Abstract
There are conflicting reports on the correlation between manganese (Mn) levels and breast cancer. The purpose of the present study is to clarify the association between Mn levels and breast cancer using a meta-analysis approach. We searched articles indexed in Pubmed and the Chinese Journal Full-text Database (CJFD) published as of August 2014 that met our predefined criteria. Eleven eligible studies involving 1302 subjects were identified. Overall, pooled analysis indicated that subjects with breast cancer had lower Mn levels than the healthy controls (SMD = -1.51, 95% CI = [-2.47, -0.56]). Further subgroup analysis found a similar pattern in China (SMD = -1.32, 95% CI = [-2.33, -0.32]) and Korea (SMD = -4.08, 95% CI = [-4.63, -3.54]), but not in Turkey (SMD = -0.96, 95% CI = [-3.19, 1.27]). Further subgroup analysis also found a similar pattern in different sample specimens (serum: SMD = -1.24, 95% CI = [-2.31, -0.16]; hair: SMD = -1.99, 95% CI = [-3.91, -0.06]) and different types of Mn measurement (inductively coupled plasma-atomic absorption spectrometry (ICP-AAS): SMD = -1.14, 95% CI = [-2.24, -0.04]; graphite furnace atomic absorption spectroscopy (GFAAS): SMD = -1.94, 95% CI = [-2.38, -1.49]; inductively coupled plasma-atomic emission spectrometry (ICP-AES): SMD = -3.77, 95% CI = [-4.70, -2.85]). No evidence of publication bias was observed. In conclusion, this meta-analysis supports a significant association between deficient Mn levels and breast cancer. However, the subgroup analysis found that there was contradiction regarding races and geography, like China and Turkey. Thus this finding needs further confirmation by trans-regional multicenter, long-term observation in a cohort design to obtain better understanding of causal relationships between Mn levels and breast cancer, through measuring Mn at baseline to investigate whether the highest Mn category versus lowest was associated with breast cancer risk.
Keywords: Manganese, serum, hair, breast cancer, meta-analysis
Introduction
Breast cancer is the most common type of cancer among women, inflicting 16% of all female cancer patients. While its incidence continues to rise, the mortality rate from breast cancer has remained almost unchanged in the past five decades [1-3]. The high incidence of breast cancer highlights the need for a better understanding of the underlying mechanisms of breast tumorigenesis. Despite great progress in the monitoring and treatment of this disease, the exact etiology of breast cancer is not fully understood.
Breast cancer is a multifactorial disease as the intensive epidemiological, clinical, and genetic studies have identified a number of biological and social traits as risk factors associated with breast cancer [4-7]. Some meta-analyses have found that trace elements disturbances, such as iron or selenium deficiency, have been recognized as playing a role in carcinogenesis in breast tissues [8,9]. Manganese (Mn), as a trace element, is required for the activity of several enzymes [10-12]. Some clinical studies find that there is an inverse association between Mn concentration and breast cancer [13,14]. A lower levels of Mn in patients with breast cancer than in healthy controls indicated that Mn might share the anti-carcinogenic properties. However, some studies suggest that there is no relationship between Mn concentration and breast cancer [15]. Although Mn deficiency is plausibly linked to breast cancer, the inconsistency among the findings of previous studies precludes definitive recommendations at present.
Meta-analysis is an important tool for revealing trends that might not be apparent. Therefore, we performed a comprehensive and critical meta-analysis of the studies, in order to draw a more clear and evidence-based conclusion on the association between Mn levels and breast cancer.
Methods
Search strategy
We searched all articles indexed in Pubmed and the Chinese Journal Full-text Database (CJFD) published up to August 2014. Literature searches were performed using medical subject heading (MeSH) or free text words. The searching keywords were: (manganese OR Mn) AND breast cancer. Reference lists of all eligible studies were screened to identify potentially eligible studies. Emails were sent to the authors of identified studies for additional information if necessary. We accepted studies written in English or Chinese.
Selection criteria
Three authors (Fei Shen, Jie Cao, Bo Xu) conducted the search independently. Titles and abstracts were screened for subject relevance. Studies that could not be definitely excluded based on abstract information were also selected for full text screening. Two authors (Fei Shen, Jie Cao) independently selected eligible studies for inclusion possibility. Where there was a disagreement for study inclusion, a discussion was held (with Bo Xu) to reach a consensus.
Eligible studies should meet the following criteria: (1) human study; (2) case-control study or cohort study or randomized clinical trial; (3) studies focusing on the association between Mn levels and breast cancer; (4) studies providing data of Mn levels for both subjects with breast cancer and healthy controls; (5) subjects with no other diseases and no drugs intake which might influence the levels of Mn.
Exclusion criteria included: (1) animal study; (2) in vitro or laboratory study; (3) review or case report; (4) studies not providing Mn levels for both subjects with breast cancer and healthy controls; (5) subjects with diseases and drugs intake which might influence the levels of Mn; (6) sample size less than 20.
Data extraction and quality assessment
Two authors (Wen-Song Cai, Jiang-Lin Li) independently extracted data using a standard form. The following information was extracted from each included study: first author’s family name, year of publication, type of study, country, demography of subjects (number of patients and age), data on levels of Mn and type of Mn measurement.
The qualities of all included studies were assessed using the Newcastle-Ottawa Scale (NOS). The assessment tool focused on three aspects, including participant selection, comparability and exposure. The studies would be assigned stars of 9 if all items were satisfied. Two authors (Fei Shen, Zhe Feng) assessed the quality independently.
Statistical analysis
The extracted data were used to perform meta-analysis to obtain the standardized mean difference (SMD) and 95% confidence intervals (CI). Heterogeneity between studies was tested through the Chi-square and I-square tests. If the I2 value was greater than 50% and the p value was less than 0.05, the meta-analysis was considered as homogeneous. The SMDs were calculated using either fixed-effects models or, in the presence of heterogeneity, random-effects models.
Subgroup analyses were used to identify associations between Mn levels and other relevant study characteristics as possible sources of heterogeneity. Publication bias was measured using Begg’s test and visualization of funnel plot. The stability of the study was also detected by sensitivity analysis, through re-meta-analysis with one involved study excluded each time. All statistical analyses were performed with Stata version 11.0 (StataCorp, College Station, TX).
Results
Literature search
The literature search yielded a total of 274 primary studies, including 184 studies published in English and 90 in Chinese. These studies were included for full-text assessment, of which 263 were excluded for one of the following reasons: (1) irrelevant to our topic (n = 206), (2) non-original studies (reviews, etc.) (n = 34), (3) non-human studies (n = 21), (4) articles not providing Mn levels for both subjects with breast cancer and healthy controls (n = 2). Overall, 11 eligible studies (6 published in Chinese and 5 in English) with 1302 subjects from 11 case-control studies were considered in the analysis [13-23]. A flow diagram of the study selection process is presented in Figure 1.
Figure 1.
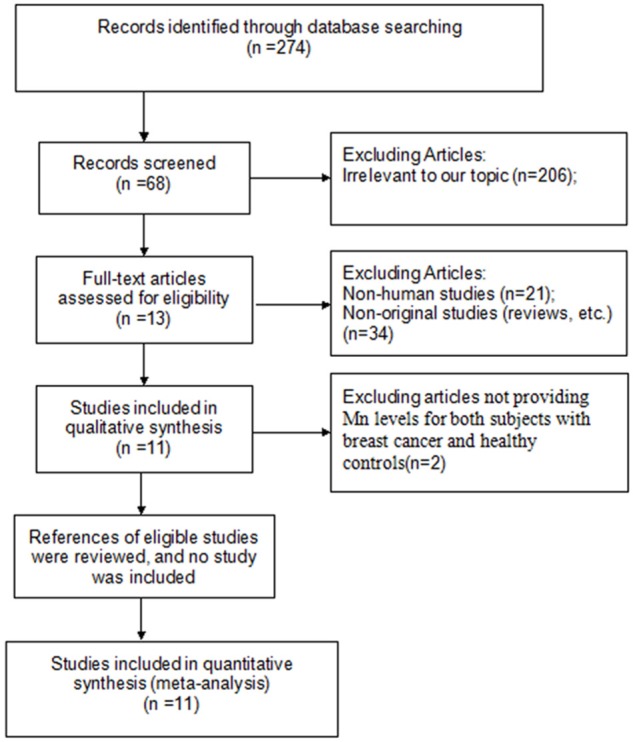
Flow diagram of screened and included papers.
Study characteristics and quality assessment
The detailed characteristics of the included studies and the results of the quality assessment were summarized in Table 1. The number of subjects in each study ranged from 50 to 285. The earliest study was published in 2000, and the latest in 2013. By geographic location, one case-control study was conducted in Korea, two case-control studies were conducted in Turkey, and eight case-control studies in China. In seven studies, Mn status was based on analysis of serum, whereas in the remaining four, hair was the sample specimen used. Eight studies measured Mn status by inductively coupled plasma-atomic absorption spectrometry (ICP-AAS), two studies by graphite furnace atomic absorption spectroscopy (GFAAS), and one studies by inductively coupled plasma-atomic emission spectrometry (ICP-AES). The overall study quality averaged 6.7 stars (range, 5-7) on a scale of 0 to 9.
Table 1.
Characteristics of subjects in eligible studies
| Studies | Country | Age (year) | Type of Mn measurement | Sample specimen | Breast cancer | Healthy controls | Weight (%) | SMD (95% CI) | Quality score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Breast cancer | Healthy control | N | Mn concentration (mean ± SD) | N | Mn concentration (mean ± SD) | |||||||
| Bai 2000 | China | 32~65 | 32~65 | ICP-AAS | Hair | 38 | 1.01 ± 0.62 ng/g | 38 | 6.78 ± 4.19 ng/g | 9.11 | -1.93 (-2.47, -1.38) | 6 |
| Kilic 2004 | Turkey | 53±9 | 55 ± 7 | GFAAS | Hair | 26 | 0.67 ± 0.52 μg/g | 27 | 1.66 ± 0.4 μg/g | 8.97 | -2.12 (-2.80, -1.44) | 7 |
| Dai 2005 | China | 28~58 | 23~68 | ICP-AAS | Serum | 54 | 0.18 ± 0.07 μmol/l | 56 | 1.44 ± 0.84 μmol/l | 9.18 | -2.10 (-2.56, -1.63) | 7 |
| Liang 2005 | China | 32~68 | 22~58 | ICP-AAS | Serum | 28 | 0.08 ± 0.01 μmol/l | 22 | 1.52 ± 0.88 μmol/l | 8.88 | -2.47 (-3.22, -1.73) | 7 |
| Wu 2006 | China | NA | NA | ICP-AES | Serum | 25 | 5.5 ± 0.47 μg/l | 26 | 9.09 ± 1.25 μg/l | 8.63 | -3.77 (-4.70, -2.85) | 5 |
| Li 2006 | China | 20~70 | 20~70 | ICP-AAS | Serum | 78 | 1.71 ± 0.11 μg/dl | 61 | 1.65 ± 0.18 μg/dl | 9.28 | 0.41 (0.08, 0.75) | 7 |
| Li 2008 | China | 49.9 ± 10.7 | 20~70 | ICP-AAS | Serum | 93 | 1.75 ± 0.16 μg/dl | 81 | 1.64 ± 0.17 μg/dl | 9.30 | 0.67 (0.36, 0.97) | 7 |
| Joo 2009 | Korea | 47.1 ± 9.6 | 47.8 ± 5.1 | ICP-AAS | Hair | 40 | 0.21 ± 0.04 μg/g | 144 | 0.44 ± 0.0 μg/g | 9.11 | -4.08 (-4.63, -3.54) | 7 |
| Wang 2010 | China | 25~60 | 26~60 | ICP-AAS | Serum | 25 | 0.24 ± 0.41 μmol/l | 260 | 0.22 ± 0.2 μmol/l | 9.23 | 0.10 (-0.31, 0.51) | 7 |
| Cihan 2011 | Turkey | 50.3 ± 8.8 | 47.4 ± 10.1 | ICP-AAS | Hair | 52 | 0.989 ± 0.924 μg/g | 52 | 0.863 ± 0.675 μg/g | 9.25 | 0.16 (-0.23, 0.54) | 7 |
| Feng 2012 | China | 48.7 ± 8.4 | 47.2 ± 7.6 | GFAAS | Serum | 56 | 8.3 ± 1.57 μg/l | 20 | 10.95 ± 1.14 μg/l | 9.07 | -1.80 (-2.39, -1.21) | 7 |
ICP-AAS, inductively coupled plasma-atomic absorption spectrometry. ICP-AES, inductively coupled plasma-atomic emission spectrometry. GFAAS, graphite furnace atomic absorption spectroscopy.
Mn and breast cancer
The random-effects meta-analysis results indicated that subjects with breast cancer had lower Mn levels in serum and hair than healthy controls (SMD = -1.51, 95% CI = [-2.47, -0.56]) (Figure 2). The 11 sets of results (listed at Table 1) showed a statistically significant amount of heterogeneity (I2 = 97.8%, P < 0.001).
Figure 2.
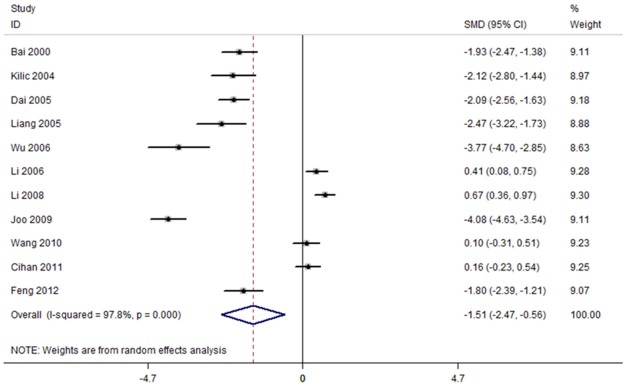
Forest plot of studies in Mn levels for subjects with breast cancer versus healthy controls. The combined SMD and 95% confidence intervals (CIs) were calculated using the random-effects model.
The subgroup analysis showed that geographical location had an influence on the levels of Mn in patients with breast cancer and healthy controls. Further subgroup analysis found that subjects with breast cancer had lower Mn levels than healthy controls in China (SMD = -1.32, 95% CI = [-2.33, -0.32]) and Korea (SMD = -4.08, 95% CI = [-4.63, -3.54]), but not in Turkey (SMD = -0.96, 95% CI = [-3.19, 1.27]) (Figure 3A). In addition, the further subgroup analysis found a similar pattern in different sample specimens (serum: SMD = -1.24, 95% CI = [-2.31, -0.16]; hair: SMD = -1.99, 95% CI = [-3.91, -0.06]) (Figure 3B) and different types of Mn measurement (ICP-AAS: SMD = -1.14, 95% CI = [-2.24, -0.04]; GFAAS: SMD = -1.94, 95% CI = [-2.38, -1.49]; ICP-AES: SMD = -3.77, 95% CI = [-4.70, -2.85]) (Figure 3C). Summary of further subgroup analysis is given in Table 2.
Figure 3.
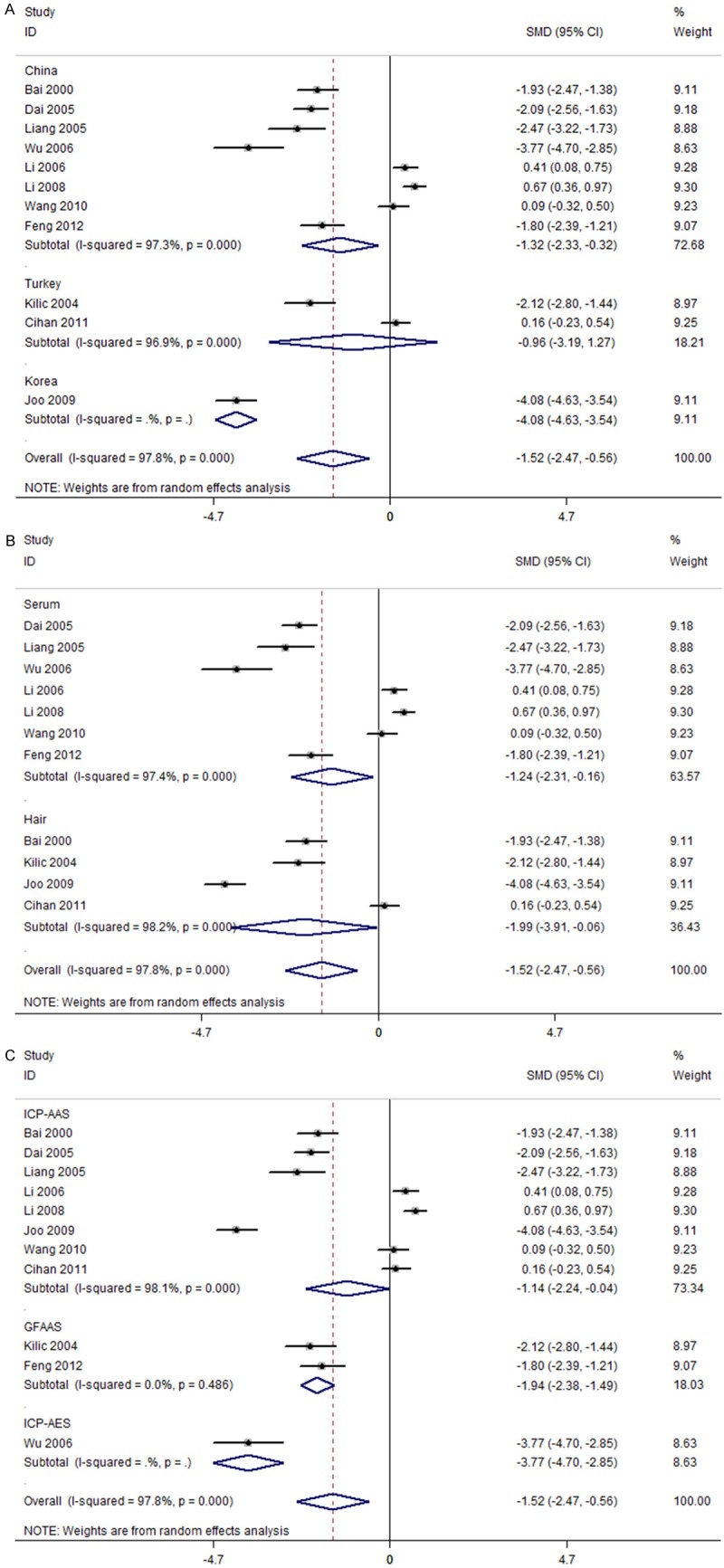
Subgroup analysis of Mn levels for subjects with breast cancer versus healthy controls. A: Stratified by geographical location; B: Stratified by sample specimen; C: Stratified by type of Mn measurement.
Table 2.
Differences between studies by subgroup analysis
| Subgroups | No. of case-control studies | SMD (95% CI) | I2 (%) | P |
|---|---|---|---|---|
| Geographical Location | ||||
| China | 8 | -1.32 (-2.33, -0.32) | 97.3 | 0.01 |
| Turkey | 2 | -0.96 (-3.19, 1.27) | 96.9 | 0.397 |
| Korea | 1 | -4.08 (-4.63, -3.54) | 0 | < 0.001 |
| Sample specimen | ||||
| Serum | 7 | -1.24 (-2.31, -0.16) | 97.4 | 0.024 |
| Hair | 4 | -1.99 (-3.91, -0.06) | 98.2 | 0.043 |
| Type of Mn measurement | ||||
| ICP-AAS | 8 | -1.14 (-2.24, -0.04) | 98.1 | 0.042 |
| GFAAS | 2 | -1.94 (-2.38, -1.49) | 0 | < 0.001 |
| ICP-AES | 1 | -3.77 (-4.70, -2.85) | 0 | < 0.001 |
Publication bias and sensitivity analysis
Publication bias was determined by Begg’s test and visualization of funnel plot. There was no evidence of publication bias (P = 0.436) (Figure 4). Sensitivity analysis showed that excluding any one study from the pooled analysis did not vary the results substantially (Table 3).
Figure 4.
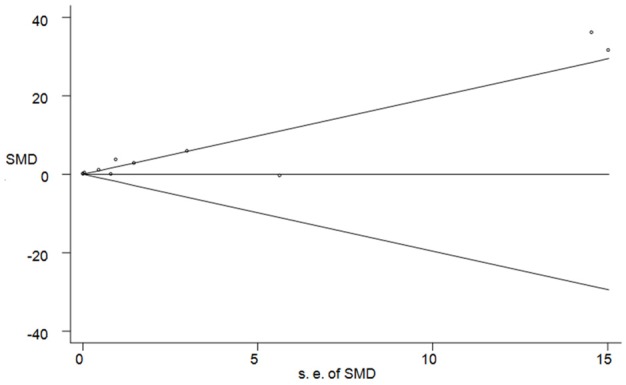
Funnel plot for studies in Mn levels for subjects with breast cancer versus healthy controls.
Table 3.
The heterogeneity of the included studies through sensitivity analysis
| Excluded study arm | SMD (95% CI) | I2 (%) | p value |
|---|---|---|---|
| Before excluding | -1.51 (-2.47, -0.56) | 97.8 | < 0.001 |
| Bai 2000 | -1.48 (-2.50, -0.46) | 97.9 | < 0.001 |
| Kilic 2004 | -1.46 (-2.47, -0.45) | 97.9 | < 0.001 |
| Dai 2005 | -1.46 (-2.47, -0.44) | 97.8 | < 0.001 |
| Liang 2005 | -1.42 (-2.42, -0.42) | 97.9 | < 0.001 |
| Wu 2006 | -1.30 (-2.27, -0.34) | 97.8 | < 0.001 |
| Li 2006 | -1.71 (-2.76, -0.67) | 97.7 | < 0.001 |
| Li 2008 | -1.74 (-2.74, -0.74) | 97.4 | < 0.001 |
| Joo 2009 | -1.25 (-2.09, -0.40) | 96.9 | < 0.001 |
| Wang 2010 | -1.68 (-2.74, -0.62) | 97.9 | < 0.001 |
| Cihan 2011 | -1.69 (-2.75, -0.63) | 97.9 | < 0.001 |
| Feng 2012 | -1.49 (-2.51, -0.47) | 97.9 | < 0.001 |
Discussion
The present study indicated that patients with breast cancer had a lower levels of Mn in serum and hair than healthy controls (SMD = -1.51, 95% CI = [-2.47, -0.56]). Similarly, previous study also had shown that the concentrations of Mn in hair samples of patients diagnosed as having nasopharyngeal cancer were less than that of healthy subjects [24]. It supported a clear negative correlation between Mn content and breast cancer. A low concentration of Mn clearly indicated that Mn might share the similar anticarcinogenic properties, as does the other trace elements, such as iron and selenium [8,9]. Roomi et al. [25] investigated the in vivo effect of dietary supplementation with a nutrient mixture containing Mn on the development of lung tumors, and found that the mean number of tumors and the mean lung weights in the mice on the supplemented diet were significantly reduced, by 49% (P < 0.0001) and 18% (P = 0.0025), respectively, compared to mice on the control diet, indicating that dietary supplementation with Mn has inhibitory potential on the development of mouse lung tumors. Mn is an essential trace element for human health, being absolutely necessary for development, metabolism, and the antioxidant system [26]. Mn plays very important role in a number of biological processes. It is a functional cofactor of various enzymes crucial for the various cellular activities [27-30]. For example, manganese superoxide dismutase (Mn-SOD) contains Mn in its active site, which has the cancer-fighting properties [31,32]. High intracellular Mn can compensate for the loss of SOD and provide protection against oxidative stress [33]. Study also finds that there is negative correlation between the serum levels of Mn and oxidant/antioxidant status in patients with breast cancer [23]. Thus, the Mn levels are closely related to oxidant/antioxidant status in both physiological and pathological conditions. Therefore, we speculated that Mn disturbance might increase the risk of breast cancer through disrupting the balance of oxidant/antioxidant system.
Further subgroup analysis found subjects with breast cancer had a lower level of Mn than healthy controls in serum (SMD = -1.24, 95% CI = [-2.31, -0.16]), and the similar pattern was also found in hair (SMD = -1.99, 95% CI = [-3.91, -0.06]). Scalp hair has been widely accepted for the evaluation of trace element exposure and has been performed by clinical laboratories since it was first used to assess systemic levels of the elements in 1929 [34]. Researchers have found correlations between hair trace elements and diseases, metabolic disorders, environmental exposures, and nutritional status [35-37]. Hair can provide a more permanent record of trace elements associated with normal and abnormal metabolism. In addition, hair is easily collected, conveniently stored, and easily treated [38]. While blood tests show the recent and current body status, hair represents a longer time frame, which can incorporate many years [34]. Since the elements are present in the hair at higher levels, more sensitive and accurate analysis results can be expected [39]. As such, it has been suggested that hair mineral analysis can be a cost-effective means of screening an individual’s nutritional status [40]. Therefore, the analysis of human hair instead of serum instead of serum can be a useful way to understand the permanent record of Mn levels in patients with breast cancer.
However, our results showed strong heterogeneity among the studies (I2 = 97.8%, P < 0.001). Heterogeneity indicates differences in results across the studies. There are two sources of heterogeneity: one is within-study variability which means a difference within a study of estimating the same effect size and it always exists in meta-analysis because of sampling error, the other is between-study variability which means differences among studies in estimating effect size among different population. In present study, the between-study variability was the main source of heterogeneity, because the further subgroup analysis indicated that the geographical location was the possible source of heterogeneity. We found that the subjects with breast cancer had lower Mn levels than healthy controls in China (SMD = -1.32, 95% CI = [-2.33, -0.32]) and Korea (SMD = -4.08, 95% CI = [-4.63, -3.54]), but not in Turkey (SMD = -0.96, 95% CI = [-3.19, 1.27]). And the differences of Mn levels between patients with breast cancer and healthy controls were similar in different sample specimens (serum: SMD = -1.24, 95% CI = [-2.31, -0.16]; hair: SMD = -1.99, 95% CI = [-3.91, -0.06]) and different types of Mn measurement (ICP-AAS: SMD = -1.14, 95% CI = [-2.24, -0.04]; GFAAS: SMD = -1.94, 95% CI = [-2.38, -1.49]; ICP-AES: SMD = -3.77, 95% CI = [-4.70, -2.85]). These findings can well explain that the between-study variability was the main source of heterogeneity.
To the best of our knowledge, this is the first meta-analysis to estimate the association between Mn levels and breast cancer. We made sure to minimize the bias by means of study procedure. Not only did we search Pubmed and the Chinese Journal Full-text Database (CJFD) to identify potential studies, but also we manually examined all reference lists from relevant studies. Sensitivity analysis showed that excluding any one study from the pooled analysis did not vary the results substantially. Publication bias was also absent, as determined by visualization of funnel plot and Begg’s test. However, the possible limitations of our study must be considered. First, only 1302 subjects from 11 case-control studies and no randomized clinical trial included in the meta-analysis might weaken the quality of the results. In addition, heterogeneity could not be omitted because of methodological diversities between studies, thus the conclusion should be more conservative. Third, all the included studies were from Asia, such conception focalises on a unique area rather than general. Despite these limitations, our findings point out new directions for future research. First, ours study does raise new question why there is no relationship with Mn deficiency and breast cancer among Turkish women, just like Chinese and Korean women do. Therefore we suggest that a community-based, long-term observation in a cohort design should be performed to obtain better understanding of causal relationships between Mn and breast cancer in Turkey, through measuring Mn at baseline to investigate whether the highest Mn category versus lowest was associated with breast cancer risk. Second, our results only represented the Asian and showed the ethnic/geographical paradox, therefore trans-regional multicenter study is needed for the investigation of the interrelationship between Mn and breast cancer of different human races or regions.
Conclusion
In summary, this meta-analysis supports a significant association between deficient Mn concentration and breast cancer. However, the subgroup analysis found that there was contradiction regarding races and geography, like China and Turkey. Thus this finding needs further confirmation by trans-regional multicenter, long-term observation in a cohort design to obtain better understanding of causal relationships between Mn levels and breast cancer, through measuring Mn at baseline to investigate whether the highest Mn category versus lowest was associated with breast cancer risk.
Acknowledgements
All authors designed the study together and performed the experiment together; all authors analyzed the data and wrote the paper; all authors approved the final manuscript.
Disclosure of conflict of interest
None.
References
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Wolff MS, Weston A. Breast cancer risk and environmental exposures. Environ Health Perspect. 1997;105(Suppl 4):891–896. doi: 10.1289/ehp.97105s4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Ou YL, Liu YQ, Xie Q, Liu QF, Wu Q, Fan TQ, Yan LL, Wang JY. Correlations of trace element levels in the diet, blood, urine, and feces in the Chinese male. Biol Trace Elem Res. 2012;145:127–135. doi: 10.1007/s12011-011-9177-8. [DOI] [PubMed] [Google Scholar]
- 6.Almeida AA, Lopes CM, Silva AM, Barrado E. Trace elements in human milk: correlation with blood levels, inter-element correlations and changes in concentration during the first month of lactation. J Trace Elem Med Biol. 2008;22:196–205. doi: 10.1016/j.jtemb.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Fournier A, Mesrine S, Dossus L, Boutron-Ruault MC, Clavel-Chapelon F, Chabbert-Buffet N. Risk of breast cancer after stopping menopausal hormone therapy in the E3N cohort. Breast Cancer Res Treat. 2014;145:535–543. doi: 10.1007/s10549-014-2934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonseca-Nunes A, Jakszyn P, Agudo A. Iron and cancer risk--a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomarkers Prev. 2014;23:12–31. doi: 10.1158/1055-9965.EPI-13-0733. [DOI] [PubMed] [Google Scholar]
- 9.Babaknejad N, Sayehmiri F, Sayehmiri K, Rahimifar P, Bahrami S, Delpesheh A, Hemati F, Alizadeh S. The relationship between selenium levels and breast cancer: a systematic review and meta-analysis. Biol Trace Elem Res. 2014;159:1–7. doi: 10.1007/s12011-014-9998-3. [DOI] [PubMed] [Google Scholar]
- 10.Bairati C, Goi G, Bollini D, Roggi C, Luca M, Apostoli P, Lombardo A. Effects of lead and manganese on the release of lysosomal enzymes in vitro and in vivo. Clin Chim Acta. 1997;261:91–101. doi: 10.1016/s0009-8981(97)06515-7. [DOI] [PubMed] [Google Scholar]
- 11.Crowley JD, Traynor DA, Weatherburn DC. Enzymes and proteins containing manganese: an overview. Met Ions Biol Syst. 2000;37:209–278. [PubMed] [Google Scholar]
- 12.Dismukes GC. Manganese Enzymes with Binuclear Active Sites. Chem Rev. 1996;96:2909–2926. doi: 10.1021/cr950053c. [DOI] [PubMed] [Google Scholar]
- 13.Wu HD, Chou SY, Chen DR, Kuo HW. Differentiation of serum levels of trace elements in normal and malignant breast patients. Biol Trace Elem Res. 2006;113:9–18. doi: 10.1385/BTER:113:1:19. [DOI] [PubMed] [Google Scholar]
- 14.Kilic E, Saraymen R, Demiroglu A, Ok E. Chromium and manganese levels in the scalp hair of normals and patients with breast cancer. Biol Trace Elem Res. 2004;102:19–25. doi: 10.1385/BTER:102:1-3:019. [DOI] [PubMed] [Google Scholar]
- 15.Benderli Cihan Y, Sozen S, Ozturk Yildirim S. Trace elements and heavy metals in hair of stage III breast cancer patients. Biol Trace Elem Res. 2011;144:360–379. doi: 10.1007/s12011-011-9104-z. [DOI] [PubMed] [Google Scholar]
- 16.LI X, Duan A, Li J, Liu A, Zhao X. Study of serum trace elements in patients with breast tumor and recurred breast cancer in Baotou area. Oncology Progress. 2008;6:641–644. [Google Scholar]
- 17.Bai N, Xia F, Zhang Z, Li H. Determination of eight trace elements in breast cancer, normal breast tissue and hair. Journal of Capical University of Medical Science. 2000;21:351–352. [Google Scholar]
- 18.Dai D, Yu R. Variation of trace elements in breast cancer patients before and after treatment. Chinese Journal of Clinical Laboratory Science. 2005;23:461. [Google Scholar]
- 19.Li X, Ding J, Li X, Ba C, Duan A, Gao F, Wang Y. Clinical value of determination of trace elements in serum in breast cancer. Inner Mongolia Medical Journal. 2006;38:799–800. [Google Scholar]
- 20.Liang C, Qi H, Dan Z. Variation of serum trace elements in patients with breast cancer before and after operation. Maternal and Child Health Care of China. 2005;20:2946. [Google Scholar]
- 21.Joo NS, Kim SM, Jung YS, Kim KM. Hair iron and other minerals’ level in breast cancer patients. Biol Trace Elem Res. 2009;129:28–35. doi: 10.1007/s12011-008-8281-x. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Zhang L, Qi X. Analysis of serum trace elements in patients with gynecological malignancies. Medical Journal of West China. 2010;22:2046–2047. [Google Scholar]
- 23.Feng JF, Lu L, Zeng P, Yang YH, Luo J, Yang YW, Wang D. Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int J Clin Oncol. 2012;17:575–583. doi: 10.1007/s10147-011-0327-y. [DOI] [PubMed] [Google Scholar]
- 24.Leung PL, Huang HM. Analysis of trace elements in the hair of volunteers suffering from naso-pharyngeal cancer. Biol Trace Elem Res. 1997;57:19–25. doi: 10.1007/BF02803866. [DOI] [PubMed] [Google Scholar]
- 25.Caple F, Williams EA, Spiers A, Tyson J, Burtle B, Daly AK, Mathers JC, Hesketh JE. Inter-individual variation in DNA damage and base excision repair in young, healthy non-smokers: effects of dietary supplementation and genotype. Br J Nutr. 2010;103:1585–1593. doi: 10.1017/S0007114509993540. [DOI] [PubMed] [Google Scholar]
- 26.Avila DS, Puntel RL, Aschner M. Manganese in health and disease. Met Ions Life Sci. 2013;13:199–227. doi: 10.1007/978-94-007-7500-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malthankar GV, White BK, Bhushan A, Daniels CK, Rodnick KJ, Lai JC. Differential lowering by manganese treatment of activities of glycolytic and tricarboxylic acid (TCA) cycle enzymes investigated in neuroblastoma and astrocytoma cells is associated with manganese-induced cell death. Neurochem Res. 2004;29:709–717. doi: 10.1023/b:nere.0000018841.98399.ce. [DOI] [PubMed] [Google Scholar]
- 28.Rajendiran M, Caudle T, Kirk ML, Setyawati I, Kampf JW, Pecoraro VL. Evaluating hydrogen bond interactions in enzymes containing Mn(III)-histidine complexation using manganese-imidazole complexes. J Biol Inorg Chem. 2003;8:283–293. doi: 10.1007/s00775-002-0414-7. [DOI] [PubMed] [Google Scholar]
- 29.Sosa-Torres ME, Kroneck PM. Interaction of cyanide with enzymes containing vanadium, manganese, non-heme iron, and zinc. Met Ions Life Sci. 2009;6:363–393. doi: 10.1039/BK9781847559159-00363. [DOI] [PubMed] [Google Scholar]
- 30.Hoseini SM, Hedayati A, Ghelichpour M. Plasma metabolites, ions and thyroid hormones levels, and hepatic enzymes activity in Caspian roach (Rutilus rutilus caspicus) exposed to waterborne manganese. Ecotoxicol Environ Saf. 2014;107:84–89. doi: 10.1016/j.ecoenv.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Plymate SR, Haugk KH, Sprenger CC, Nelson PS, Tennant MK, Zhang Y, Oberley LW, Zhong W, Drivdahl R, Oberley TD. Increased manganese superoxide dismutase (SOD-2) is part of the mechanism for prostate tumor suppression by Mac25/insulin-like growth factor binding-protein-related protein-1. Oncogene. 2003;22:1024–1034. doi: 10.1038/sj.onc.1206210. [DOI] [PubMed] [Google Scholar]
- 32.Kurokawa H, Sakimoto M, Yamashita Y, Murata T, Kajiyama M. Manganese superoxide dismutase (Mn-SOD) correlates with prognosis of patients with oral squamous cell carcinoma. Fukuoka Igaku Zasshi. 1998;89:321–327. [PubMed] [Google Scholar]
- 33.Reddi AR, Jensen LT, Naranuntarat A, Rosenfeld L, Leung E, Shah R, Culotta VC. The overlapping roles of manganese and Cu/Zn SOD in oxidative stress protection. Free Radic Biol Med. 2009;46:154–162. doi: 10.1016/j.freeradbiomed.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bass DA, Hickock D, Quig D, Urek K. Trace element analysis in hair: factors determining accuracy, precision, and reliability. Altern Med Rev. 2001;6:472–481. [PubMed] [Google Scholar]
- 35.Park SB, Choi SW, Nam AY. Hair tissue mineral analysis and metabolic syndrome. Biol Trace Elem Res. 2009;130:218–228. doi: 10.1007/s12011-009-8336-7. [DOI] [PubMed] [Google Scholar]
- 36.Tan C, Chen H, Xia C. The prediction of cardiovascular disease based on trace element contents in hair and a classifier of boosting decision stumps. Biol Trace Elem Res. 2009;129:9–19. doi: 10.1007/s12011-008-8279-4. [DOI] [PubMed] [Google Scholar]
- 37.Berschneider F, Schwenke I. [Mineral and trace element determination in porcine hair as diagnostic procedure for nutrition disorders with these elements] . Arch Exp Veterinarmed. 1969;23:247–251. [PubMed] [Google Scholar]
- 38.Seidel S, Kreutzer R, Smith D, McNeel S, Gilliss D. Assessment of commercial laboratories performing hair mineral analysis. JAMA. 2001;285:67–72. doi: 10.1001/jama.285.1.67. [DOI] [PubMed] [Google Scholar]
- 39.Miekeley N, Dias Carneiro MT, da Silveira CL. How reliable are human hair reference intervals for trace elements? Sci Total Environ. 1998;218:9–17. doi: 10.1016/s0048-9697(98)00185-5. [DOI] [PubMed] [Google Scholar]
- 40.Druyan ME, Bass D, Puchyr R, Urek K, Quig D, Harmon E, Marquardt W. Determination of reference ranges for elements in human scalp hair. Biol Trace Elem Res. 1998;62:183–197. doi: 10.1007/BF02783970. [DOI] [PubMed] [Google Scholar]


