Abstract
A green method of Silver nanoparticles (AgNPs) preparation has been established. This method depends on reduction of silver nitrate with soluble starch. The formation of AgNPs was observed by the color change from colorless to dark brown through the starch addition into silver nitrate solution. It was observed that use of starch makes convenient method for the synthesis of silver nanoparticles and can reduce silver ions into the produced silver nanoparticles within one hour of reaction time without using any harsh conditions. The prepared silver nanoparticles were characterized by using UV-visible spectroscopy and evaluated for its antimicrobial activity. The synthesized green AgNPs showed a potential antibacterial activity that was stronger against Gram positive pathogenic bacteria (Staphylococus aureus and Streptococus pyogenes) than against Gram negative pathogenic bacteria (Salmonella typhi, Shigellasonnei and Pseudomonas aeruginosa). Inhibition zones diameter of antibacterial activity depends upon nanoparticles concentration as AgNPs exhibited greater inhibition zone for S.aureus (16.4 mm) followed by P. aeruginosa and S. pyogenes while the least activity was observed for S. typhi (10.4 mm) at 40 μl/ disc. These results suggested that AgNPs can be used as an effective antiseptic agents in medical fields and process of synthesis creates new opportunities in process development for the synthesis of safe and eco-friendly AgNPs.
Keywords: Silver Nanoparticles, soluble starch, UV-Vis, antibacterial activity
Introduction
Nanoscience and nanotechnology has seen major development in the bio-fabrication process of metal nanoparticles (MNPs) [1]. The MNPs are broadly applied in the science and technology such as medicine, biology, biotechnology, chemistry, physics, and catalysis, electronics and material sciences. In addition, Silver nanoparticles (AgNPs) are considered as an effective antiseptic and antimicrobial agents for treating wounds and may have a potential commercial application as medical tools and health care products [2,3]. AgNPs have drawn intensive interest in recent years due to the low-cost, high-efficiency, unique optical and electronic properties which lead to potential applications in industrial fields such as catalyst [4], antimicrobial agents [5], conductive coating and sensors [6]. Recently, a large number of physical, chemical, biological, and hybrid methods are available to synthesize different types of nanoparticles [7]. Though popular physical and chemical methods for nanoparticle synthesis, the use of toxic compounds limits their applications. To overcome the problem of toxicity in synthesis, safe eco-friendly green methods have a major role for producing nanoparticles [8]. Eco-friendly approach for the synthesis of nanoparticles has several advantages such as simplicity, cost effectiveness, compatibility for biomedical and pharmaceutical application as well as for large scale commercial production. Nowadays, many environmentally friendly or green methods have been investigated, using non-toxic chemicals instead to synthesize silver nanoparticles [9].
In a “green” synthetic strategy, it is important to use nontoxic chemicals, environmentally benign solvents, and renewable materials. In our research, starch was selected as the protecting agent because it is renewable and it can form dispersion in H2O. Several authors [10] have reported this “green” synthetic strategy for the production of uniform and monodisperse nanoparticles using starch as capping agent. Starch is one of the most widely-used materials due to its naturally occurring and environmentally friendly polymer. As far as the authors know, no literature has been published about synthesizing the AgNPs nanoparticles with starch as capping agent and reducing agent in the same time. Hence, the present study was designed to prepare AgNPs using bio-green method with starch as capping and reducing agent of Ag+ to Ag to synthesize the silver nanoparticles. The formed AgNPs were characterized using ultraviolet-visible spectroscopy. Understanding of the particle stabilization mechanism in situ are focused in this study.
Material and methods
Materials
All chemicals used in the present study are of high purity and are obtained from Sigma and Merck. All glassware’s were washed with HNO3 and distilled water and dried in oven.
Bio-green synthesis of silver nanoparticles
In a typical one-step synthesis protocol, 1.0 g of soluble starch was added to 100 mL of deionized water and gently heated with string on hotplate. The synthesis of nanoparticles by bio-green method was carried by adding 10 ml of soluble starch to 50 ml of 1 mM silver nitrate solution and kept for 3 h on hotplate with stirring. The overall reaction process was carried out in dark to avoid unnecessary photochemical reactions. The color change of the silver nitrate solution from colorless to brownish yellow was observed by naked eye (Figure 1) and the bio reduced sample component was confirmed by UV-Visible spectroscopy. The obtained AgNPs were purified through repeated centrifugation at 11,500 rpm for 20 min and washed with distilled water. AgNPs were collected and redispersed in deionized water for characterization.
Figure 1.

Biosynthesis of AgNPs using starch visible observations.
UV-visible spectroscopy
The UV-vis analysis was done by sampling the aqueouscomponent at different time intervals and the absorption maximawas scanned over the 200-600 nm wavelength range using UV-1700 Shimadzu UV-Visible spectrophotometer. The deionized water was used as the blank.
Antibacterial activity of silver nanoparticle
Bacterial strains
The antibacterial activity of Ag nanoparticle was evaluated using Gram positive (Staphylococus aureus and Streptococus pyogenes) and three strains of Gram negative (Salmonella typhi, Shigellasonnei and Pseudomonas aeruginosa) bacteria. The microorganisms were provided from the culture collection of Botany and Microbiology Dept. King Saud University, Riyadh, K.S.A. The identity of the bacterial strains was confirmed morphologically and through using of biochemical techniques.
Inoculums preparation
Bacterial inoculums were prepared by subculturing microorganisms into nutrient agar slants at 37°C for 18 hrs, the growth was harvested using 5 ml of sterile saline water, diluted and their absorbance were adjusted at 580 um to 25% using spectrophotometer. The viable cell count at this absorbance was approximately 107 CFU/ml of each bacterial strain.
Antibacterial assay
Agar disk diffusion method is used to evaluate antimicrobial activity of the sensitized Ag nanoparticle. Ten ml of nutrient agar medium was poured into sterile petridishes (as a basal layer) followed with 15 ml of seeded medium previously inoculated with bacterial suspension (100 ml of medium/1 ml of 107 CFU) to attain 105 CFU/ml of medium. Sterile filter paper discs (6 mm in diameter) were loaded with 10, 20 and 40 µl of the nanoparticle solution and placed on the top of the nutrient agar plates. Filter paper discs loaded with 10 µg of Gentamycin was used as positive control. The plates were incubated at 37°C for 24 hrs and the presence of inhibition zones were recorded, measured by Vernier caliper and considered as indication for antibacterial activity.
Results and discussion
Silver nanoparticle preparation and characterization
The characterization and measurements of the AgNPs produced by the green reduction method using starch are represented in this study. The effects of the reaction time, mechanism of the formation of the AgNPs and the influence of the essential functional groups involved in reducing the silver ions to nanoparticles, have been considered, and are discussed in the following sections.
Formation of AgNPs from 1 mM solution of silver nitrate and starch filtrate can be easily visualized by the color change of the mixture which is turned from green to yellowish brown color as showed in Figure 1. It is well known that AgNPs shows a yellowish brown color in aqueous solution; this color arises from excitation of surface plasmon vibrations in the metal nanoparticles [11], it is due to collective oscillation of free electrons present in the reducedAgNPs [12]. Suchvisual observations on a change in biomass color due to the synthesis of gold nanoparticles have been reported earlier [13].
UV-Vis spectroscopy is usually the first technique which is used in characterization of metallic nanoparticles because of surface Plasmon resonance (SPR) phenomenon [14]. Figure 2 shows the UV-vis absorption spectra of the Ag nanoparticles at different time intervals (10, 20, 30, 60, and 90 min). Typical AgNPs having λmax values which are in the visible range of 400-500 nm [15]. The absorption peak of obtained AgNPs is centered around 410-430 nm. This observation clearly indicates the successful reduction of Ag using soluble starch. Surface Plasmon peak observed confirms the influence of starch in reducing Ag+ ions to Ag NPs from aqueous AgNO3 solution. The formation of AgNPs was found to increase with reaction time. Wide beak at short reaction time (10 min) due to the presence a wide distribution particle size. Slight modification in the size and shape of nanoparticles gave slight shift in the peak to shorter wavelength with increasing time [16]. From the figure it is observed that the absorption peak becomes sharp as the time increases from 10-90 min. This indicates the formation of the poly dispersed AgNPs initially that become mono-dispersed spherical nanoparticles with increasing reaction time. Therefore, mixing time at room temperature is an important factor in determining the size distribution of AgNPs.
Figure 2.
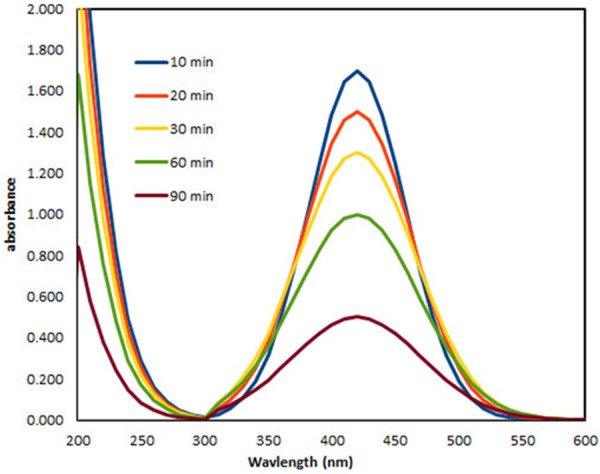
UV-vis spectra of AgNPs synthesized by starch at different time intervals.
Mechanism of the formation of silver nanoparticle
Starch (C6H10O5) n, which is a common type of carbohydrate is a polymer of glucose and is found in the cereal grains (wheat, rice, corn, oats, barley) as well as in tubers such as potatoes. Water soluble, amylose and water insoluble, amylopectin are the main constituents of the starch. Under our experimental conditions, amylase is present in the aqueous solution of soluble starch. The helical structure of the amylose chain (soluble starch) is depicted in Figure 3. Hot water changes amylase slowly into smaller molecules. This reaction, an example of hydrolysis, is catalyzed by acids giving still simpler molecules, like glucose, C6H12O6. Glucose is established as a very good reducing agent for silver nitrate to silver metal.
Figure 3.
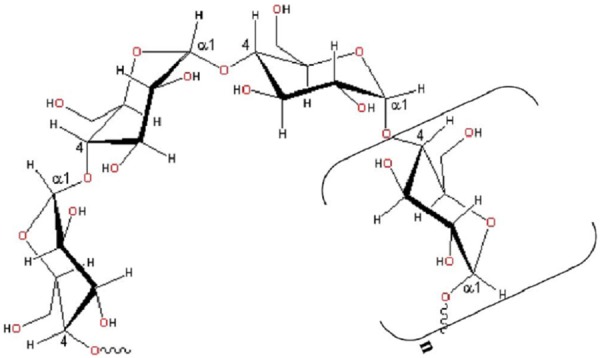
Illustration of the helical structure of amylose chain formed by a-(1-4) linkages between D-glucose units. Square brackets indicate the monomer.
The concept of green nanoparticles preparation using β-D-glucose as the reducing agent was first reported by Raveendranet al. [17] where starch played the role of stabilizer. In the present work, silver nanoparticles are prepared using soluble starch acting as both the reducing and stabilizing agents. Soluble starch, the amylose component of starch, is a linear polymer formed by the α-(1, 4) linkages between D-glucoseunits and adopts a left-handed helical conformation in aqueous solution. Despite its slight branching, amylose behaves essentially like a linear polymer, forming films and complexes with ligands [18]. In this report ,the aldehyde terminal of soluble starch is used to reduce silver nitrate while the starch itself stabilized he silver nanoparticles.
Antibacterial assay
The antibacterial activity of Ag nanoparticle was screened because of their great medicinal relevance. There are a numerous techniques to evaluate antibacterial susceptibility test of silver nanoparticles. A gar disc diffusion method was used in this project because its reliability, low cost and simplicity. Evaluation of antibacterial activity of Ag nanoparticle was recorded in Table 1 and illustrated in Figures 4 and 5. The result revealed that AgNPs exhibited a potential antibacterial activity against all the tested bacterial strains. The antibacterial activity was increased with increasing silver nanoparticles concentration. AgNPs showed a broad spectrum of activity against all pathogenic bacterial strains at the tested concentrations of 10 – 40 µl/ disc except S. typhi and S. sonnie which were insensitive at concentration of 10 µl/disc. The Ag nanoparticle exhibited greater inhibition zone for S. aureus (16.4 mm) followed by P. aeruginosa and S. pyogenes while the least activity was observed for S. typhi (10.4 mm) at 40 µl/disc.
Table 1.
Antibacterial activity of AgNPs against some Gram (+ve) and Gram (-ve) pathogenic bacteria
| Conc. of Ag nanoparticle (µl/disc) | Inhibition zones (mm) | ||||
|---|---|---|---|---|---|
|
| |||||
| Gram (+ve) pathogenic bacteria | Gram (-ve) pathogenic bacteria | ||||
|
| |||||
| S. aureus | S. pyogenes | S. typhi | S. sonnei | P. aeruginosa | |
| 10 | 12.2±0.11 | 11.4±0.15 | 0.00 | 0.00 | 12.3±0.20 |
| 20 | 14.3±0.15 | 13.2±0.25 | 7.2±0.26 | 8.3±0.15 | 13.5±0.10 |
| 40 | 16.4±0.21 | 15.3±0.15 | 10.4±0.10 | 12.4±0.25 | 16.3±0.20 |
| (+ve) Control (10 µg/disc) | 30.3±0.1o | 31.2±0.15 | 27.5±0.20 | 21.3±0.20 | 25.6±0.20 |
Data are means of three replicates (n=3) ± standard error.
Figure 4.
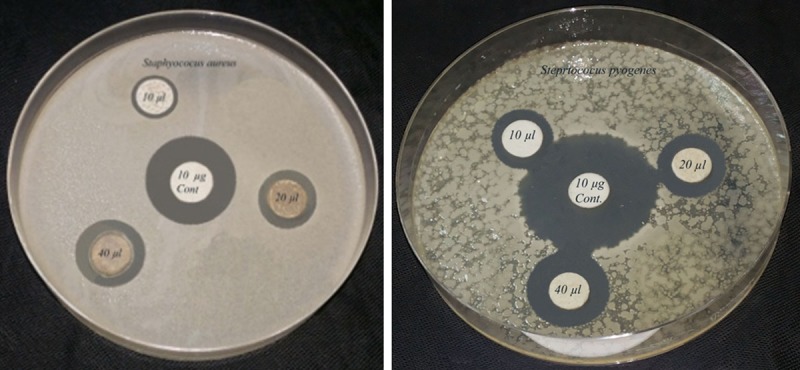
Antibacterial activity of AgNPs against Gram (+ve) pathogenic bacteria.
Figure 5.
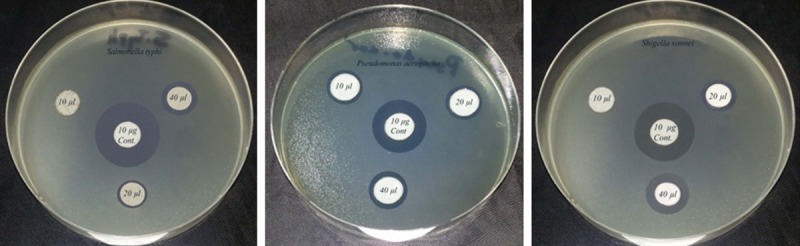
Antibacterial activity of AgNPs against Gram (-ve) pathogenic bacteria.
In our result silver nanoparticle showed a strong antimicrobial activity against. S.aureus, P.aeruginosa and S. pyogenes. In contrast, the inhibitory effect of silver nanoparticle was mild in S. sonnie and week in S. typhi especially at concentration of 20 µl/disc. These results suggested that antimicrobial activity of silver nanoparticle may be associated with characteristics of certain bacterial species. Bacteria have differences in their cell wall structure and their ability of capsule formation, hence they are different in their susceptibility. Some researchers suggested that, antimicrobial activity of silver nanoparticle is associated with the structure of bacterial cell wall and the variation of antibacterial activity may derive from differences in bacterial cell wall structure and thickness of peptidoglycan layer of bacterial cell wall [19]. Other researchers attributed the inhibitory effect of silver nanoparticle to their nano-size that enable them to attached easily to bacterial cell membrane and reach to nuclear content of the bacterial cell disturbing their structure and rendering them more permeable. Leaking of ions and other cell contents with disturbing bacterial enzymatic activity may cause bacterial cell death [20].
Conclusion
Nano size metals have brought a new revolution to the modernera of science; particularly AgNPs have been exploited in various fields of chemistry, physics, biology and medicine. In conclusion we introduce a simple, fast, and economical biological procedure to synthesize Ag nanoparticles using soluble starch. starch was used as a reducing and capping agents. The starch in the solution mixture avoids use of relatively toxic organic solvents. In addition, the binding interactions between starch and silver nanoparticle are weak and can be reversible at higher temperatures, allowing separation of the synthesized particles. The characterization of Ag+ ions exposed to sucrose by UV-vis techniques confirm the reduction of silver ions to silver nanoparticles. The reducing agent involved in the present approach resulted from the hydrolysis of amylose which is main component of soluble starch in the solution to α-glucose. Our method for AgNPs preparation showed a potential antibacterial activity that was stronger against Gram positive pathogenic bacteria (S.aureus and S. pyogenes) than against Gram negative bacteria especially S.typhi. These results suggested that AgNPs can be used as effective growth inhibitors to various pathogenic bacteria making them applicable to medical field and to be used as antiseptic agents.
Acknowledgements
This project was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center.
Disclosure of conflict of interest
None.
References
- 1.Balantrapu K, Goia D. Silver nanoparticles for printable electronics and biological applications. J Mater Res. 2009;24:2828–2836. [Google Scholar]
- 2.Jones SA, Bowler PG, Walker M, Parsons D. Controlling wound bioburden with a novel silver-containing Hydrofiber dressing. Wound Repair Regen. 2004;12:288–294. doi: 10.1111/j.1067-1927.2004.012304.x. [DOI] [PubMed] [Google Scholar]
- 3.Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine. 2007;3:168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Bhatte K, Tambade P, Dhake K, Bhanage B. Silver nanoparticles as an efficient, heterogeneous and recyclable catalyst for synthesis of β enaminones. Catal Comm. 2010;11:1233–1237. [Google Scholar]
- 5.Musarrat J, Dwivedi S, Singh BR, Al-Khedhairy AA, Azam A. Bioresour. Technol. 2011;101:8872–8876. doi: 10.1016/j.biortech.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 6.Kosmala R, Wright R, Zhang Q, Kirby MP. Chem. Phys. 2011;129:1075–1080. [Google Scholar]
- 7.Mahdavi M, Ahmad MB, Haron MJ, Namvar F, Nadi B, Ab Rahman MZ, Amin J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules. 2013;18:7533–7548. doi: 10.3390/molecules18077533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arockiya Aarthi Rajathi F, Parthiban C, Ganesh Kumar V, Anantharaman P. Biosynthesis of antibacterial gold nanoparticles using brown alga, stoechospermum marginatum (kützing) Spectrochim Acta Part A Mol Biomol Spectrosc. 2012;99:166–173. doi: 10.1016/j.saa.2012.08.081. [DOI] [PubMed] [Google Scholar]
- 9.Shameli K, Ahmad MB, Zamanian A, Sangpour P, Shabanzadeh P, Abdollahi Y, Zargar M. Green biosynthesis of silver nanoparticles using Curcuma longa tuber powder. Int J Nanomed. 2012;7:5603–5610. doi: 10.2147/IJN.S36786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oluwafemi OS. A novel “green” synthesis of starch-capped CdSe nanostructures. Colloids Surf B Biointerfaces. 2009;73:382–386. doi: 10.1016/j.colsurfb.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Kanipandian N, Kannan S, Ramesh R, Subramanian P, Thirumurugan R. Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Materials Research Bulletin. 2014;49:494–502. [Google Scholar]
- 12.Noginov MA, Zhu G, Bahoura M, Adegoke JC, Small C, Ritzo BA, Drachev VP, Shalaev VM. The effect of gain and absorbance on surface Plasmon in metal nanoparticles. Appl Phys. 2006;B86:455–460. [Google Scholar]
- 13.Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, Murali S. Colloids Surf. B: Biointerfaces. 2003;28:313–318. [Google Scholar]
- 14.Smitha SL, Philip D, Gopchandran KG. Spectrochim. Acta A. 2009;74:735–739. doi: 10.1016/j.saa.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Sastry M, Mayyaa KS, Bandyopadhyay K. pH dependent changes in the optical properties of carboxylic acid derivatized silver colloid particles. Colloids Surf A. 1997;127:221–228. [Google Scholar]
- 16.Raghunandan Bedre D, Mahesh D, Basavaraja S, Balaji SD, Manjunath SY, Venkataraman J Nanopart Res. 2011;13:2021–2028. [Google Scholar]
- 17.Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125:13940–13941. doi: 10.1021/ja029267j. [DOI] [PubMed] [Google Scholar]
- 18.Walter RH. Polysaccharide Association Structures in Food. New York: Marcel Dekker; 1998. [Google Scholar]
- 19.Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PK, Chiu JF, Che CM. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5:916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- 20.Kora AJ, Arunachalam J. Assessment of antibacterial activity of silver nanoparticles on Pseudomonas aeruginosa and its mechanism of action. World Journal of Microbiology and Biotechnology. 2011;27:1209–1216. [Google Scholar]


