).
Results
Selection of the nine clinical trial studies
We retrieved 270 articles from MEDLINE bibliographical database. 252 papers that were neither RCTs, nor original studies were excluded from this study. Studies that did not involve either of the target drug Atrasentan or Zibotentan were also excluded. After reviewing of the remaining 18 articles, only 9 studies met our inclusion criteria and are outlined in Figure 1. Among these 9 articles, 5 studies evaluated Zibotentan treated patients [4,27-30]. Three of them described the results of phase III trials, while the other 2 studies described the results of phase II trials. All these studies were conducted on patients with hormone-refractory prostate cancer. The rest (four) of the studies evaluated Atrasentan treated patients [3,31-33], including 3 phase III trials and one phase II trials. Detailed information about these studies is provided in Table 1. The Jaded scoring system was used to assess the quality of the methods in these studies.
Figure 1.
Flowchart showing the literature searching and selection.
Table 1.
Nine randomized controlled trials included in the meta-analysis
| Trial | Study design | Number | HR for OS | HR for PFS | HR for PSA | Jadad Score |
|---|---|---|---|---|---|---|
| Joel 2012 | zibotentan | 299 | 0.87 | 1.01 | N | 5 |
| phase III | placebo | 295 | ||||
| Karim 2013 | Docetaxel+zibotentan | 524 | 1.00 | 1.00 | N | 5 |
| phase III | Docetaxel+placebo | 528 | ||||
| Miller 2013 | zibotentan | 703 | 1.13 | 0.89 | N | 3 |
| phase III | placebo | 712 | ||||
| Nicholas 2010 | zibotentan | 107 | 0.83 | 1.06 | N | 3 |
| phase II | placebo | 107 | ||||
| Nicholas 2008 | zibotentan | 107 | 0.55 | 0.88 | N | 3 |
| phase II | placebo | 107 | ||||
| Michael 2007 | atrasentan | 408 | 0.97 | 0.89 | 0.86 | 4 |
| phase III | placebo | 401 | ||||
| David 2013 | Docetaxel+atrasentan | 498 | 1.04 | 1.02 | N | 4 |
| phase III | Docetaxel+placebo | 496 | ||||
| Joel 2008 | atrasentan | 467 | 0.92 | 0.92 | 0.92 | 4 |
| phase III | placebo | 474 | ||||
| Michael 2003 | atrasentan | 89 | N | 0.80 | 0.75 | 4 |
| phase II | placebo | 104 |
Effect of Zibotentan on hormone-refractory prostate cancer
To determine the effect of Zibotentan on hormone-refractory prostate cancer, we pooled the overall survival (OS) and progression-free survival (PFS) and compared to the controls treated with placebo. The results showed that Zibotentan did not significantly improve the OS (pooled HR for OS, 0.86, 95% CI 0.70-1.06, Figure 2A) and PFS (pooled HR for PFS, 0.98, 95% CI 0.91-1.06, Figure 2B) of the patients. Heterogeneity was found across the five studies for OS (I2=76.5%, P=0.002), we then used a random model for meta-analysis to calculate the overall survival. No heterogeneity was shown for PFS (I2=0.0%, P=0.627) and a fixed model was applied for analysis of the progression-free survival. The fun- nel plots were symmetrical and the results of Begg’s test in our meta-analyses of OS were shown (Pr>|z|=0.462, P>0.05) and PFS (Pr>|z|=0.806, P>0.05, Figure 5).
Figure 2.
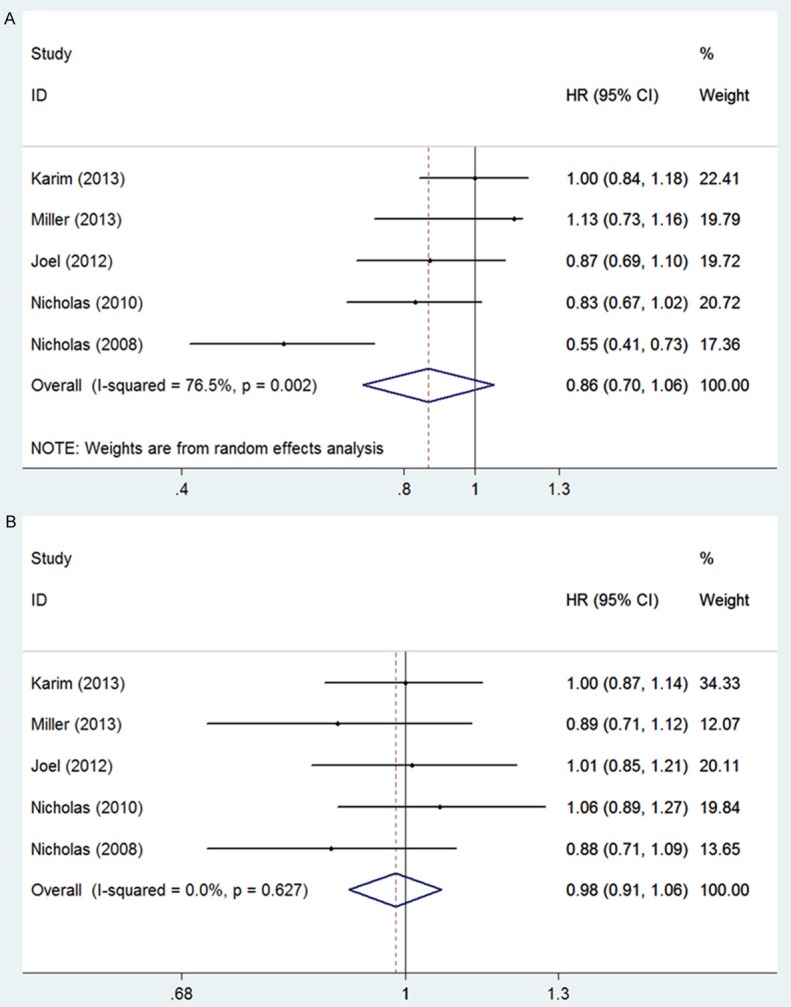
Meta-analysis of effects of Zibotentan on hormone-refractory prostate cancer A: OS (pooled HR for OS, 0.86, 95% CI 0.70-1.06); B: PFS (pooled HR for PFS, 0.98, 95% CI 0.91-1.06).
Figure 5.
Funnel plot analysis of potential publication bias.
The efficacy of Atrasentan
No statistically significant difference was detected in overall survival (pooled HR for OS, 0.99, 95% CI 0.90-1.08, Figure 3A) and progression-free survival (pooled HR for PFS, 0.94, 95% CI 0.86-1.02, Figure 3B) between the Atrasentan treated group and the placebo-treated group. As to the time to first 50% increase in PSA, Atrasentan significantly extended the time of PSA progression (HR, 0.87; 95% CI, 0.78-0.97, Figure 4A), while the bone pain relative risk (RR =0.68, 95% CI, 0.48-0.97, Figure 4B) significantly decreased in Atrasentan-treated group compared to the control group. No heterogeneity was shown for overall survival, progression-free survival, Time to first 50% increase in PSA and bone pain relative risk and a fixed model was applied for analysis of these parameters. A Begg’s funnel plot and a Begg’s test were used to assess for publication bias for these studies. The result showed no obvious asymmetry, indicating no publication bias.
Figure 3.
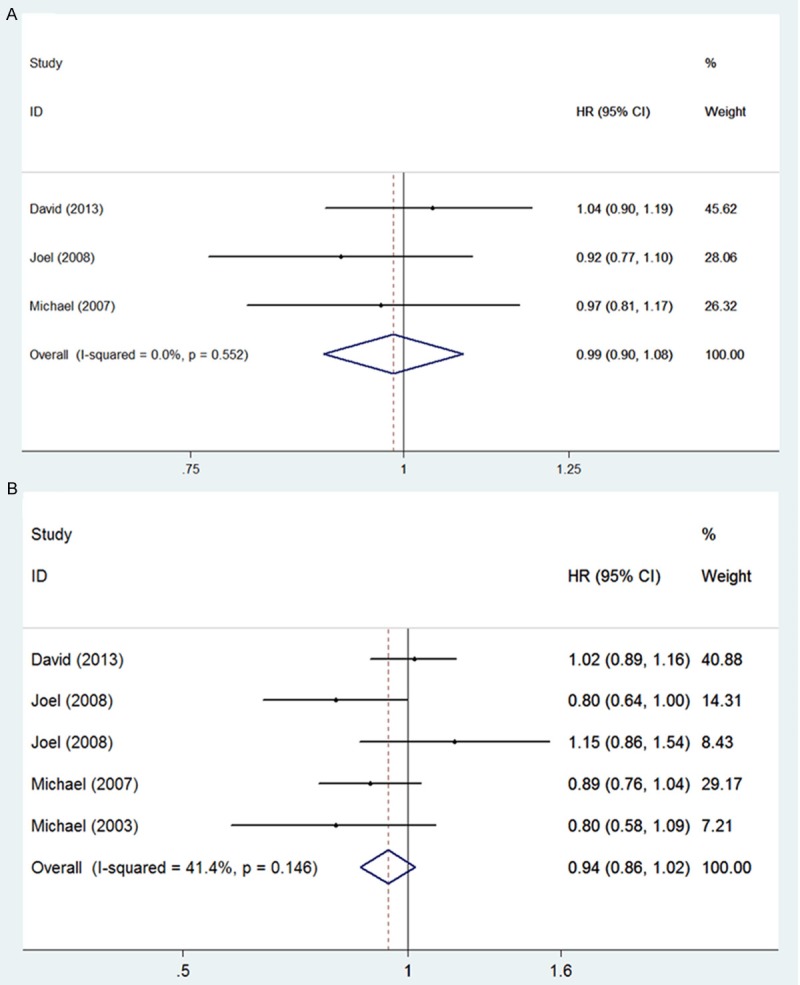
Meta-analysis of effects of Atrasentan on hormone-refractory prostate cancer A: OS (pooled HR for OS, 0.99, 95% CI 0.90-1.08); B: PFS (pooled HR for PFS, 0.94, 95 % CI 0.86-1.02).
Figure 4.
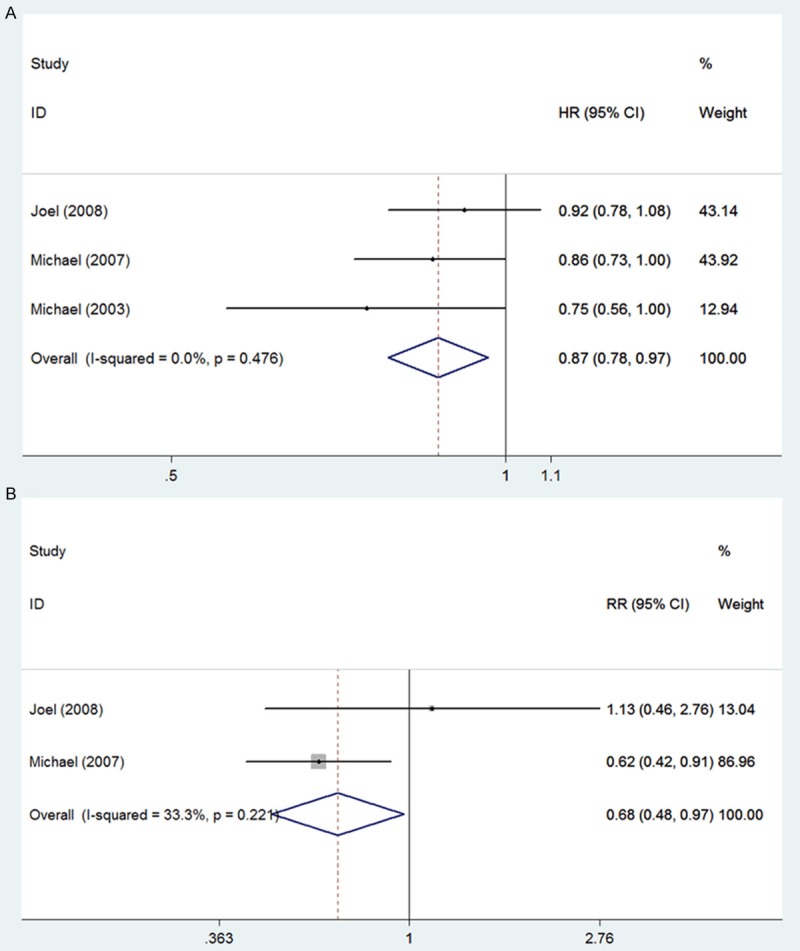
Meta-analysis of effects of Atrasentan on hormone-refractory prostate cancer A: TTPSA (pooled HR for TTPSA, 0.87, 95% CI 0.78-0.97); B: Bone pain relative risk (pooled RR for bone pain, 0.68, 95% CI 0.48-0.97).
Discussion
The endothelins (ET) are a group of proteins that act through G-protein coupled receptors. Recently, the ET axis, i.e. the ET-1 acting through the endothelin A receptor (ETA) pathway, has been implicated an essential role in the pathogenesis of many cancers, especially prostate cancer. ET-1 may work through activation of multiple pathways that involve angiogenesis, osteogenesis, cell proliferation, migration, invasion, and epithelial-mesenchymal transition [34]. In recent years, multiple phase III clinical trials using ETA receptor antagonists have been conducted in prostate cancer patients, but, the results were very inconsistent. Joel and his colleagues have shown that treatment with Atrasentan resulted in a significantly slower rate of PSA increase and a significant attenuation of BALP activity. The time to initial presentation with skeletal metastases showed a tendency to delay, but no significant improvement in survival or time to cancer progression was detected [32]. However, a recent study of a phase II clinical trial indicated that ZD4054, another ETA receptor antagonist called Zibotentan, offers a promising result of improving the overall survival in patients with HRPC and bone metastases [4]. These results demonstrated the inconsistency of these clinical trials using ETA receptor antagonists as therapeutics for HRPC.
According to this meta-analysis, neither Zibotentan nor Atrasentan showed statistically significant improvement of OS and PFS in treated patients compared to the ones with placebo therapy. Treatment with Atrasentan resulted in a significantly slower rate of PSA increase, a decrease of bone pain relative risk, and a delay in the time to initial presentation with skeletal metastases. The discrepancy between phase II clinical trial and phase III is partially due to median drug exposure in the phase II study was shorter. Data about subsequent systemic anticancer therapy usage was also lower than in phase III study. Although Asian populations with prostate cancer have better OS rates than Caucasian patients, indicating a possible genetic influence, contribution of geographical basis for these differences may not be excluded, as discontinuations because of adverse events were more frequent at US sites than other regions [30,32,35,36].
In summary, there are many ETA receptor antagonists that have been reported, but the number of available randomized studies that examined their role in HRPC treatment are still lacking and may not reach the needed statistics power [37]. Notably, these studies were randomized controlled trials with significant homogeneity and should give rise to convincing data. Based on current meta-analysis, endothelin inhibitors do not have an established role in advanced prostate cancer. However, the slow increase of PSA level and decrease of the bone-related BALP activity in Atrasentan treated patients worth further investigation regarding its efficacy in controlling bone pain and skeletal complications in patients with HRPC.
Acknowledgements
This project received support from the Jiangsu Key Laboratory of Medical Science and Laboratory Medicine, School of Medicine, Jiangsu University (Q.Z.), the Innovation Program of Jiangsu Province (No. 480, 2013, Q.Z.) and the NSFC grants (Nos. 31271399, 31440058, and 81472047, Q.Z., J.G., Y.L.).
Disclosure of conflict of interest
None.
References
- 1.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, Sleep DJ, Isaacson JD, Nelson JB. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–1966. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 4.James ND, Caty A, Payne H, Borre M, Zonnenberg BA, Beuzeboc P, McIntosh S, Morris T, Phung D, Dawson NA. Final safety and efficacy analysis of the specific endothelin A receptor antagonist zibotentan (ZD4054) in patients with metastatic castration-resistant prostate cancer and bone metastases who were pain-free or mildly symptomatic for pain: a double-blind, placebo-controlled, randomized Phase II trial. BJU Int. 2010;106:966–973. doi: 10.1111/j.1464-410X.2010.09638.x. [DOI] [PubMed] [Google Scholar]
- 5.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 6.Horinouchi T, Terada K, Higashi T, Miwa S. Endothelin receptor signaling: new insight into its regulatory mechanisms. J Pharmacol Sci. 2013;123:85–101. doi: 10.1254/jphs.13r02cr. [DOI] [PubMed] [Google Scholar]
- 7.Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jimeno A, Carducci M. Atrasentan: targeting the endothelin axis in prostate cancer. Expert Opin Investig Drugs. 2004;13:1631–1640. doi: 10.1517/13543784.13.12.1631. [DOI] [PubMed] [Google Scholar]
- 9.Bagnato A, Spinella F, Rosano L. The endothelin axis in cancer: the promise and the challenges of molecularly targeted therapy. Can J Physiol Pharmacol. 2008;86:473–484. doi: 10.1139/Y08-058. [DOI] [PubMed] [Google Scholar]
- 10.Levin ER. Endothelins. N Engl J Med. 1995;333:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- 11.Thakkar SG, Choueiri TK, Garcia JA. Endothelin receptor antagonists: rationale, clinical development, and role in prostate cancer therapeutics. Curr Oncol Rep. 2006;8:108–113. doi: 10.1007/s11912-006-0045-1. [DOI] [PubMed] [Google Scholar]
- 12.Battistini B, Chailler P, D’Orleans-Juste P, Briere N, Sirois P. Growth regulatory properties of endothelins. Peptides. 1993;14:385–399. doi: 10.1016/0196-9781(93)90057-n. [DOI] [PubMed] [Google Scholar]
- 13.Pirtskhalaishvili G, Nelson JB. Endothelium-derived factors as paracrine mediators of prostate cancer progression. Prostate. 2000;44:77–87. doi: 10.1002/1097-0045(20000615)44:1<77::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Shichiri M, Kato H, Marumo F, Hirata Y. Endothelin-1 as an autocrine/paracrine apoptosis survival factor for endothelial cells. Hypertension. 1997;30:1198–1203. doi: 10.1161/01.hyp.30.5.1198. [DOI] [PubMed] [Google Scholar]
- 15.Eberl LP, Egidy G, Pinet F, Juillerat-Jeanneret L. Endothelin receptor blockade potentiates FasL-induced apoptosis in colon carcinoma cells via the protein kinase C-pathway. J Cardiovasc Pharmacol. 2000;36(Suppl 1):S354–356. doi: 10.1097/00005344-200036051-00103. [DOI] [PubMed] [Google Scholar]
- 16.Eberl LP, Valdenaire O, Saintgiorgio V, Jeannin JF, Juillerat-Jeanneret L. Endothelin receptor blockade potentiates FasL-induced apoptosis in rat colon carcinoma cells. Int J Cancer. 2000;86:182–187. doi: 10.1002/(sici)1097-0215(20000415)86:2<182::aid-ijc6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Nelson JB, Nguyen SH, Wu-Wong JR, Opgenorth TJ, Dixon DB, Chung LW, Inoue N. New bone formation in an osteoblastic tumor model is increased by endothelin-1 overexpression and decreased by endothelin A receptor blockade. Urology. 1999;53:1063–1069. doi: 10.1016/s0090-4295(98)00658-x. [DOI] [PubMed] [Google Scholar]
- 18.Boyce BF, Yoneda T, Guise TA. Factors regulating the growth of metastatic cancer in bone. Endocr Relat Cancer. 1999;6:333–347. doi: 10.1677/erc.0.0060333. [DOI] [PubMed] [Google Scholar]
- 19.Mundy GR. Endothelin-1 and osteoblastic metastasis. Proc Natl Acad Sci U S A. 2003;100:10588–10589. doi: 10.1073/pnas.2035063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salani D, Di Castro V, Nicotra MR, Rosano L, Tecce R, Venuti A, Natali PG, Bagnato A. Role of endothelin-1 in neovascularization of ovarian carcinoma. Am J Pathol. 2000;157:1537–1547. doi: 10.1016/S0002-9440(10)64791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bek EL, McMillen MA. Endothelins are angiogenic. J Cardiovasc Pharmacol. 2000;36(Suppl 1):S135–139. doi: 10.1097/00005344-200036051-00043. [DOI] [PubMed] [Google Scholar]
- 22.Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. J Biol Chem. 2002;277:27850–27855. doi: 10.1074/jbc.M202421200. [DOI] [PubMed] [Google Scholar]
- 23.Qin H, Pan F, Li J, Zhang X, Liang H, Ruan Z. Whole brain radiotherapy plus concurrent chemotherapy in non-small cell lung cancer patients with brain metastases: a meta-analysis. PLoS One. 2014;9:e111475. doi: 10.1371/journal.pone.0111475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay J. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Chen C, Wang Y, Liu J, Lin R. Effect of black tea consumption on blood cholesterol: a meta-analysis of 15 randomized controlled trials. PLoS One. 2014;9:e107711. doi: 10.1371/journal.pone.0107711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B, Lv ZF, Wang YH, Wang H, Liu XQ, Xie Y, Zhou XJ. Standard triple therapy for Helicobacter pylori infection in China: A meta-analysis. World J Gastroenterol. 2014;20:14973–14985. doi: 10.3748/wjg.v20.i40.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fizazi KS, Higano CS, Nelson JB, Gleave M, Miller K, Morris T, Nathan FE, McIntosh S, Pemberton K, Moul JW. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2013;31:1740–1747. doi: 10.1200/JCO.2012.46.4149. [DOI] [PubMed] [Google Scholar]
- 28.James ND, Caty A, Borre M, Zonnenberg BA, Beuzeboc P, Morris T, Phung D, Dawson NA. Safety and efficacy of the specific endothelin-A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: a double-blind, placebo-controlled, randomised, phase 2 trial. Eur Urol. 2009;55:1112–1123. doi: 10.1016/j.eururo.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Miller K, Moul JW, Gleave M, Fizazi K, Nelson JB, Morris T, Nathan FE, McIntosh S, Pemberton K, Higano CS. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:187–192. doi: 10.1038/pcan.2013.2. [DOI] [PubMed] [Google Scholar]
- 30.Nelson JB, Fizazi K, Miller K, Higano C, Moul JW, Akaza H, Morris T, McIntosh S, Pemberton K, Gleave M. Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer. 2012;118:5709–5718. doi: 10.1002/cncr.27674. [DOI] [PubMed] [Google Scholar]
- 31.Quinn DI, Tangen CM, Hussain M, Lara PN Jr, Goldkorn A, Moinpour CM, Garzotto MG, Mack PC, Carducci MA, Monk JP, Twardowski PW, Van Veldhuizen PJ, Agarwal N, Higano CS, Vogelzang NJ, Thompson IM Jr. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol. 2013;14:893–900. doi: 10.1016/S1470-2045(13)70294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson JB, Love W, Chin JL, Saad F, Schulman CC, Sleep DJ, Qian J, Steinberg J, Carducci M. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478–2487. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carducci MA, Padley RJ, Breul J, Vogelzang NJ, Zonnenberg BA, Daliani DD, Schulman CC, Nabulsi AA, Humerickhouse RA, Weinberg MA, Schmitt JL, Nelson JB. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J. Clin. Oncol. 2003;21:679–689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 34.Bagnato A, Loizidou M, Pflug BR, Curwen J, Growcott J. Role of the endothelin axis and its antagonists in the treatment of cancer. Br J Pharmacol. 2011;163:220–233. doi: 10.1111/j.1476-5381.2011.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robbins AS, Koppie TM, Gomez SL, Parikh-Patel A, Mills PK. Differences in prognostic factors and survival among white and Asian men with prostate cancer, California, 1995-2004. Cancer. 2007;110:1255–1263. doi: 10.1002/cncr.22872. [DOI] [PubMed] [Google Scholar]
- 36.Fujimoto H, Nakanishi H, Miki T, Kubota Y, Takahashi S, Suzuki K, Kanayama HO, Mikami K, Homma Y. Oncological outcomes of the prostate cancer patients registered in 2004: report from the Cancer Registration Committee of the JUA. Int J Urol. 2011;18:876–881. doi: 10.1111/j.1442-2042.2011.02895.x. [DOI] [PubMed] [Google Scholar]
- 37.Shao N, Wang S, Yao C, Xu X, Zhang Y, Zhang Y, Lin Y. Sequential versus concurrent anthracyclines and taxanes as adjuvant chemotherapy of early breast cancer: a meta-analysis of phase III randomized control trials. Breast (Edinburgh, Scotland) 2012;21:389–393. doi: 10.1016/j.breast.2012.03.011. [DOI] [PubMed] [Google Scholar]


