Abstract
Although there are possible cardiovascular adverse effects associated with the azithromycin treatment according to some case reports and cohort studies, there is no experimental study evaluating cardiotoxicity in repeated pharmacological doses of this drug. In our study, 15 mg/kg and 30 mg/kg azithromycin were orally administered to rats for 14 days to evaluate the cardiotoxicity of this drug. ECGs of the azithromycin-treated and control animals were recorded. Blood samples were assayed to determine LDH and CK-MB levels. Additionally, CAT, SOD, GSH and MDA levels of heart tissues were measured. According to our ECG recordings, decreased heart rate, prolonged PR and QT intervals, QRS complex and T wave abnormalities were observed in 30 mg/kg azithromycin-administered group significantly when compared with control group. Plasma CK-MB and LDH levels were increased in 30 mg/kg azithromycin-administered group significantly when compared to the control group. In heart tissues, CAT, SOD and GSH levels were decreased while MDA levels were increased in both azithromycin-administered groups significantly when compared with the control group. In conclusion, our findings supported the possible cardiotoxicity risk with azithromycin treatment and also, oxidative stress, which was induced by azithromycin in our study, was thought to be occurred secondary to cardiac toxicity of the drug.
Keywords: Azithromycin, CK-MB, LDH, ECG, oxidative stress
Introduction
The macrolides, such as erythromycin, clarithromycin, azithromycin and telithromycin, are the most commonly used members of the clinically important antibiotics to treat infections caused by gram-positive bacteria like Staphylococcus aureus, Streptococcus pneumoniae and Streptococcus pyogenes [1,2]. Despite having various advantages in the treatment of several diseases, the macrolide antibiotics, when used both alone and in combination with other medications, may rarely cause cardiotoxic effects, such as prolonged QT interval and polymorphic ventricular tachycardia [2,3].
Azithromycin, which is a broad spectrum second generation macrolide group of antibiotic with minimal adverse effects and a pharmacokinetic/pharmacodynamic profile that allow simple dose regimens, is frequently used in the treatment of bacterial infections [4,5]. Besides, there has been an increasing interest in azithromycin due to its additional effects on host-defense reactions and chronic human diseases in recent years [5]. Azithromycin is the best seller antibiotic in the USA, ranked 15th among the mostly-prescribed medications [6]. Even though rare incidence/occurrence of cardiotoxicity has been suggested with the azithromycin treatment when compared with other macrolide derivatives, the cases of cardiovascular adverse effects associated with azithromycin have attracted attention recently [7,8].
According to the data from the adverse effect notification system of FDA (Food and Drug Administration), 20 cases of Torsades de pointes associated with azithromycin have been reported [9]. Furthermore, according to the results of the cohort studies conducted on the patients who received azithromycin treatment, it was reported that there had been sudden deaths due to ventricular arrhythmia as a result of azithromycin use [7].
Although there are case reports drawing attention to the cardiovascular adverse effects associated with the azithromycin treatment, there is no experimental study evaluating cardiotoxicity in repeated pharmacological doses of azithromycin. Therefore, it was aimed to evaluate the cardiotoxicity profile of azithromycin in repeated pharmacological doses in rats, by cardiac biomarker levels, such as plasma lactate dehydrogenase (LDH) and creatine kinase-MB (CK-MB), electrocardiogram (ECG) recordings and histopathological analysis of the heart tissue in the present study. Additionally, it was also aimed to evaluate the oxidative status in a possible pathology caused by azithromycin administration by determining the levels of catalase (CAT), superoxide dismutase (SOD), glutathione (GSH) and malondialdehyde (MDA) in the heart tissues of azithromycin-administered rats.
Materials methods
Materials
The chemicals used were obtained from the following sources: ketamine (Ketalar, Pfizer, Turkey); xylazine (Bayer, Turkey), azithromycin (DEVA Holding, Turkey). LDH levels were measured by LDH Assay kit (BioAssay Systems, CA, USA) and CK-MB levels were measured by CK-MB Assay kit (BioCheck, CA, USA) according to the manufacturer’s instructions. Heart SOD, CAT and GSH levels were determined by ELISA kits from Cayman Chemical Company, USA. For the measurements of MDA levels of heart tissues ELISA kits from Cusabio Biotech CO. Ltd., P. R. China were used.
Animals
Male Wistar rats weighing 250-300 g were obtained from Anadolu University Research Center for Animal Experiments. Rats were housed under controlled temperature (24°C) and lighting (12/12-hour light dark cycle) with free access to food and water. The experimental protocol was approved by the Local Ethical Committee on Animal Experimentation of Anadolu University, Eskisehir, Turkey.
The experimental groups of animals were as follows: Group 1, control animals treated orally with distilled water at a volume of 1 ml/100 g for 14 days (n = 10) (C); Group 2, animals treated orally with 15 mg kg- azithromycin at a volume of 1 ml/100 g for 14 days (n = 10); and Group 3, animals treated orally with 30 mg kg- azithromycin at a volume of 1 ml/100 g for 14 days (n = 10). The doses of azithromycin were determined according to the previous studies [10-12]. Azithromycin was dissolved in distilled water for oral administration of treatment groups.
At the end of 14 days, the following experimental protocol was performed.
Methods
ECG recordings
Rats were anesthetized by intraperitoneal injection of 60 mg kg- ketamine and 5 mg kg- xylazine. ECGs were recorded through needle electrodes which were located right wrist, right ankle and left ankle (Lead II) using Biopac MP36 data acquisition system (Biopac Systems, Santa Barbara, CA). The changes in heart rate, PR interval, QT interval, QRS duration, QRS amplitude and T amplitude were determined from ECGs of the animals.
The records were analyzed off-line and included measures of QT (interval between the beginning of the Q wave and the end of the T wave of ECG), PR (interval between the beginning of the P wave and the end of the R wave) and QRS (interval from the beginning of the Q wave to the end of the S wave), T wave (interval between the beginning to the end of the isoelectric line) (baseline).
Biochemical analysis
Blood samples for biochemical analyses were collected from the right ventricle of the anesthetized animals by using a syringe. The animals were killed by withdrawing high amounts of blood from the heart and heart tissues were removed. The blood samples were centrifuged at 2000 × g for 15 minutes at +4°C after they were rested at room temperature for 30 minutes. LDH and CK-MB levels were assayed according to the manufacturer’s instructions.
Heart tissues were excised from animals. The tissues were washed with phosphate buffered saline (PBS) solution, pH 7.4. The heart samples were stored in a refrigerator at -80°C until they were used in biochemical analysis. The tissues were used for the determination of GSH, SOD, CAT and MDA levels in groups.
GSH assay
The tissues were diluted at the ratio of 1:20 (w: v) with cold buffer (50 mM MES, pH 6-7, containing 1 mM EDTA) and were homogenized. The homogenates were centrifuged at 10000xg for 15 minutes at +4°C. The supernatants were removed and were deproteinated. Then, the samples were used for total GSH assay.
CAT assay
The tissues were diluted at the ratio of 1:20 (w:v) with cold buffer (50 mM potassium phosphate, pH 7, containing 1 mM EDTA) and were homogenized. The homogenates were centrifuged at 10000xg for 15 minutes at +4°C. The supernatants were removed and were used for the assay.
SOD assay
The tissues were diluted at the ratio of 1:20 (w:v) with HEPES buffer (pH 7.2, containing 1 mM EGDA, 210 mM mannitol, and 70 mM sucrose) and were homogenized. The homogenates were centrifuged at 10000xg for 15 minutes at +4°C. The supernatants were removed and were used for the assay.
MDA assay
The tissues were diluted 1:20 (w:v) with cold buffer (1x phosphate buffered saline, pH 7.4) and were homogenized. The homogenates were centrifuged at 5000xg for 5 minutes at +4°C. The supernatants were removed and were used for the assay.
Light microscopic analysis
The heart samples were fixed in 10% buffered formalin solution for 48 hours and embedded in paraffin. Then, 5 μm thick slices were stained with haematoxylin and eosin and examined by light microscopy. All sections were observed under Olympus BH-2 microscope (Olympus Corp., Tokyo, Japan).
Statistical analysis
All data were expressed as mean ± standard deviation. Statistical analysis of the groups were performed on GraphPad 4.0 package program and by using One-way ANOVA. Tukey’s test was used as the post hoc test. P < 0.05 was considered as statistically significant.
Results
Plasma CK-MB and LDH levels
In the present study, CK-MB levels were increased significantly in both azithromycin-administered groups compared to the control group. LDH levels were also increased significantly in our 30 mg/kg azithromycin-administered group compared to control and 15 mg/kg azithromycin-administered groups (Table 1).
Table 1.
Effects of azithromycin on cardiac parameters
| C | 15 mg/kg AZI | 30 mg/kg AZI | |
|---|---|---|---|
| CK-MB (ng ml-) | 47.05 ± 8.41 | 61.35 ± 5.52* | 66.66 ± 9.6* |
| LDH (IU L-) | 37.41 ± 11.99 | 43.34 ± 12.87 | 60.95 ± 9.66*,+ |
Definition of abbreviations: CK-MB, creatine kinase-myocardial band; LDH, lactate dehydrogenase. C: distilled water-administered rats for 14 days; 15 mg/kg AZI: 15 mg- kg azithromycin-administered rats for 14 days; 30 mg/kg AZI: 30 mg- kg azithromycin-administered rats for 14 days.
Different from the control group (P < 0.05);
Different from the 15 mg- kg AZI group (P < 0.05).
ECG results
According to our ECG recordings, heart rate was significantly decreased in 30 mg/kg azithromycin-administered group compared to control and 15 mg/kg azithromycin-administered groups. In 30 mg/kg azithromycin-administered group, PR and QT-intervals were prolonged significantly compared to the control group. Also QT intervals of 30 mg/kg azithromycin-administered group were found to be significantly longer than QT intervals of 15 mg/kg azithromycin-administered group. Furthermore, QRS amplitudes of 30 mg/kg azithromycin-administered group were significantly increased compared to the control group. T-amplitudes of 30 mg/kg azithromycin-administered group were significantly increased compared to the control group. Also, these increases were significantly different from 15 mg/kg azithromycin-administered group (Figure 1 and Table 2).
Figure 1.
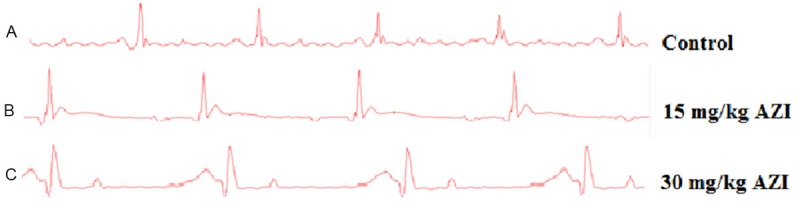
Effect of azithromycin on electrocardiographic patterns and changes [recorded from lead II]. A. The control group showed a “normal” pattern on ECG. B. PR interval was prolonged and QRS complex was widened. C. QRS complex was significantly widened, PR and QT intervals were significantly prolonged.
Table 2.
Effects of azithromycin on ECG parameters
| C | 15 mg/kg AZI | 30 mg/kg AZI | |
|---|---|---|---|
| Heart rate (Beats/min) | 320.63 ± 49.2 | 324.38 ± 14.78 | 265.25 ± 31.82 (*,+) |
| P-R interval (msec) | 48.13 ± 4.55 | 50.5 ± 3.59 | 55.13 ± 3.37 (*) |
| Q-T interval (msec) | 52.29 ± 4.7 | 54.5 ± 5.1 | 61.13 ± 4.58 (*,+) |
| QRS amplitude (mV) | 0.42 ± 0.07 | 0.58 ± 0.13 | 0.69 ± 0.16 (*) |
| QRS duration (msec) | 22 ± 5.24 | 25.88 ± 3.48 | 26.13 ± 2.95 |
| T wave amplitude (mV) | 0.09 ± 0.01 | 0.1 ± 0.02 | 0.13 ± 0.02 (*,+) |
C: distilled water-administered rats for 14 days; 15 mg/kg AZI: 15 mg- kg azithromycin-administered rats for 14 days; 30 mg/kg AZI: 30 mg- kg azithromycin-administered rats for 14 days.
Different from the control group (P < 0.05);
Different from the 15 mg/kg AZI group (P < 0.05).
GSH levels
It was found that GSH levels were significantly decreased in azithromycin-administered groups compared to the control group. There was no difference between the two treatment groups (Table 3).
Table 3.
The mean GSH, SOD, CAT and MDA levels of heart tissues in groups
| C | 15 mg/kg AZI | 30 mg/kg AZI | |
|---|---|---|---|
| MDA (pmol/ml) | 1085.14 ± 122.27 | 1562.43 ± 124.29 (***) | 1370.36 ± 145.38 (*,+) |
| GSH (ug/gTissue) | 20.3 ± 1.07 | 14.91 ± 2.33 (*) | 14.06 ± 1.76 (*) |
| SOD (Uml) | 1.63 ± 0.12 | 1.14 ± 0.13 (*) | 1.59 ± 0.45 (+) |
| CAT (nmol/min/ml) | 388.27 ± 24.18 | 354.21 ± 21.40 (*) | 349.21 ± 18.24 (*) |
Definition of abbreviations: GSH, glutathione; SOD, superoxide dismutase; CAT, catalase; MDA, malondialdehyde. C: distilled water-treated rats for 14 days; 15 mg/kg AZI”: 15 mg- kg azithromycin-administered rats for 14 days; 30 mg/kg AZI: 30 mg- kg azithromycin-administered rats for 14 days.
Different from the control group (P < 0.05);
Different from control group (P < 0.001);
Different from the 15 mg- kg AZI group (P < 0.05).
Catalase level
Catalase activity was found to be significantly lower in azithromycin-administered groups compared to the control group. But, no differences were found between the two treatment groups (Table 3).
SOD level
Heart SOD levels were decreased in 15 mg/kg azithromycin-administered group compared to the control group. Also, SOD levels were significantly decreased in 15 mg/kg azithromycin-administered group compared to 30 mg/kg azithromycin-administered group (Table 3).
MDA level
It was found that MDA levels were significantly decreased (P < 0.01) in azithromycin-administered groups compared to the control group. Also, MDA levels were significantly decreased in 30 mg/kg azithromycin-administered group compared to 15 mg/kg azithromycin-administered group (Table 3).
Light microscopic analysis of the heart
Light microscopic examination of heart tissues of control group showed normal morphological appearances (Figure 2). In the heart tissue of 15 mg/kg azithromycin-administered group, normal myocardial cells accompanied by minimal vacuolization of the cytoplasm were observed (Figure 3). In the cardiac tissue of 30 mg/kg azithromycin-administered group, necrosis and hypertrophy of cardiac muscle cells with infiltration of inflammatory cells were observed. In addition, there was an increase in interstitial edema (Figure 4).
Figure 2.
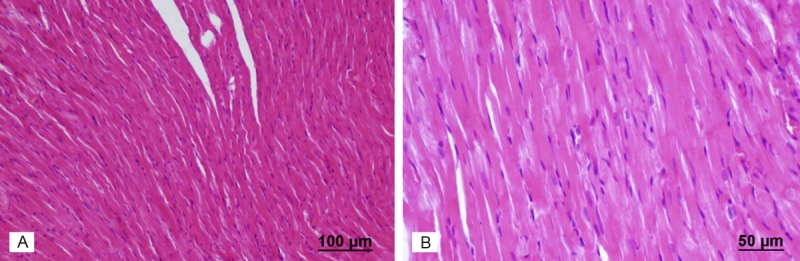
Assessment of heart light microscopy analysis in control rats. The heart tissues of rats from the control group revealed normal myofibrillar structure. A. Haematoxylin and eosin stain, scale bar-100 μm; B. Haematoxylin and eosin stain, scale bar-50 μm.
Figure 3.
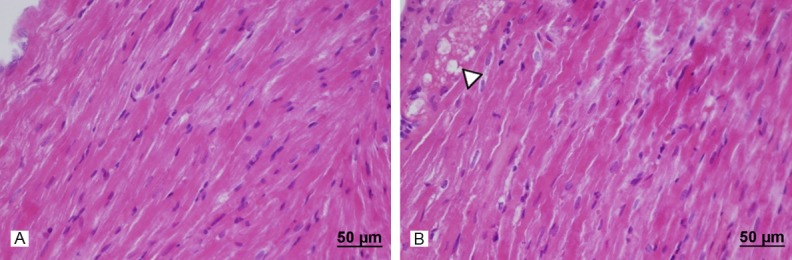
Assessment of heart light microscopy analysis in 15 mg- kg azithromycin-administered rats. The heart tissues of azithromycin-administered rats revealed normal cardiac muscle fibers without necrosis and absence of any damage. But, normal myocardial cells were accompanied by minimal vacuolization of the cytoplasm (►). A. Haematoxylin and eosin stain, scale bar-50 μm; B. Haematoxylin and eosin stain, scale bar-50 μm.
Figure 4.
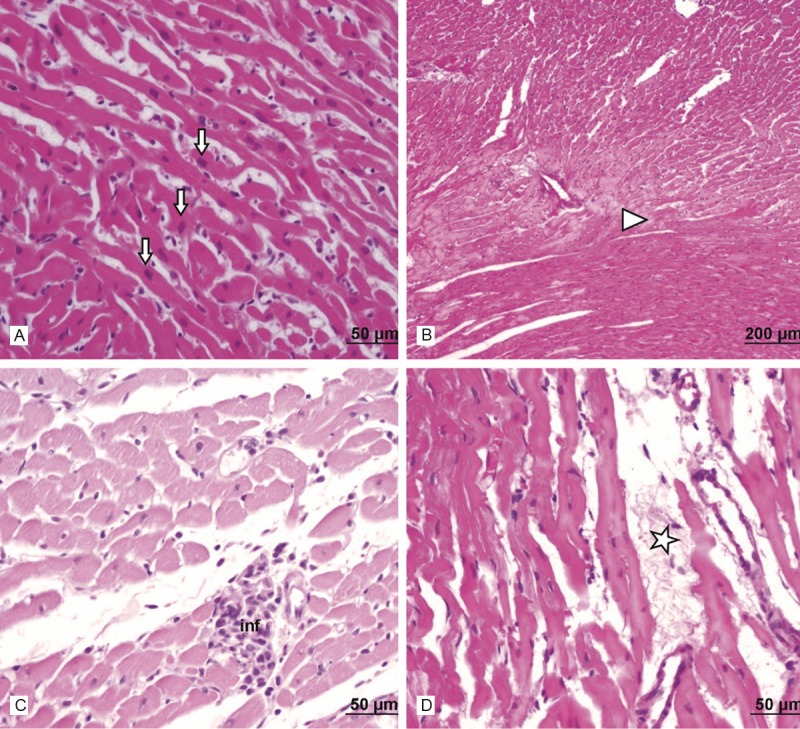
Assessment of heart light microscopy analysis in 30 mg- kg mg- kg azithromycin-administered rats. In the heart tissues of azithromycin-administered rats, light microscopic examination showed necrosis (►) and hypertrophy (↓) of cardiac muscle cells, infiltration of inflammatory cells (inf) and interstitial edema (*). A. Haematoxylin and eosin stain, scale bar-50 μm; B. Haematoxylin and eosin stain, scale bar-200 μm; C. Haematoxylin and eosin stain, scale bar-50 μm; D. Haematoxylin and eosin stain, scale bar-50 μm.
Discussion
The cardiovascular adverse effects of azithromycin, which is used frequently in the treatment of bacterial infections, were investigated in repeated pharmacological doses in our study. Particularly, pathological cardiovascular symptoms attracted attention in our 30 mg/kg azithromycin-administered group. In our study, it was determined that plasma CK-MB and LDH levels, which are cardiac biomarkers, were increased, heart rate was decreased, PR and QT intervals were prolonged, and the amplitude and duration of QRS complex as well as the amplitude of T wave were increased with azithromycin administration in rats. Necrosis and hypertrophy of cardiac muscle cells, infiltration of inflammatory cells and interstitial edema were observed in the histological sections of the heart tissue in 30 mg/kg azithromycin-administered group. On the other hand, when the oxidative status in the heart tissue after azithromycin treatment was evaluated, it was found that catalase, superoxide dismutase and glutathione levels were decreased while the malondialdehyde levels were increased.
The myocardial damage occurs mostly in cases where there is an abrupt decline in the coronary blood flow, and then cardiac biomarkers are released from the damaged myocardium into the circulation [13]. LDH, total CK, aspartate aminotransferase (AST) and troponins are the biomarkers used for determining such damage. The rise of the cardiac biomarkers in blood is interpreted as the messenger of a myocardial damage. The short half-lives of CK-MB and LDH lead to their role in determining the acute heart damage [13-15]. Our findings, which indicated the significant increases of CK-MB levels in both azithromycin-administered groups and the significant increases of LDH levels in 30 mg/kg azithromycin-administered group, can be interpreted as an acute myocardial damage caused by azithromycin via inducing the ischemia development in the myocardial tissue. In a retrospective study conducted by Coloma et al. (2013), it was shown that azithromycin treatment could cause acute myocardial infarction [16].
In our study, the heart rate significantly declined in the 30 mg/kg azithromycin-administered group was administered in comparison with the control group. There are also case reports supporting our study and drawing attention to the development of bradycardia in the patients under the treatment of azithromycin [17,18]. Drugs are known to be the major causes of bradycardia [19,20]. Bradyarrhythmia, which is induced by drugs, can be associated with sinus and/or atrioventricular node dysfunction and conduction disturbances [19,21,22]. In general, the bradycardic cases resulting from sinus node dysfunction and atrioventricular conduction disturbances can be determined by ECG findings [20]. A reflection of the action potential of heart cells, ECG is the electrical expression of the heart rate in the form of PQRST complex. The P wave stands for atrium depolarization, while the QRS wave represents the ventricular depolarization and the T wave shows the ventricular repolarization [23]. Several drugs may cause changes in ECG, even in the patients with no cardiac pathology history/medical record. The early functional cardiotoxic effects of drugs can be assessed by electrophysiological changes. The differences in ECG findings are regarded as the determinants/indicators of cardiotoxicity [24]. Drugs may induce the changes in the ECG recordings along with their membrane-depressant effects and their effects on the autonomic nervous system and its cardiovascular components. Secondarily, most of the drugs may cause changes in the ECG through different mechanisms, such as hypoxia and electrolyte and metabolic imbalances [25].
In our 30 mg/kg azithromycin-administered group, it was determined that PR and QT intervals were prolonged, and the amplitude and duration of QRS complex were increased along with the amplitude of the T wave when compared with the control group. In a previous study by Ohtani et al., intravenously injected azithromycin for 90 minutes evoked QT interval prolongation at 100 mg/kg/h concentration, but didn’t affect PR or QRS interval in rats [26]. In our study, long term of azithromycin administration can be considered as a fact that caused more arrhythmogenic effects because of this agent’s well known wide tissue distribution [27].
The prolongation of the PR interval in ECG may be basically due to the delay of the conduction in the atrioventricular node as well as being associated with the delay of the conduction in the atrium, bundle of his, branches and Purkinje fibers [28]. The PR interval analyzed in ECG is used for evaluating the β-adrenergic receptor function and calcium channel activation [25]. At this point, in our study, the molecular basis of the prolongation in the PR interval induced by azithromycin can be clarified via in vitro studies and through the evaluation of the effects of azithromycin on the β-adrenergic receptor and calcium channels.
The prolongation of QT interval in ECG is regarded as a major indicator in determining cardiotoxic effects of drugs [29]. This prolongation also poses a risk for Torsades de pointes or polymorphic ventricular tachycardia [21,30]. Furthermore, the prolongation of QT interval is evaluated as a biomarker of the early stages of acute myocardial ischemia [31]. Potassium efflux from the myocardial cells, is directly responsible for the QT interval [25,32,33]. The blockage or dysfunction of the potassium channel hERG (human ether-a-go-go-related gene), which has a major role in the myocyte repolarization, leads to the prolongation of QT interval in humans [6,34]. The expression of ERG in rats with similar functionality as in humans has been still debatable, however many reports are available which indicates IKr-like current and ERG expression in rats with properties similar to those described in other species [35,36]. In previous studies, azithromycin was shown to be a weak blocker/inhibitor of this potassium channel in high concentrations in vitro [6,34]. Otherwise, peak plasma levels of azithromycin in healthy volunteers receiving short-term azithromycin have been reported to be less than the drug’s estimated hERG channel blocker/inhibitor concentration. Therefore, underlying mechanism of azithromycin-related long QT might be different from azithromycin-induced hERG channel inhibition [37]. Since, hERG channel is not the only reason for QT prolongation, there are other mechanisms involved and azithromycin might not share similar proarrhythmic features like other macrolides as shown in previous studies [37,38]. At this point, it must be emphasized that the effect of azithromycin on potassium channels (hERG and other Kv channel) should be identified through further detailed studies. On the other hand, another point not to be sneezed at is that bradycardia is one of the major factors giving rise to the prolongation of QT interval [21,30]. The bradycardic condition observed in our 30 mg/kg azithromycin-administered group might have triggered the prolongation of QT interval.
The duration and amplitude of QRS complex are crucial in the diagnosis of conduction anomalies, ventricular hypertrophy and myocardial ischemia. Hence, it is possible to associate the anomalies in QRS complex with myocardial infarction, ventricular hypertrophy and intranodal conduction anomalies [33]. The inhibition of rapid sodium channels slows down the upslope of depolarization and causes the QRS complex to expand. It is also possible to associate the changes of QRS complex with the inhibition of calcium influx and potassium efflux [25,32]. Thus, in our study, the abnormalities observed in QRS complex indicated that azithromycin may have an inhibitory efficiency/effect on the sodium channels. The effect of this agent on the sodium channels can also be evaluated in further detailed studies.
The prolongation of QT interval also triggers the T wave abnormalities [32]. Therefore, it is also possible to associate the changes in the T wave amplitude with ischemia [31,39]. In our study, the monitored increase of T wave amplitude in 30 mg/kg azithromycin-administered group was seemed to support the cardiotoxic effect of the agent, accompanied the prolongation in QT interval, and was interpreted as one of the ischemic markers in ECG.
Separately, another important point to be addressed is that when myocardium is hypertrophied, the electrical activation passes to a broader part of myocardial tissue and thus increases the amplitude of QRS complex. On the other hand, the dispersion of the electrical activity all along the heart also takes longer in the abnormally thickened myocardium, so the duration of QRS increases. In addition, the repolarization phase is affected by the myocardial hypertrophy, in which case the abnormal T wave formation can be observed [40]. It is also possible to support and associate the QRS complex and T wave abnormalities determined in our 30 mg/kg azithromycin-administered group with the results of our histopathological findings of heart tissue. It was determined that the hypertrophic processes in the myocardial cells were triggered in the histological sections of this group. The QRS complex and the significant increases in the T wave amplitude observed in the 30 mg/kg azithromycin-administered group in our study can be regarded as the indicators of the changes in the intracardiac blood flow and the weakened of the ventricular function.
In our study, it was determined that, the MDA levels were increased, whereas catalase, superoxide dismutase and glutathione levels were decreased in the azithromycin-administered groups when compared with the control group. The significant differences determined in terms of the biomarkers used in the evaluation of oxidative status indicated that oxidative stress in rats was induced by the administration of azithromycin. Oxidative stress occurs as a result of the imbalance between free radicals and antioxidants in the organism [41]. The oxidative stress increasing in the organs like liver, heart and kidneys may induce functional, biochemical and structural damage [42,43]. The reactive oxygen species in the heart, besides being generated throughout the oxidative phosphorylation process, may occur by the enzymatic reactions catalyzed by xanthine oxidase, NAD[P]H oxidases and cytochrome P450 [44]. On the other hand, it was shown that only a small amount of azithromycin, when taken orally, was metabolized and it was mainly excreted from the organism without any change (~ 75%) [5,45]. The inactive descladinose and the active 9a-N-desmethyl metabolites are frequently recovered because of acidic degradation in the gut. Also, hepatic biotransformation of this drug is marginal. Importantly, azithromycin is neither metabolized by nor an inhibitor of CYP3A4 [5].
The oxidative stress which could not be directly associated with azithromycin and/or azithromycin biotransformation was interpreted in our study as the result of an induced myocardial damage. In our study, the myocardial ischemic findings, which were supported by plasma LDH and CK-MB levels, and long QT, QRS complex and T wave anomalies, suggested the importance of the reactive oxygen species in ischemia-induced tissue damage and reperfusion injury. Ischemic heart is under oxidative stress, which is exacerbated by reperfusion. The early reperfusion under the clinical conditions of myocardial ischemia is defensive against an irreversible cardiac damage. Yet, reperfusion may lead to some harmful effects induced by oxidative stress, such as ventricular arrhythmia and contractile dysfunction. The harmful effects of oxidative stress occur due to a disruption in the antioxidant defense system and an increase in the generation/production of reactive oxygen species in the cases of ischemia-reperfusion. In this case, the changes in the cardiac functions and the functions of ion channels, ionic pumps, ion exchangers, connexin-43 remodeling and inflammatory processes have been suggested to be associated with the oxidative stress [44]. In this respect, it can be put forward in our study that the oxidative stress occurred secondarily along with the cardiac damage induced by azithromycin.
At this point, we have to mention the popularity and validity of rat models to predict cardiotoxicity. It is because of such characteristics that the rat/ mouse models are often the very first in vivo models that are the most commonly used models in basic and applied toxicology studies [46]. As a result, our study, in which we conducted independently of the cardiovascular risk factors and evaluated multiple parameters of cardiotoxicity, showed that azithromycin in repeated pharmacological doses was determined to cause toxic effects in terms of plasma cardiac biomarkers and ECG parameters. These functional changes were also suggested to be reflected by the structural changes through the histopathological analysis of the heart tissue. At this point, it can be emphasized that it is necessary to determine the presence of cardiovascular risk factors, such as gender, age, history/medical record of a cardiovascular disease, the presence of a long QT interval, hypokalemia, hypomagnesemia and bradycardia, in the patients under azithromycin treatment, since these individuals can be more sensitive to the cardiovascular toxic effects associated with azithromycin than healthy ones. Besides, during the use of azithromycin in these patients, it may be essential to arrange the correct dose, to receive the daily ECG recordings and to avoid the use of this drug with the drugs posing similar adverse effects. According to our study, it might be suggested to evaluate the effects of azithromycin on potassium, calcium and sodium channels, considering the fact that drugs cause changes in ECG by blocking these channels in myocardial cells in further studies.
Disclosure of conflict of interest
None.
References
- 1.Gaynor M, Mankin AS. Macrolide antibiotics: binding site, mechanism of action, resistance. Curr Top Med Chem. 2001;3:949–961. doi: 10.2174/1568026033452159. [DOI] [PubMed] [Google Scholar]
- 2.Grandy JK. Clinical update: Macrolides and cardiovascular death. J Pharm Pharm Sci. 2013;16:551–63. [Google Scholar]
- 3.Guo D, Cai Y, Chai D, Liang B, Bai N, Wang R. The cardiotoxicity of macrolides: a systematic review. Pharmazie. 2010;65:631–640. [PubMed] [Google Scholar]
- 4.Duran JM, Amsden GW. Azithromycin: indications for the future? Expert Opin Pharmacother. 2000;1:489–505. doi: 10.1517/14656566.1.3.489. [DOI] [PubMed] [Google Scholar]
- 5.Parnham MJ, Erakovic-Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143:225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Giudicessi JR, Ackerman MJ. Azithromycin and risk of sudden cardiac death: guilty as charged or falsely accused? Cleve Clin J Med. 2013;80:539–544. doi: 10.3949/ccjm.80a.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2013;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svanström H, Pasternak B, Hviid A. Use of Azithromycin and Death from Cardiovascular Causes. N Engl J Med. 2013;368:1704–1712. doi: 10.1056/NEJMoa1300799. [DOI] [PubMed] [Google Scholar]
- 9.Maisch NM, Kochupurackal JG, Sin J. Azithromycin and the risk of cardiovascular complications. J Pharm Pract. 2013 doi: 10.1177/0897190013516503. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Carević O, Djokić S. Comparative studies on the effects of erythromycin A and azithromycin upon extracellular release of lysosomal enzymes in inflammatory processes. Agents Actions. 1988;25:124–131. doi: 10.1007/BF01969103. [DOI] [PubMed] [Google Scholar]
- 11.Baronas ET, Lee JW, Alden C, Hsieh FY. Biomarkers to monitor drug-induced phospholipidosis. Toxicol Appl Pharmacol. 2007;218:72–78. doi: 10.1016/j.taap.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Mahgoub A, El-Medany A, Mustafa A, Arafah M, Moursi M. Azithromycin and erythromycin ameliorate the extent of colonic damage induced by acetic acid in rats. Toxicol Appl Pharmacol. 2005;205:43–52. doi: 10.1016/j.taap.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Walker DB. Serum Chemical Biomarkers of Cardiac Injury for Nonclinical Safety Testing. Toxicol Pathol. 2006;34:94–104. doi: 10.1080/01926230500519816. [DOI] [PubMed] [Google Scholar]
- 14.Wallace KB, Hausner E, Herman E, Holt GD, MacGregor JT, Metz AL, Murphy E, Rosenblum IY, Sistare FD, York MJ. Serum troponins as biomarkers of drug-induced cardiac toxicity. Toxicol Pathol. 2004;32:106–121. doi: 10.1080/01926230490261302. [DOI] [PubMed] [Google Scholar]
- 15.Tonomura Y, Mori Y, Torii M, Uehara T. Evaluation of the usefulness of biomarkers for cardiac and skeletal myotoxicity in rats. Toxicology. 2009;266:48–54. doi: 10.1016/j.tox.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Coloma PM, Schuemie MJ, Trifirò G, Furlong L, van Mulligen E, Bauer-Mehren A, Avillach P, Kors J, Sanz F, Mestres J, Oliveira JL, Boyer S, Helgee EA, Molokhia M, Matthews J, Prieto-Merino D, Gini R, Herings R, Mazzaglia G, Picelli G, Scotti L, Pedersen L, van der Lei J, Sturkenboom M. EU-ADR consortium. Drug-induced acute myocardial infarction: identifying ‘prime suspects’ from electronic healthcare records-based surveillance system. PLoS One. 2013;8:e72148. doi: 10.1371/journal.pone.0072148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilelli JA, Smith KM, Pettignano R. Life-threatening bradyarrhythmia after massive azithromycin overdose. Pharmacotherapy. 2006;26:147–150. doi: 10.1592/phco.2006.26.1.147. [DOI] [PubMed] [Google Scholar]
- 18.Santos N, Oliveira M, Galrinho A, Oliveira JA, Ferreira L, Ferreira R. QT interval prolongation and extreme bradycardia after a single dose of azithromycin. Ev Port Cardiol. 2010;29:139–142. [PubMed] [Google Scholar]
- 19.Ovsyshcher IE, Barold SS. Drug induced bradycardia: to pace or not to pace? Pacing Clin Electrophysiol. 2004;27:1144–1147. doi: 10.1111/j.1540-8159.2004.00597.x. [DOI] [PubMed] [Google Scholar]
- 20.Kappenberger L, Linde C, Toff WD. Chapter 27: Bradycardia. Oxford Medicine; 2006. pp. 807–830. [Google Scholar]
- 21.Yap YG, Camm AJ. Drug induced qt prolongation and torsades de pointes. Heart. 2003;89:1363–72. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Ryu HM, Bae MH, Kwon YS, Lee JH, Park Y, Heo JH, Lee YS, Yang DH, Park HS, Cho Y, Chae SC, Kim YN, Jun JE, Park WH. Prognosis and natural history of drug-related bradycardia. Korean Circ J. 2009;39:367–371. doi: 10.4070/kcj.2009.39.9.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sameni R, Clifford GD. A review of fetal ecg signal processing; issues and promising directions. Open Pacing Electrophysiol Ther J. 2010;3:4–20. doi: 10.2174/1876536X01003010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller N. Cardiotoxicity and Drug Development. Beremans Limited; 2005. Fair of face, full of hear. [Google Scholar]
- 25.Lionte C, Bologa C, Sorodoc L. Toxic and Drug-Induced Changes of the Electrocardiogram. In: Millis RM, editor. Advances in Electrocardiograms-Clinical Applications. Shanghai: Intech; 2012. pp. 271–296. [Google Scholar]
- 26.Ohtani H, Taninaka C, Hanada E, Kotaki H, Sato H, Sawada Y, Iga T. Comparative pharmacodynamic analysis of Q-T interval prolongation induced by the macrolides clarithromycin, roxithromycin, and azithromycin in rats. Antimicrob Agents Chemother. 2000;44:2630–2637. doi: 10.1128/aac.44.10.2630-2637.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blondeau JM. The evolution and role of macrolides in infectious diseases. Expert Opin Pharmacother. 2002;3:1131–1151. doi: 10.1517/14656566.3.8.1131. [DOI] [PubMed] [Google Scholar]
- 28.Aro AL, Anttonen O, Kerola T, Junttila MJ, Tikkanen JT, Rissanen HA, Reunanen A, Huikuri HV. Prognostic significance of prolonged PR interval in the general population. Eur Heart J. 2014;35:123–129. doi: 10.1093/eurheartj/eht176. [DOI] [PubMed] [Google Scholar]
- 29.Braam SR, Tertoolen L, van de Stolpe A, Meyer T, Passier R, Mummery CL. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010;4:107–116. doi: 10.1016/j.scr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Namboodiri N. Bradycardia-induced torsade de pointes-an arrhythmia less understood. Indian Pacing Electrophysiol J. 2010;10:435–438. [PMC free article] [PubMed] [Google Scholar]
- 31.Jiménez-Candil J, Martín-Luengo C. QT interval and acute myocardial ischemia: past promises, new evidences. Rev Esp Cardiol. 2008;61:561–563. doi: 10.1157/13123059. [DOI] [PubMed] [Google Scholar]
- 32.Holstege CP, Eldridge DL, Rowden AK. ECG manifestations: the poisoned patient. Emerg Med Clin North Am. 2006;24:159–77. doi: 10.1016/j.emc.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Homoud MK. Introduction to Electrocardiography: The Genesis and Conduction of Cardiac Rhythm. Tufts-New England Medical Center. 2008 [Google Scholar]
- 34.Thomsen MB, Beekman JD, Attevelt NJ, Takahara A, Sugiyama A, Chiba K, Vos MA. No proarrhythmic properties of the antibiotics moxifloxacin or azithromycin in anaesthetized dogs with chronic-AV block. Br J Pharmacol. 2006;149:1039–48. doi: 10.1038/sj.bjp.0706900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wymore RS, Gintant GA, Wymore RT, Dixon JE, McKinnon D, Cohen IS. Tissue and species distribution of mRNA for the IKr-like K+ channel, erg. Circ Res. 1997;80:261–8. doi: 10.1161/01.res.80.2.261. [DOI] [PubMed] [Google Scholar]
- 36.Karmakar S, Padman A, Swamy Mane N, Sen T. Hypokalemia: a potent risk for QTc prolongation in clarithromycin treated rats. Eur J Pharmacol. 2013;709:80–84. doi: 10.1016/j.ejphar.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 37.Hancox JC, Hasnain M, Vieweg WV, Crouse EL, Baranchuk A. Azithromycin, cardiovascular risks, QTc interval prolongation, torsade de pointes, and regulatory issues: A narrative review based on the study of case reports. Ther Adv Infect Dis. 2013;1:155–65. doi: 10.1177/2049936113501816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDermott JS, Salmen HJ, Cox BF, Gintant GA. Importance of species selection in arrythmogenic models of Q-T interval prolongation. Antimicrob Agents Chemother. 2002;46:938–939. doi: 10.1128/AAC.46.3.938-939.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gambill CL, Wilkins ML, Haisty WK, Anderson ST, Maynard C, Wagner NB, Selvester RH, Wagner GS. T wave amplitudes in normal populations. Variation with ECG lead, sex, and age. J Electrocardiol. 1995;28:191–197. doi: 10.1016/s0022-0736(05)80257-2. [DOI] [PubMed] [Google Scholar]
- 40. http://www.learntheheart.com/ecg-review/ecg-topic-reviews-and-criteria/left-ventricular-hypertrophy-review/
- 41.Doshi SB, Khullar K, Sharma RK, Agarwal A. Role of reactive nitrogen species in male infertility. Reprod Biol Endocrinol. 2012;10:109. doi: 10.1186/1477-7827-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva FM, Marques A, Chaveiro A. Reactive oxygen species: a double-edged sword in reproduction. The Open Vet Sci J. 2010;4:127–133. [Google Scholar]
- 43.Deavall DG, Martin EA, Horner JM, Roberts R. Drug-induced oxidative stress and toxicity. J Toxicol. 2012;2012:645460. doi: 10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leiris J, Rakotovao A, Boucher F. Oxidative stress and ischemia. Heart Metab. 2008;31:5–7. [Google Scholar]
- 45.Hunter RP, Koch DE, Coke RL, Goatley MA, Isaza R. Azithromycin metabolite identification in plasma, bile, and tissues of the ball python (Python regius) J Vet Pharmacol Ther. 2003;26:117–121. doi: 10.1046/j.1365-2885.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 46.Farraj AK, Hazari MS, Cascio WE. The utility of the small rodent electrocardiogram in toxicology. Toxicol Sci. 2011;121:11–30. doi: 10.1093/toxsci/kfr021. [DOI] [PubMed] [Google Scholar]


