Abstract
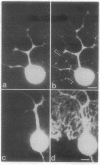
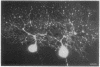
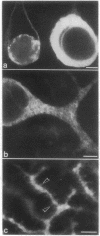
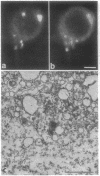
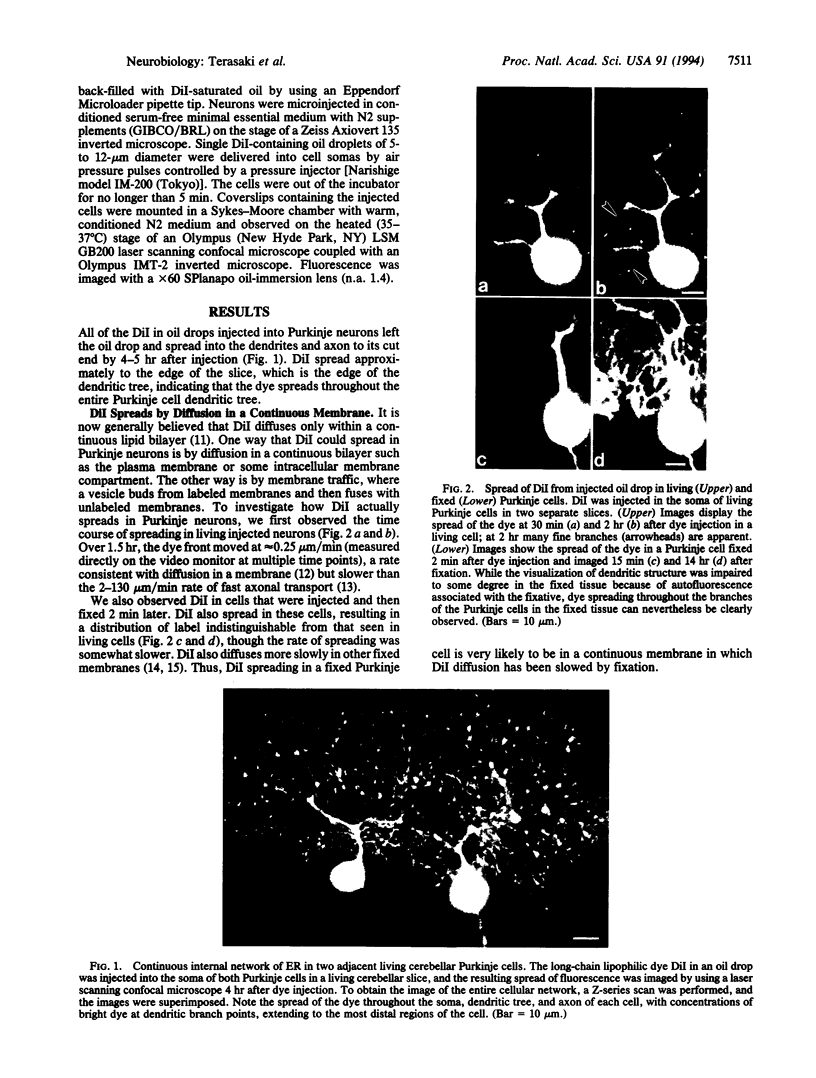
Purkinje neurons in rat cerebellar slices injected with an oil drop saturated with 1,1'-dihexadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate [DiIC16(3) or DiI] to label the endoplasmic reticulum were observed by confocal microscopy. DiI spread throughout the cell body and dendrites and into the axon. DiI spreading is due to diffusion in a continuous bilayer and is not due to membrane trafficking because it also spreads in fixed neurons. DiI stained such features of the endoplasmic reticulum as densities at branch points, reticular networks in the cell body and dendrites, nuclear envelope, spines, and aggregates formed during anoxia nuclear envelope, spines, and aggregates formed during anoxia in low extracellular Ca2+. In cultured rat hippocampal neurons, where optical conditions provide more detail, DiI labeled a clearly delineated network of endoplasmic reticulum in the cell body. We conclude that there is a continuous compartment of endoplasmic reticulum extending from the cell body throughout the dendrites. This compartment may coordinate and integrate neuronal functions.
Full text
PDF
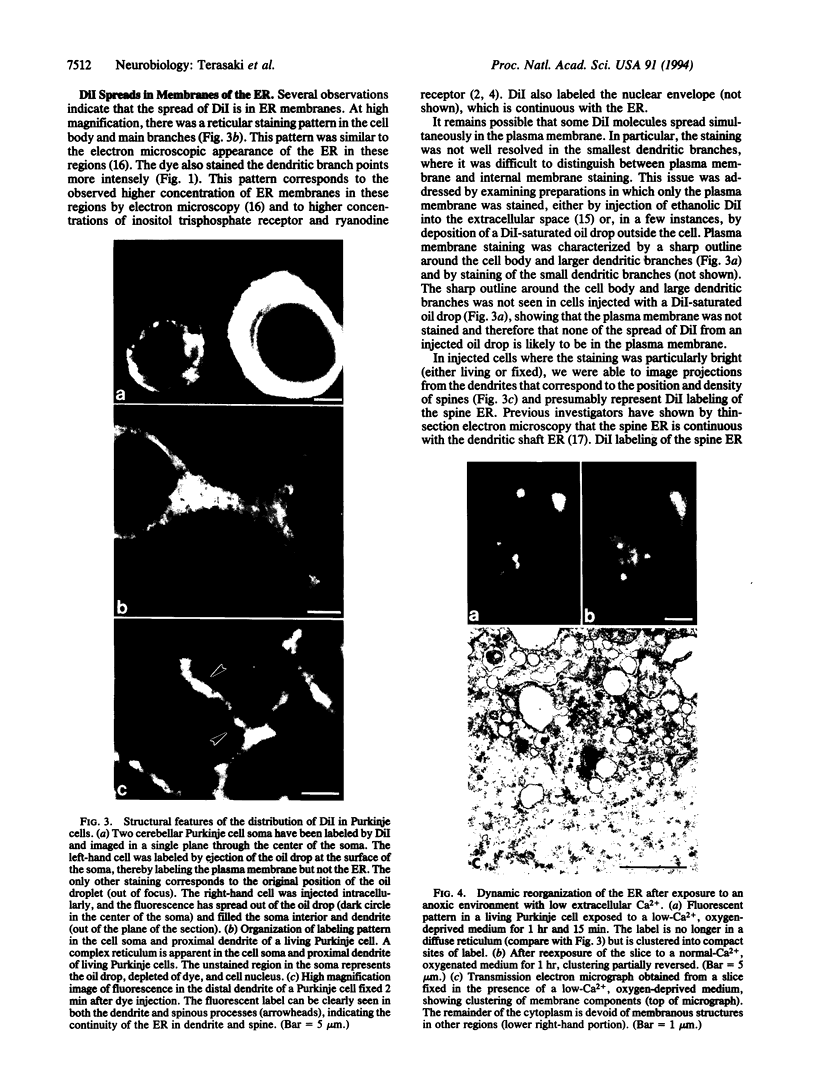


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellisman M. H., Deerinck T. J., Ouyang Y., Beck C. F., Tanksley S. J., Walton P. D., Airey J. A., Sutko J. L. Identification and localization of ryanodine binding proteins in the avian central nervous system. Neuron. 1990 Aug;5(2):135–146. doi: 10.1016/0896-6273(90)90304-x. [DOI] [PubMed] [Google Scholar]
- Feng J. J., Carson J. H., Morgan F., Walz B., Fein A. Three-dimensional organization of endoplasmic reticulum in the ventral photoreceptors of Limulus. J Comp Neurol. 1994 Mar 8;341(2):172–183. doi: 10.1002/cne.903410204. [DOI] [PubMed] [Google Scholar]
- Garthwaite G., Hajos F., Garthwaite J. Morphological response of endoplasmic reticulum in cerebellar Purkinje cells to calcium deprivation. Neuroscience. 1992;48(3):681–688. doi: 10.1016/0306-4522(92)90411-t. [DOI] [PubMed] [Google Scholar]
- Godement P., Vanselow J., Thanos S., Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987 Dec;101(4):697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- Grafstein B., Forman D. S. Intracellular transport in neurons. Physiol Rev. 1980 Oct;60(4):1167–1283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- Harris K. M., Stevens J. K. Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1988 Dec;8(12):4455–4469. doi: 10.1523/JNEUROSCI.08-12-04455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig M. G., Hume R. I. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol. 1986 Jul;103(1):171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. A., Terasaki M. Structural changes of the endoplasmic reticulum of sea urchin eggs during fertilization. Dev Biol. 1993 Apr;156(2):566–573. doi: 10.1006/dbio.1993.1103. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. The role of ionic currents in establishing developmental pattern. Philos Trans R Soc Lond B Biol Sci. 1981 Oct 7;295(1078):553–566. doi: 10.1098/rstb.1981.0160. [DOI] [PubMed] [Google Scholar]
- Lindsey J. D., Ellisman M. H. The neuronal endomembrane system. III. The origins of the axoplasmic reticulum and discrete axonal cisternae at the axon hillock. J Neurosci. 1985 Dec;5(12):3135–3144. doi: 10.1523/JNEUROSCI.05-12-03135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martone M. E., Zhang Y., Simpliciano V. M., Carragher B. O., Ellisman M. H. Three-dimensional visualization of the smooth endoplasmic reticulum in Purkinje cell dendrites. J Neurosci. 1993 Nov;13(11):4636–4646. doi: 10.1523/JNEUROSCI.13-11-04636.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyja E., Kida E. Protective effect of the calcium entry blocker nimodipine on hippocampal rat cultures submitted to anoxia. Neuropatol Pol. 1990;28(1-2):25–39. [PubMed] [Google Scholar]
- Rambourg A., Droz B. Smooth endoplasmic reticulum and axonal transport. J Neurochem. 1980 Jul;35(1):16–25. doi: 10.1111/j.1471-4159.1980.tb12484.x. [DOI] [PubMed] [Google Scholar]
- Remis T., Benuska J., Binovský A., Masárová M. The ultrastructural picture of cerebral cortex of rat after hypoxia. I. Findings after inhalation of 5% O2 and 95% N2. Z Mikrosk Anat Forsch. 1989;103(2):297–308. [PubMed] [Google Scholar]
- Ross C. A., Meldolesi J., Milner T. A., Satoh T., Supattapone S., Snyder S. H. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989 Jun 8;339(6224):468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Samoilov M. O., Vorob'ev V. S., Malunova L. B. Ul'trastrukturnye izmeneniia kory bol'shikh polusharii golovnogo mozga koshki cherez 30-60 min posle anoksii. Tsitologiia. 1987 Sep;29(9):1027–1031. [PubMed] [Google Scholar]
- Satoh T., Ross C. A., Villa A., Supattapone S., Pozzan T., Snyder S. H., Meldolesi J. The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: quantitative immunogold labeling reveals concentration in an ER subcompartment. J Cell Biol. 1990 Aug;111(2):615–624. doi: 10.1083/jcb.111.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O., Reeves T. M. Protein-synthetic machinery beneath postsynaptic sites on CNS neurons: association between polyribosomes and other organelles at the synaptic site. J Neurosci. 1988 Jan;8(1):176–184. doi: 10.1523/JNEUROSCI.08-01-00176.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K., Stukenbrok H., Metcalf A., Mignery G. A., Südhof T. C., Volpe P., De Camilli P. Ca2+ stores in Purkinje neurons: endoplasmic reticulum subcompartments demonstrated by the heterogeneous distribution of the InsP3 receptor, Ca(2+)-ATPase, and calsequestrin. J Neurosci. 1992 Feb;12(2):489–505. doi: 10.1523/JNEUROSCI.12-02-00489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M., Jaffe L. A. Organization of the sea urchin egg endoplasmic reticulum and its reorganization at fertilization. J Cell Biol. 1991 Sep;114(5):929–940. doi: 10.1083/jcb.114.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Podini P., Clegg D. O., Pozzan T., Meldolesi J. Intracellular Ca2+ stores in chicken Purkinje neurons: differential distribution of the low affinity-high capacity Ca2+ binding protein, calsequestrin, of Ca2+ ATPase and of the ER lumenal protein, Bip. J Cell Biol. 1991 May;113(4):779–791. doi: 10.1083/jcb.113.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton P. D., Airey J. A., Sutko J. L., Beck C. F., Mignery G. A., Südhof T. C., Deerinck T. J., Ellisman M. H. Ryanodine and inositol trisphosphate receptors coexist in avian cerebellar Purkinje neurons. J Cell Biol. 1991 Jun;113(5):1145–1157. doi: 10.1083/jcb.113.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Otsu H., Yoshimori T., Maeda N., Mikoshiba K., Tashiro Y. Stacks of flattened smooth endoplasmic reticulum highly enriched in inositol 1,4,5-trisphosphate (InsP3) receptor in mouse cerebellar Purkinje cells. Cell Struct Funct. 1991 Oct;16(5):419–432. doi: 10.1247/csf.16.419. [DOI] [PubMed] [Google Scholar]