Abstract
The pathophysiological effects of spinal cord injury (SCI) occur as a result of oxidative stress and inflammatory mechanisms. In the present study we analyzed the protective role of ginseng on spinal injury in wistar rats. To evaluate the redox status, we investigated various parameters including estimation of reactive oxygen species, lipid peroxidation content, protein carbonyl and sulphydryl content, myeloperoxidase activity, antioxidant status (superoxide dismutase, catalase, glutathione peroxidase, glutathione-s-transferase). Expression of antioxidant transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) was determined through immunoblot. Inflammatory study was performed by evaluating the expression of nuclear factor-κB, cycloxygenase-2 by western blot analysis. Further the pro-inflammatory cytokines were determined through ELISA (IL-6, TNF-α, IL-1β). We observed a significant enhancement in oxidative stress and inflammatory markers in rats with SCI injury. Ginseng treatment significantly down regulated the oxidative stress by enhancing the antioxidant status in SCI rats. Significant inhibition of inflammation was observed through down regulation of inflammatory proteins and pro-inflammatory cytokines. Thus our findings show that Ginseng significantly ameliorated spinal cord injury in wistar rats by modulating oxidative stress and inflammation.
Keywords: Ginseng, oxidative stress, inflammation, spinal cord injury, antioxidant, cytokines, antioxidant
Introduction
Spinal cord injury induced effects cause permanent damage to the neurons which usually occurs through series of biochemical events. These biochemical events are complex which is initiated through primary mechanical injury followed by secondary effects [1,2]. Secondary injury predominantly involves redox imbalance which leads to outburst of reactive species thus activating redox sensitive molecules [3]. Thus the entire process exacerbates the pathology of spinal cord injury (SCI). One of the early events which occur during spinal cord injury is depolarization of voltage dependent ion channels, leading to cellular ionic imbalance. These ionic disturbances cause accumulation of intracellular calcium, which activates signaling events such as 1. phospholipase A2 pathway resulting in production of various pro-inflammatory genes, leukotrienes and prostaglandins. 2. Increased nitric oxide production through activation of iNOS. 3. Mitochondrial dysfunction and enhanced oxidative stress. 4. Activation of calcium-mediated cysteine proteases [4]. Thus, ultimate end result of these consequences results in generation of reactive oxygen species and activation of various interrelated pathways. Thus targeting redox homeostasis through antioxidant molecules could offer potential in preventing spinal cord injury and its associated effects
Ginseng is a dietary agent with numerous health benefits. Major active components of ginseng include ginsenosides, polysaccharides, peptides, polyacetylenes, alkaloids, nitrogen-containing compounds, fatty acids and phenolic compounds [5-7]. Ginseng offers excellent antioxidant effect [8]; which in turn associated with anti-inflammatory [9], anti-cancer [10], anti-aging [11] and anti-neoplastic [12] activities. In addition, ginseng extract has been reported to involve in protection against oxidative stress conditions [13-15]. The role of ginseng in neuroprotection has not been well explored, thus the present study was designed to analyze the neuroprotective effect of ginseng against spinal cord injury in wistar rats.
Methods
Animals and treatment schedule
Male wistar rats weighing 150-200 g were housed in controlled conditions of temperature (22 ± 1°C) and relative humidity (70-72%). The animals were allowed for alternative light (12 h) and dark (12 h) cycle. Animals were fed with ad libitum water and standard diet throughout the study period. The animals were acclimatized for 10 days and used for the experiment. The study was approved by institutional animal care and use committee in Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine, China. Thirty rats were divided randomly into 3 groups - Group (I) Sham (n=6) - Laminectomy. Group (II) - Laminectomy followed by spinal cord injury (SCI). Group (III) - Laminectomy followed by SCI on day 1, followed by intraperitoneal injection of Ginseng (100 mg/kg) from day 1 (after 2 hrs) which was followed to 7 consecutive days. SCI induction: The spinal cord injury model was followed as described by Akakin et al., [16]. In detail; the rats were anesthetized with ketamine and chlorpromazine; 100 mg/kg and 1 mg/kg, ip, respectively). Followed by which the rats were positioned on a thermostat controlled heating pad in a prone position and a rectal probe was inserted. Under sterile conditions, following T5-12 midline skin incision and para-vertebral muscle dissection, spinous processes and laminar arcs of T7-10 were removed. The dura was left intact. A modified weight-drop model was performed for SCI [17]. The animals were subjected to an impact of 100 g/cm (10 g weight from 10 cm height) to the dorsal surface of the spinal cord. The force was applied via a stainless steel rod (3 mm diameter tip) rounded at the surface and dropped vertically through a 10 cm guide tube positioned perpendicular to the center of the spinal cord. The incision was sutured and the rats were placed in a warming chamber (maintained at 37°C) until they were completely awake. After the treatment period spinal tissue was removed and washed in PBS and homogenized using ice-cold Tris-HCl buffer (50 mM, pH 7.4). The homogenate was centrifuged at 3,000 rpm for 15 min at 4°C. The supernatant was aliquot and stored at -20°C and used for oxidative stress parameters. Protein estimation was performed as described previously by Lowry et al., [18].
Malondialdehyde (MDA) levels
The tissue levels of MDA were determined by estimating the amount of TBA reactants (Lipid peroxidation products) as described previously by Devasagayam et al., [19]. The tissue samples were boiled for 1 hour in the presence of TBA reagent and the formed pink color chromogen was measured spectrophotometrically at 532 nm. The results were expressed as TBARS formed/mg of protein.
Reactive oxygen species
The tissue homogenate was incubated with 2’,7’-dichlorofluorescein diacetate DCF-DA at 37°C for 15 mins. After 15 minutes, the reaction mixture was centrifuged for 15 minutes at 10,000 rpm. The supernatant was discarded and the resultant pellet was re-suspended in phosphate buffered saline and incubated for 60 min at 37°C. Followed by which the ROS levels were measured spectrofluorometrically at excitation (485 nm) and emission (528 nm) wavelength. The results were expressed as percentage of ROS generation by comparing the levels to that of control (100%) Hashimoto et al., [20].
Protein carbonyls and sulphydryl content
Protein carbonyls: The Protein carbonyls formed were measured as described by Dalle-Donne et al., [21]. Briefly, the reaction between carbonyl groups in the sample and 2,4-dinitrophenylhydrazine (DNPH) results in the formation of 2,4-dinitrophenylhydrazone which is quantified spectrophotometrically at 365 nm. The results were expressed as nmoles of protein carbonyls formed per milligram of protein. Protein sulphydryls: The tissue Protein sulphydryls were determined using Ellman’s reagent (5,5-dithiobis-2-nitrobenzoic acid) as described previously by Ellmann [22]. The results were expressed as nmoles protein sulphydryls per mg protein.
Glutathione (GSH) content
The GSH content was determined as described by Beutler, (1975) [23], with some modification. The tissue sample was added with 0.3M of Na2HPO4·2H2O and 0.2 ml of dithiobisnitrobenzoate (0.4 mg/ml in 1% sodium citrate). After incubation, the absorbance was measured at 412 nm. The results were expressed as nmoles of GSH/mg of protein.
Antioxidant enzyme activities
SOD-Superoxide dismutase activity: The SOD activity was determined as described by Sun et al., [24]. The assay is based on reduction of nitrobluetetrazolium (NBT). 1 U of SOD activity = amount required for 50% inhibition of NBT reduction. The SOD activity is expressed as U/mg of protein. Catalase (CAT) activity: The activity was determined according to the method described by Aebi, [25]. The reaction mixture contained tissue homogenate and 30 mM H2O2 in a 50 mM phosphate buffer pH 7.0. The activity was estimated by decreased in absorbance of H2O2 at 240 nm. Glutathione-S-Transferase (GST) activity: The reaction between 1-chloro-2, 4-dinitro benzene (CDNB) and reduced glutathione results in formation of dinitrophenylthioether which is measured at 340 nm (Habig et al., 1974) [26]. 1 U = Amount of enzyme producing 1 mmol of CDNB-GSH conjugate/min. Glutathione Peroxidase (GPx) activity: The GPx was performed as described by Pagia and Valentine, [27]. The oxidized glutathione (GSSG) is reduced by glutathione reductase and NADPH. The oxidation of NADPH to NADP+ is measured by a decrease in absorbance at 340 nm. GPx activity is expressed as U /mg of protein. Myeloperoxidase Activity: Tissue Myeloperoxidase activity was estimated as described previously by Hillegas et al. [28]. The spinal tissues were homogenized in potassium phosphate buffer and centrifuged at 1500 rpm for 15 minutes. The supernatant was discarded and the pellet was resuspended in 0.5% hexadecyltrimethylammonium bromide (HETAB) in phosphate buffer. Followed by 3 freeze thaw cycles, the suspension was further centrifuged at 15,000 rpm for 10 minutes. To an aliquot of the sample, added the reaction mixture containing 50 mM PB, o-dianisidine, and 20 mM H2O2 solution. The absorbance was measured at 460 nm for 3 minutes at regular interval of 1 min. The enzyme activity was expressed as Units/mg of protein.
Inflammatory cytokines by ELISA: IL-6, TNF-α and IL-1β levels
The serum interleukins levels of TNF-α and IL-1β levels were detected using TNF-α and IL-1β ELISA kit (Sigma). The levels of interleukins were expressed as pg/ml. The absorbance was measured using an ELISA reader (MTP-800 Microplate reader; Corona Electric, Tokyo, Japan).
Immunoblot
Whole cell lysate was used for COX-2 expression while nuclear extract was used for NF-κB p65 and Nrf-2 expression. The sample aliquots containing 50 μg of protein were separated on 8~12% SDS-polyacrylamide gels and transferred onto polyvinylidene fluoride (PVDF) membranes using glycine transfer buffer (192 mM glycine, 25 mM Tris-HCl (pH 8.8), 20% MeOH (v/v)). After proteins are transferred to membranes, the nonspecific sites were blocked with 5% nonfat dried milk for 1 hour at RT. The membrane was washed with TBST thrice and incubated with specific primary mouse monoclonal antibodies against Nrf-2, COX-2, NF-κB p65 (1:1000) at 4°C overnight. Each membrane was further incubated for 30 min with secondary peroxidase-conjugated goat anti-mouse or -rabbit IgG (1:5000). The bands were visualized with an enhanced chemiluminescence (ECL) system according to the manufacturer’s instructions. Densitometric analyses of the western blot bands were performed using optical density scanning and Image J software (GE Healthcare Life Sciences).
Statistical analysis
Data are expressed as mean ± standard deviation. The data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. All biochemical experiments were performed thrice in triplicates to ensure reproducibility.
Results
Ginseng inhibits oxidative stress through reducing reactive oxygen species and lipid peroxide levels
Compared to control rats, spinal cord injury rats showed a significant enhancement ROS (P<0.001) and lipid peroxide content (P<0.001). SC Injury followed by ginseng treatment significantly reduced (P<0.001) these levels when compared to SCI rats (Figure 1).
Figure 1.
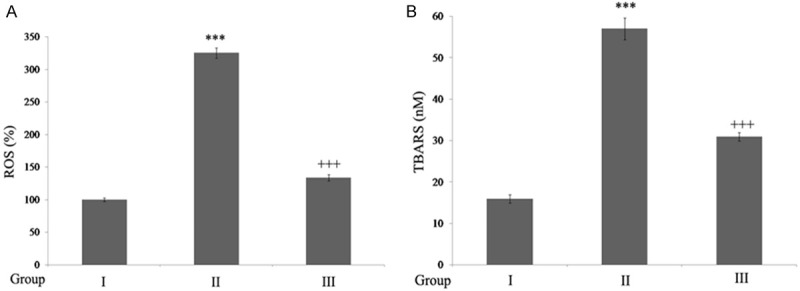
Ginseng reduces oxidative stress in SCI-rats. A. Ginseng inhibits ROS generation: The results are expressed as ROS generated (%) when compared to sham rats. **P<0.001, when compared to sham group. ++P<0.001, when compared to SCI-rats. B. Ginseng reduces lipid peroxide: The results are expressed as nanomoles of TBARS formed/mg of protein. ***P<0.001, when compared to sham group. +++P<0.001, when compared to SCI-rats. Group I (sham); Group II (SCI-injury rats); Group III (Ginseng + SCI-injury rats); Results are given as the mean ± SEM for 6 rats in each group. (One way ANOVA followed by Tukey’s multiple comparison).
Ginseng inhibits protein damage through reducing protein carbonylation and sulphydryl levels
The results show a significant increase (P<0.001) in the protein carbonyl and sulphydryl content in rats with SC injury when compared to the control rats. Rats treated with ginseng showed significant decline in carbonyls and sulphydryls when compared to SCI rats (Figure 2).
Figure 2.
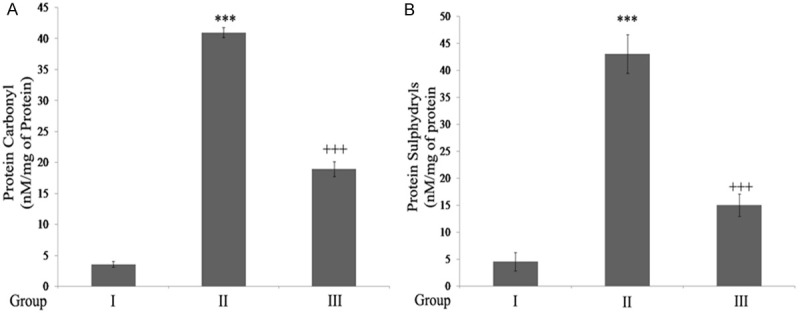
Ginseng inhibits protein carbonyls and sulphydryls in SCI rats. A. Protein Carbonylation. B. Protein Sulphydryls: ***P<0.001, when compared to sham group. +++P<0.001, when compared to SCI-rats. Group I (sham); Group II (SCI-injury rats); Group III (Ginseng + SCI-injury rats); Results are given as the mean ± SEM for 6 rats in each group. (One way ANOVA followed by Tukey’s multiple comparison).
Ginseng inhibits myeloperoxidase activity
Figure 3 shows the effect Ginseng on Myeloperoxidase activity. The results show that myeloperoxidase activity was increased significantly (P<0.001) in rats with SCI. Ginseng treatment significantly reduced (P<0.001) the activity compared to SCI-rats.
Figure 3.
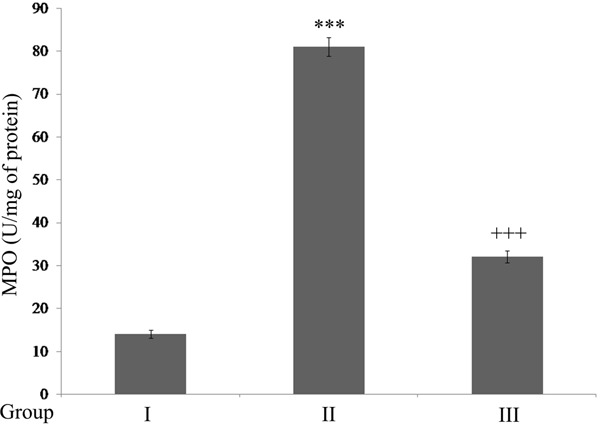
Ginseng inhibits Myeloperoxidase activity in SCI rats. Results are expressed as U/mg of protein. ***P<0.001, when compared to sham group. +++P<0.001, when compared to SCI-rats. Group I (sham); Group II (SCI-injury rats); Group III (Ginseng + SCI-injury rats); Results are given as the mean ± SEM for 6 rats in each group. (One way ANOVA followed by Tukey’s multiple comparison).
Ginseng modulates antioxidant status through Nrf-2 up regulation
(Table 1) Rats with SC injury showed significant decline in antioxidant activities compared to control rats. However, ginseng treatment increased the antioxidant status when compared to SCI rats. Figure 4A shows rats with SC injury showed a significant down regulation in Nrf-2 levels compared t control rats. However, ginseng treatment showed significant up regulation in Nrf-2 expression post SC injury.
Table 1.
Ginseng enhances antioxidant status in wistar rats
| Parameters | Group I | Group II | Group III |
|---|---|---|---|
| GSH | 87 ± 1.01 | 51 ± 1.23*** | 69 ± 2.00+++ |
| SOD | 34 ± 0.91 | 12 ± 1.93*** | 27 ± 1.61+++ |
| CAT | 1.94 ± 0.02 | 1.07 ± 0.01*** | 1.56 ± 0.01+++ |
| GPX | 4.37 ± 0.1 | 3.02 ± 0.01*** | 3.62 ± 0.01+++ |
| GST | 39 ± 1.14 | 12 ± 1.20*** | 28 ± 1.52+++ |
Group I (sham); Group II (SCI-injury rats); Group III (Ginseng + SCI-injury rats); Results are given as the mean ± SEM for 6 rats in each group.
indicate P<0.001 in comparison to sham group and SCI-rats respectively.
indicate P<0.001 in comparison to sham group and SCI-rats respectively.
GSH-expressed as nmol of GSH/mg of protein. SOD, CAT, GST and GPx expressed in Units/mg protein. One way ANOVA followed by Tukey’s multiple comparison.
Figure 4.
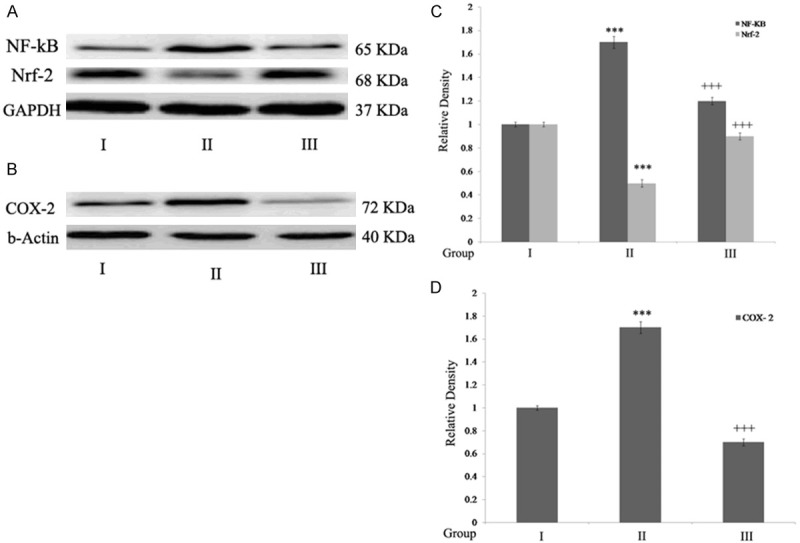
Ginseng up regulates Nrf-2 expression and exerts anti- inflammatory effect. A. NF-κB, Nrf-2 expression; B. COX- 2 expression; C, D. Densitometry analysis. Statistical analyses were carried out by student t-test. Results were expressed as mean ± SEM. ***P<0.001, when compared to sham group. +++P<0.001, when compared to SCI-rats. Group I (sham); Group II (SCI-injury rats); Group III (Ginseng + SCI-injury rats); Results are given as the mean ± SEM for 6 rats in each group. (One way ANOVA followed by Tukey’s multiple comparison).
Ginseng exerts anti-inflammatory effect during SC injury
SC injury showed significant up regulation (P<0.001) of NF-κB and COX-2 expression and pro-inflammatory cytokines (P<0.001) when compared to that of control rats. Treatment with Ginseng showed anti-inflammatory effect by down regulating NF-κB, COX-2, IL-6, TNF-α and IL-1β levels (P<0.001) compared to SCI rats (Figures 4A, 4B and 5).
Figure 5.
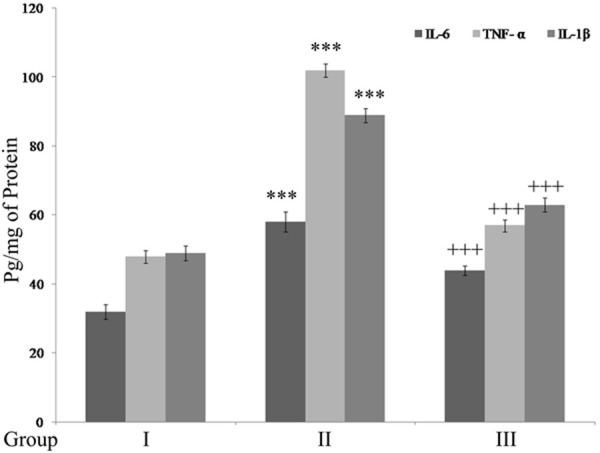
Ginseng down-regulates pro-inflammatory cytokines. Results are expressed as pg/mg of protein. ***P<0.001, when compared to sham group. +++P<0.001, when compared to SCI-rats. Group I (sham); Group II (SCI-injury rats); Group III (Ginseng + SCI-injury rats); Results are given as the mean ± SEM for 6 rats in each group. (One way ANOVA followed by Tukey’s multiple comparison).
Discussion
It is well noted that oxidative stress and associated inflammatory mechanisms play a major role in SC injury and its post effects. The present study highlights some of the novel insights on SCI-induced injury and the role of Ginseng in ameliorating oxidative stress and inflammation. We could observe a significant increase in various oxidative stress markers during SC injury such as enhanced ROS levels, lipid peroxide content and protein damage in the form of carbonylation and sulfhydryl levels. The results obtained from the present study on generation of reactive oxygen species and its associated oxidative damage is consistent with earlier reports [29]. Further, the SCI rats showed a redox imbalance by down regulation of Nrf-2 and subsequent depletion of enzymatic and non-enzymatic antioxidants. Normally, cells are equipped with antioxidants to combat cellular stress and offer cytoprotection. However, when oxidative stress exceeds to that of the cellular antioxidant defense, then redox status rises. Oxidative stress is defined as the imbalance between oxidants and antioxidants in favor of the oxidants, ultimately leading to cellular damage [30]. In addition to this we examined the myeloperoxidase activity, marker of oxidative stress and inflammation and the enzyme functions by formation of hypochlorite [31]. It has been suggested that hypochlorite oxidation directly modifies the functional residues of proteins and results in oxidation of thiol groups leading to protein carbonyl formation [32]. Increase in MPO activity in kidney, lungs and urinary bladder during SCI-Injury induced cellular damage has been documented earlier [33,34]. The protective role of ginseng against oxidative stress and cellular pathogenesis in SCI injury might be attributed to its antioxidant potential by up regulating Nrf-2 levels and antioxidant mechanisms. Similar antioxidant protection has been reported earlier [35].
Oxidative stress-induced inflammatory response is one of the major events in secondary injury; which ultimately leads to apoptosis with eventual loss of neurological function [36]. The present study shows that SCI caused significant up regulation of inflammatory markers and pro-inflammatory cytokines in wistar rats. Impact of inflammatory cytokines as potent mediators of inflammation in SCI injury has been reported earlier; confirming important role of cytokines in SCI [37]. In the present study we observed that ginseng showed a significant anti-inflammatory effect by reducing NF-κB, COX-2 and pro-inflammatory cytokines in SCI rats. Protective role of ginseng in exerting anti-inflammation is consistent with earlier reports [38-40].
In the present study, we could observe that SC injury showed high levels of oxidative stress with increase in inflammatory mediators. However, ginseng treatment significantly down regulated the levels of pro-inflammatory mediators by enhancing antioxidant status and exerting anti-inflammatory mechanisms.
Disclosure of conflict of interest
None.
References
- 1.Piao MS, Lee JK, Jang JW, Kim SH, Kim HS. A mouse model of photochemically induced spinal cord injury. J Korean Neurosurg Soc. 2009;46:479–483. doi: 10.3340/jkns.2009.46.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu SH, Cho DC, Kim KT, Nam KH, Cho HJ, Sung JK. The neuroprotective effect of treatment of valproic Acid in acute spinal cord injury. J Korean Neurosurg Soc. 2012;51:191–198. doi: 10.3340/jkns.2012.51.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury part I pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254–264. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hall ED, Springer JE. Neuroprotection and acute spinal cord injury a reappraisal. Neuro Rx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 6.Choi KT. Botanical characteristics pharmacological effects and medicinal components of Korean panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee SY, Kim YK, Park N, Kim CS, Lee CY, Park SU. Chemical constituents and biological activities of the berry of Panax ginseng. J Med Plants Res. 2010;5:349–353. [Google Scholar]
- 8.Kang KS, Kim HY, Baek SH, Yoo HH, Park JH, Yokozawa T. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30:724–748. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 9.Park J, Cho JY. Anti-inflammatory effects of ginsenosides from Panax ginseng and their structural analogs. Afr J Biotechnol. 2009;8:3682–3690. [Google Scholar]
- 10.Kang JH, Song KH, Woo JK, Park MH, Rhee MH, Choi C, Oh SH. Ginsenoside Rp1 from Panax ginseng exhibits anti-cancer activity by down-regulation of the IGF-1R/Akt pathway in breast cancer cells. Plant Foods Hum Nutr. 2011;66:298–305. doi: 10.1007/s11130-011-0242-4. [DOI] [PubMed] [Google Scholar]
- 11.Cho S, Won CH, Lee DH, Lee MJ, Lee S, So SH, Lee SK, Koo BS, Kim NM, Chung JH. Red ginseng root extract mixed with Torilus fructus and Corni fructus improves facial wrinkles and increases type I procollagen synthesis in human skin a randomized double-blind placebo-controlled study. J Med Food. 2009;12:1252–1259. doi: 10.1089/jmf.2008.1390. [DOI] [PubMed] [Google Scholar]
- 12.Lee YS, Chung IS, Lee IR, Kim KH, Hong WS, Yun YS. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acidic polysaccharide ginsan isolated from Panax ginseng. Anticancer Res. 1997;17:323–331. [PubMed] [Google Scholar]
- 13.Zhang D, Yasuda T, Yu Y, Zheng P, Kawabata T, Ma Y, Okada S. Ginseng extract scavenges hydroxyl radical and protects unsaturated fatty acids from decomposition caused by iron-mediated lipid peroxidation. Free Radic Biol Med. 1996;20:145–150. doi: 10.1016/0891-5849(95)02020-9. [DOI] [PubMed] [Google Scholar]
- 14.Lim JH, Wen TC, Matsuda S, Tanaka J, Maeda N, Peng H, Aburaya J, Ishihara K, Sakanaka M. Protection of ischemic hippocampal neurons by ginsenoside Rb1 a main ingredient of ginseng root. Neurosci Res. 1997;28:191–200. doi: 10.1016/s0168-0102(97)00041-2. [DOI] [PubMed] [Google Scholar]
- 15.Voces J, Alvarez AI, Vila L, Ferrando A, Cabral de Oliveira C, Prieto JG. Effects of administration of the standardized Panax ginseng extract G115 on hepatic antioxidant function after exhaustive exercise. Comp Biochem Physiol C. 1999;123:175–184. doi: 10.1016/s0742-8413(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 16.Akakin D, Kiran D, Ozkan N, Ersahin M, Ozdemir-Kumral ZN, Yegen B, Sener G. Protective effects of melatonin against spinal cord injury induced oxidative damage in rat kidne A morphological and biochemical study. Acta Histochem. 2013;115:827–834. doi: 10.1016/j.acthis.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column a preliminary report. JAMA. 1911;57:878–880. [Google Scholar]
- 18.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Devasagayam TPA. Lipid peroxidation in rat uterus. Biochimica Biophysica Acta. 1986;876:507–514. doi: 10.1016/0005-2760(86)90038-x. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, Shido O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J Nutr. 2005;135:549–555. doi: 10.1093/jn/135.3.549. [DOI] [PubMed] [Google Scholar]
- 21.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 22.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 23.Beutler E. Reduced glutathione (GSH) In: Beutler E, editor. Red cell metabolism, a manual of biochemical methods. New York (NY): Grune and Straton; 1975. pp. 112–114. [Google Scholar]
- 24.Sun Y, Oberley LW, Ying L. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 25.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York and London: Academic Press; 1974. pp. 673–677. [Google Scholar]
- 26.Habig WH, Pabst MJ, Jakoby W. Glutathione-S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 27.Paglia DE, Valentine WN. Studies on the quantitative andqualitative characterisation of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 28.Hillegas LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24:285–295. doi: 10.1016/0160-5402(90)90013-b. [DOI] [PubMed] [Google Scholar]
- 29.Koc RK, Akedmir H, Karakucuk E, Oktem IS, Menku A. Effect of methylprednisolone tirilazad mesylate and vitamin E on lipid peroxidation after experimental spinal cord injury. Spinal Cord. 1999;37:29–32. doi: 10.1038/sj.sc.3100732. [DOI] [PubMed] [Google Scholar]
- 30.Sies H. Oxidative stress oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 31.Loria V, Dato I, Graziani F, Biasucci LM. Myeloperoxidase A New Biomarker of Inflammation in Ischemic Heart Disease and Acute Coronary Syndromes. Mediators Inflamm. 2008;2008:135625. doi: 10.1155/2008/135625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schraufstatter IU, Browne K, Harris A, Hyslop PA, Jackson JH, Quehenberger O, Cochrane CG. Mechanisms of hypochlorite HOCl injury to target cells. J Clin Invest. 1990;85:554–562. doi: 10.1172/JCI114472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kothari N, Keshari RS, Bogra J, Kohli M, Abbas H, Malik A, Dikshit M, Barthwal MK. Increased myeloperoxidase enzyme activity in plasma is an indicator of inflammatio and onset of sepsis. J Crit Care. 2011;26:435, e1–7. doi: 10.1016/j.jcrc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Mu X, Azbill RD, Springer JE. Riluzole improves measures of oxidative stress following traumatic spinal cord injury. Brain Res. 2000;870:66–72. doi: 10.1016/s0006-8993(00)02402-1. [DOI] [PubMed] [Google Scholar]
- 35.Fu Y, Ji LL. Chronic ginseng consumption attenuates age-associated oxidative stress in rats. J Nutr. 2003;33:3603–3609. doi: 10.1093/jn/133.11.3603. [DOI] [PubMed] [Google Scholar]
- 36.Genovese T, Esposito E, Mazzon E, Di Paola R, Caminiti R, Bramanti P, Cappelani A, Cuzzocrea S. Absence of endogenous interleukin-10 enhances secondary inflammatory process after spinal cord compression injury in mice. J Neurochem. 2009;108:1360–1372. doi: 10.1111/j.1471-4159.2009.05899.x. [DOI] [PubMed] [Google Scholar]
- 37.Gál P, Kravcuková P, Mokrý M, Kluchová D. Chemokines as possible targets in modulation of the secondary damage after acute spinal cord injury a review. Cell Mol Neurobiol. 2009;29:1025–1035. doi: 10.1007/s10571-009-9392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smolinski AT, Pestka JJ. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin chamomile ginsenoside Rb1 ginseng and parthenolide feverfew. Food Chem Toxicol. 2003;41:1381–1390. doi: 10.1016/s0278-6915(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 39.Hwang JW, Oh JH, Yoo HS, Lee YW, Cho CK, Kwon KR, Yoon JH, Park J, Her S, Lee ZW, Jang IS, Choi JS. Mountain ginseng extract exhibits anti-lung cancer activity by inhibiting the nuclear translocation of NF-κB. Am J Chin Med. 2012;40:187–202. doi: 10.1142/S0192415X12500152. [DOI] [PubMed] [Google Scholar]
- 40.Peralta EA, Murphy LL, Minnis J, Louis S, Dunnington GL. American Ginseng inhibits induced COX-2 and NF-κB activation in breast cancer cells. J Surg Res. 2009;157:261–267. doi: 10.1016/j.jss.2009.05.011. [DOI] [PubMed] [Google Scholar]


