Abstract
Aims: This study is to determine the effect of astragalus and salvia extract on the alteration of myocardium in a rat model of myocardial infarction. Methods: A total of 40 male Sprague-Dawley rats were randomly divided into the sham-operated group, the control group, the Astragalus group, the Salvia group, and the compatibility of Astragalus and Salvia and group. The cardiac functions were determined at 8 weeks after treatment. Hematoxylin-eosin staining was performed to observe the morphology and arrangement of cardiomyocytes. Masson’s trichrome staining was performed to investigate the distribution of myocardial interstitial collagen. Immunohistochemical staining was performed to determine the expression ofprotein kinase D1 in myocardial tissues. Results: In the sham-operated group, the Astragalus group, the Salvia group, and the compatibility of Astragalus and Salvia group, the left ventricular systolic pressure and the maximum rate of left ventricular pressure were significantly increased while the left ventricular end diastolic pressure were significantly decreased when compared with those in the control group (P < 0.05). Normal morphology and arrangement of cardiomyocytes were maintained in the compatibility of Astragalus and Salvia group. Contents of collagen fibers in myocardial tissues were decreased in the compatibility of Astragalus and Salvia group (P < 0.05). Expression levels of protein kinase D1 were significantly decreased in cardiomyocytes of the compatibility of Astragalus and Salvia group. Conclusions: Compatibility of Astragalus and Salvia extract may inhibit myocardial fibrosis and ventricular remodeling by regulation of protein kinase D1 protein in a rat model of myocardial infarction.
Keywords: Astragalus, salvia, compatibility, myocardial infarction, protein kinase D
Introduction
Myocardial infarction (MI), one of coronary artery disease, remains a leading cause of morbidity and mortality worldwide [1]. MI occurs when myocardial ischemia, which is caused by a blood clot stopping the blood flowing in myocardium [2]. Systemic thrombolysis and percutaneous coronary angioplasty have been used to ameliorate MI, which leads to the re-establishment of blood flow in the cardiac zones affected by the occlusion of a branch of the coronary artery [3]. Nevertheless, as a consequence of this procedure, the ischemia-reperfusion event increases production of reactive oxygen. The reactive oxygen attacks biomolecules such as lipids, DNA, and proteins, triggering cell death pathways [4]. Although a number of strategies have beenimplemented to ameliorate lethal reperfusion injury, the beneficial effects are less obvious in clinical settings. However, traditional Chinese medicine theory has proven to be especially effective in treating MI in the past 10 years. Promotion and activating blood circulation to remove blood stasis is one of the main treatment strategies in ameliorating MI [5,6]. According to Chinese medicine theory, the extract of Salvia can promote blood flow and resolves blood stasis [7]. The extract of Astragalus has effects on promoting angiogenesis and protecting endothelial function [8]. Therefore, compatibility of Salvia and Astragalus extract are commonly used in Chinese medicine to treat cardiovascular disease, such as angina pectoris, MI, hyperlipidemia [9]. However, the molecular mechanism of Salvia and Astragalus extract for the treatment of MI is unknown.
Protein kinase D (PKD), a member of serine/threonine kinases, plays an important role in intercellular signal transduction, especially in the signaling pathway of MI and cardiac hypertrophy [10]. PKD family consists of 3 isoforms (PKD1, PKD2, and PKD3). PKD regulates the phosphorylation of cardiac myofilaments, which is related to MI and cardiomyocyte hypertrophy [11]. In particular, the molecular mechanism of PKD1-mediated signaling pathways in the regulation of cardiac contractility, hypertrophy and remodeling has been fully elucidated [11-14]. In this study, expression of PKD1 in cardiomyocytes treated with Salvia and Astragalus extract has been determined. Furthermore, effects of compatibility of Salvia and Astragalus extract on cardiac remodeling have been studied.
Methods and materials
Reagents
The extract of Salvia purified from the dried roots of Salvia miltiorrhiza Bge. (containing 66.9% astragaloside) and the extract of Astragalus purified from the dried roots or stems of Astragalus mongholicus Bge (containing 63.7% salvianolic acid) was provided from Key Laboratory of Zhang Zhongjing Prescription, Nanyang Institute of Technology. The rabbit anti-rat PKD1 monoclonal antibodies were purchased from GIBCO BRL (Rockville, MD, USA). 3, 3’-Diaminobenzidine chromogenic reagent and biotined goat anti-rabbit IGg antibodies were purchased from Boster Co., Ltd (Wuhan, China).
Experimental animals
A total of 40 male Sprague Dawley rats, weighed 200-250 g, were obtained from Experimental Animal Center of Henan Province (Zhengzhou, Henan, China). They were kept in standard conditions with free access to food and water. All animal experiments were conducted according to the ethical guidelines of Nanyang Institute of Technology.
Establishment of MI rat model
A rat model of MI was established as previously described [2]. Briefly, male Sprague-Dawley rats (200 g to 240 g) were intraperitoneally anesthetized with pentobarbital (70 mg/kg), intubated, and connected to a respirator. The heart was exposed using an angular incision on the left side of the thoracic cavity. The pericardium was opened and ligation was performed on the left anterior descending branch of the coronary artery. Then, the thoracic cavity was closed. Sham-operated rats were threaded in the corresponding parts of the left anterior descending coronary artery instead of ligation (sham-operated group, n = 8). At 48 h after establishment of the MI rat model, survival rats were randomly divided into the control group (n = 8), the Astragalus group (n = 8), the Salvia group (n = 8), and the compatibility of Astragalus and Salvia group (n = 8). For the control group and the sham-operated group, rats were given via oral gavage with normal saline at the dosage of 20 ml/kg once a day for 8 weeks. Rats in the Astragalus group and the Salvia group were given via oral gavage with 20 mg/kg Astragalus extract and Salvia extract once a day for 8 weeks, respectively. Rats in the compatibility of Astragalus and Salvia group were given via oral gavage with 20 mg/kg compatibility of Astragalus and Salvia extract (1:1) once a day for 8 weeks.
Cardiac function analysis
Rats were anesthetized in enterocoelia with 1% pentobarbital sodium (40 mg/kg) to analyze the cardiac functions at 8 weeks after treatment. Carotid artery was separated and ligation was performed at the distal end of heart. A PE-10 catheter (0.8 mm) (Becton Dickinson, Mountain View, CA, USA) connected to a pressure transducer was inserted retrograde from the carotid artery to the left ventricular cavity. Left ventricular systolic pressure (LVSP), left ventricular end diastolic pressure (LVEDP), and the maximum rate of left ventricular pressure were detected by the multimedia biological signal acquisition and analysis system (Becton Dickinson, Mountain View, CA, USA).
Hematoxylin-eosin staining
Hematoxylin-eosin staining was performed in the apex tissues of the heart. Briefly, apex tissues of the heartafter surgery were rapidly frozen by liquid nitrogen. After being fixed in 10% formaldehyde and embedded in paraffin, the embedded tissue were cut into sections with a thickness of 4 μm at the midpoint of left ventricle long axis.After washing with running water and distilled water, sections were stained with hematoxylin for 3-5 min. Sections were washed with running water and differentiated with 1% HCl in 70% alcohol. Then sections were stained with eosin for 1-4 min after washing with running water. After dehydration and differentiation in alcohol, sections were mounted and observed under Nikon Ti-soptical microscope (Nikon Corporation, Tokyo, Japan).
Masson’s trichrome staining and collagen volume fraction (CVF) determination
Myocardial collagen could be stained by Masson’s trichrome staining. Apex tissues of the heart after surgery were rapidly frozen by liquid nitrogen. After being fixed in 10% formaldehyde and embedded in paraffin, the embedded tissue were cut into sections with a thickness of 4 μM at the midpoint of left ventricle long axis. The sections were dewaxed, dehydrated in graded alcohols and stained by hematoxylin for 3 min. After washing with running water, sections were differentiated in a 1% hydrochloric acid alcohol solution. Sections were then stained in warm Ponceau-acid fuchsin solution for 3 min, washed with distilled water, and differentiated in a 1% phosphomolybdic acid solution for 1 min. After wiping the phosphomolybdic acid residue from the slides, the sections were stained in 2%aniline blue solutionfor 1 min. Sections were dehydrated in graded alcohols, dried with cold air, and mounted in neutral resin. Changes in myocardial interstitial collagen were observed by HMIAS-2000 Imaging System (Champion Medical Imaging Co., Wuhan, China). Cardiac fibrosis was quantified bymeasuring the blue fibrotic area.CVF referred to the ratio of the area of collagen relative to the total area of the image. Five fields at high-magnification (× 400) were randomly taken and areas of collagen were calculated.
Immunohistochemical staining
Apex tissues of the heart after surgery were rapidly frozen by liquid nitrogen. After being fixed in 10% formaldehyde and embedded in paraffin, the embedded tissue were cut into sections with a thickness of 4 μM at the midpoint of left ventricle long axis. To inactivate endogenous peroxidase, 3% fresh prepared hydrogen peroxide was added at room temperature for 15 min. After washing with distilled water, the antigen was repaired by citrate buffer (pH 6.0) with microwave heating. The sections were washed with PBS and blocked with serum blocking solution for 20 min. Then the primary rabbit anti-rat PKD1 monoclonal antibodies (dilution, 1:100) were added and incubated at 4°C in dark overnight. The secondary biotined goat anti rabbit IgG was added for a 20 min incubation at room temperature. After incubation with strept avidin-biotin complex the sections were developed with 3, 3’-Diaminobenzidine chromogenic reagent. Sections were counterstained with haematoxylin. After hydrochloric acid differentiation and dimethylbenzene transparency, sections were mountedwithneutral gum. Cells with brown staining were defined as PKD1-positive. Ten fields at high-magnification (× 400) were randomly taken from every section.
Mean optical density from every field was analyzed by HMIAS-2000 Imaging System.
Real-time RT-PCR
Total RNA was isolated from heartsusing Trizol Reagent (Invitrogen) and was digested with DNase.cDNA synthesis was carried out using Super Script First-Strand Synthesis System for RT-PCR (Invitrogen). Real-time PCR was performed using SYBR green (Applied Biosystems) as dyes according to the kit. After standard curve was based on GAPDH mRNA expression, mRNA expression level in each sample was determined by the classic ∆Ct method. Meanwhile, GAPDH mRNA was used to normalize the expression level of PKD1 gene. The transcriptional variation of mRNA levels was then calculated for PKD1 gene in each groups. A mean ratio of 2 was taken as the cutoff of statistical significance.
Statistical analysis
All results were expressed as mean ± standard deviation. Gene expression was normalized to GAPDHmRNA and calculated as fold change over the sham-treated group. Statistical analysis was performed using one way ANOVA followed by post-hocmultiple comparisons test. Analysis of variance was used to analyze comparisons between groups. P value less than 0.05 was considered to be significantly different. All statistical analyses were performed with Excel and SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
The systolic and diastolic functions are improved in the compatibility of Astragalus and Salvia group
During the 8 weeks treatment, 3 rats was dead, including 2 rats in the control group and 1 rat in the Astragalus group. Rats in the control group were loss of appetite and looked tired. The gloss on the fur was decreased. Rats in the Astragalus group and the Salvia group were active and generally better than before. Rats in the sham-operated group were normal in appetite.
The systolic and diastolic functions can be reflected by the values of LVSP and LVEDP. The changes in left ventricular pressure can be reflected by the maximum rate of the left ventricular pressure. To investigate effect of Astragalus and Salvia on the cardiac functions in MI rats, cardiac function analysis was performed to measure the values of LVSP, LVEDP, and the maximum rate of left ventricular pressure. As shown in Table 1, LVSP and the maximum rate of left ventricular pressure in the sham-operated group, the Astragalus group, the Salvi-a group, and the compatibility of Astragalus and Salvia group were significantly increased when compared with those in the control group (P < 0.05). However, LVEDP in the sham-operated group, the Astragalus group, the Salvia group, and the compatibility of Astragalus and Salvia group were significantly decreased when compared with those in the control group (P < 0.05). LVSP and the maximum rate of left ventricular pressure in the compatibility of As-tragalus and Salvia group were significantly increased when compared with those in the Salvia group (P < 0.05). These results indicate that the systolic and diastolic functions are improved in the com-patibility of Astragalus and Salvia group when compared with those in the control group.
Table 1.
Hemodynamic changes of rats with MI (mean ± SD)
| Groups | Cases (n) | LVSP (mmHg) | LVEDP (mmHg) | + dp/dt max (mmHg/s) | - dp/dt max (mmHg/s) |
|---|---|---|---|---|---|
| Control | 6 | 92.3 ± 5.7 | 28.7 ± 6.9 | 2.8 ± 0.4 | 3.1 ± 0.3 |
| Sham-operated | 8 | 138.6 ± 4.3* | 4.5 ± 0.4* | 6.6 ± 0.2* | 6.8 ± 0.5* |
| Astragalus | 7 | 132.3 ± 5.2* | 14.8 ± 0.5*,# | 5.8 ± 0.7* | 5.7 ± 0.3* |
| Salvia | 8 | 124.2 ± 4.9* | 7.5 ± 0.2* | 4.4 ± 0.6* | 4.8 ± 0.2* |
| Compatibility of Astragalus and Salvia | 8 | 145.6 ± 6.4*,# | 6.3 ± 0.6*,# | 6.2 ± 0.3*,# | 6.1 ± 0.2*,# |
Note: + dp/dt max, the maximum rate of rise; - dp/dt max, the maximum rate of fall;
P < 0.05 vs. control group;
P < 0.05 vs. Salvia group.
Normal morphology and arrangement of cardiomyocytes can be maintained in the compatibility of Astragalus and Salvia group
To investigate the effects of Astragalus and Salvia in the myocardial cells of MI rats, hematoxylin-eosin staining was performed to observe the morphology and ar-rangement of cardiomyocytes. As shown in Figure 1A, cardiomyocytes in the sham-operated group was neatly arranged and well-organized. Smaller intercellular spaces and a small number of fibroblasts were showed in the field of the sham-operated group. However, in the control group, disordered arrangement, nucleolysis, blurred boundaries, and inflammatory cell infiltration occurred in the cardiomyocytes. In addition, fibroblasts were increased (Figure 1B). For the Astragalus group and the Salvia group, larger nucleus, less fibroblast, less inflammatory cells and slightly blurred boundaries occurred when compared with those in the control group (Figure 1C, 1D). The morphology of cardiomyocytes in the compatibility of Astragalus and Salvia group was similar to that in the sham-operated group. The cell boundaries were still clear. No significant fibroblasts proliferation and inflammatory cells were showed in the compatibility of Astragalus and Salvia group (Figure 1E). These results suggest that the normal morphology and arrangement of cardiomyocytes can be maintained in the compatibility of Astragalus and Salvia group.
Figure 1.
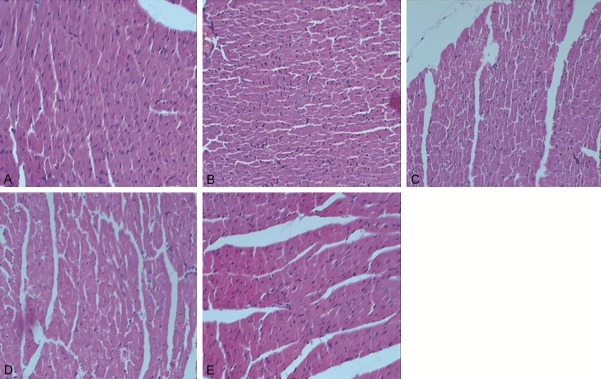
Morphology and arrangement of cardiomyocytes. Hematoxylin-eosin staining was performed to observe the morphology and arrangement of cardiomyocytes. Morphology and arrangement of cardiomyocytes in the sham-operated group (A), the control group (B), the Astragalus group (C), the Salvia group (D), and the compatibility of Astragalus and Salvia group (E) were visualized by an optical microscope at high magnification (× 400).
Collagen fibers in myocardial tissues are decreased in the compatibility of Astragalus and Salvia group
To investigate the distribution of myocardial interstitial collagen, Masson’s trichrome staining was performed to observe the sections of myocardial tissues using an ordinary optics microscope. Myocardial interstitial collagen in the sham-operated group was rarely detectible. Regular arrangement of the cardiomyocytes in myocardial tissues was shown (Figure 2A). However, for the control group, a large area of myocardial tissues was substituted by interstitial collagen. Collagen fiber network was ruptured and disorganized (Figure 2B). For the Astragalus group, the content of interstitial collagen was increased whereas the arrangement of cardiomyocytes was regularly organized (Figure 2C). For the Salvia group, collagen fibers were intermingled with myocardial tissues (Figure 2D). The distribution of collagen in the compatibility of Astragalus and Salvia group was similar to that in the sham-operated group. However, some visible fractures were detectible in the section of myocardial tissues (Figure 2E). As shown in Table 2, CVF value in the control group was significantly increased when compared with that in the sham-operated group (P < 0.01). CVF values in the Astragalus group, the Salvia group, and the compatibility of Astragalus and Salvia group were significantly decreased when compared with those in the control group. These results indicate that contents of collagen fibers in myocardial tissues are decreased in the compatibility of Astragalus and Salvia group.
Figure 2.
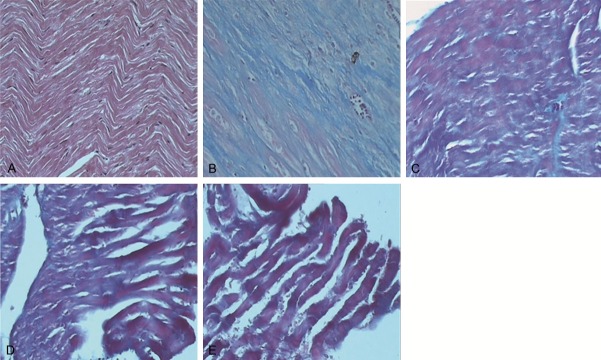
Distribution of myocardial interstitial collagen. Masson’s trichrome staining was performed to observe the distribution of collagen in myocardial tissues. Cardiomyocytes were stained to be red and collagen was stained to be blue. Collagen distributions in myocardial tissues of the sham-operated group (A), the control group (B), the Astragalus group (C), the Salvia group (D), and the compatibility of Astragalus and Salvia group (E) were analyzed by HMIAS-2000 Imaging System (× 400).
Table 2.
CVF values in myocardial tissues of rats with MI (mean ± SD)
| Groups | Cases (n) | CVF |
|---|---|---|
| Control | 6 | 24.47 ± 3.87 |
| Sham-operated | 8 | 2.38 ± 0.28** |
| Astragalus | 7 | 15.57 ± 2.99* |
| Salvia | 8 | 12.12 ± 2.64* |
| Compatibility of Astragalus and Salvia | 8 | 8.02 ± 1.97** |
P < 0.05 vs. control group;
P < 0.05 vs. control group.
PKD1 expression levels are significantly decreased in cardiomyocytes of compatibility of Astragalus and Salvia group
To determine the expression ofPKD1 in myocardial tissues of rats with MI, immunohistochemical staining was performed. Cells with brown staining were defined as PKD1-positive. Immunohistochemical staining results were shown in Figure 3 and Table 3. Positive granules were rarely detectable in cytoplasm of the sham-operated group (Figure 3A). However, in the control group, the brown-positive granules were evenly distributed in cytoplasm of myocardial cells (Figure 3B). For the Astragalus group and the Salvia group, PKD1 expression was clear but lower than that in the control group (Figure 3C, 3D). PKD1 expression in cardiomyocytes of the compatibility of Astragalus and Salvia group was further decreased (Figure 3E). As shown in Table 3, the expression levels of PKD1 in the control group were significantly increased when compared with those in the sham-operated group (P < 0.05). PKD1 expression levels in the Astragalus group and the Salvia group were decreased when compared with those in the control group (P < 0.05). Furthermore, the expression levels of PKD1 in the compatibility of Astragalus and Salvia group was decreased when compared with that in the Astragalus group and the Salvia group (P < 0.05). These results suggest that the expression levels of PKD1 are significantly decreased in cardiomyocytes of compatibility of Astragalus and Salvia group.
Figure 3.
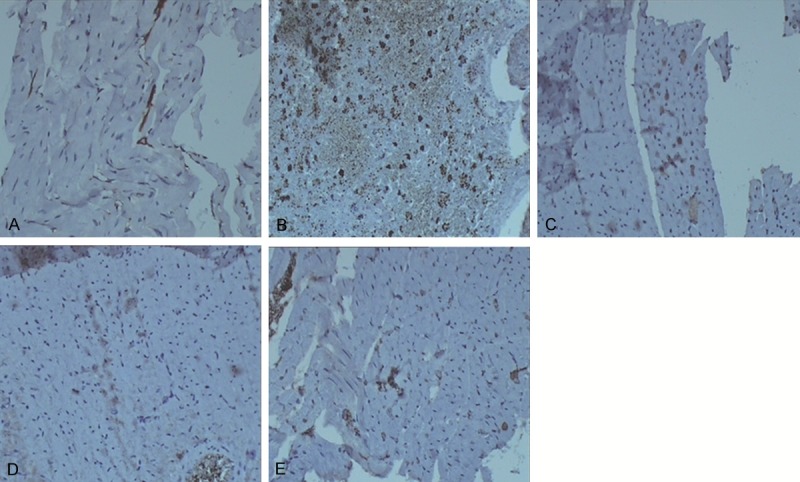
Expression of PKD1 in myocardial tissues. Immunohistochemical staining was performed to investigate the expression of PKD1 in cardiomyocytes. Cells stained brown were PKD1-positive.The expression of PKD1 in cardiomyocytes of the sham-operated group (A), the control group (B), the Astragalus group (C), the Salvia group (D), and the compatibility of Astragalus and Salvia group (E) were visualized by an optical microscope (× 100).
Table 3.
Expression levels of PKD1 in myocardial tissues of rats with MI (mean ± SD)
| Groups | Cases (n) | PKD1 expression levels |
|---|---|---|
| Control | 6 | 564.7 ± 28.87 |
| Sham-operated | 8 | 36.66 ± 2.76* |
| Astragalus | 7 | 124.46 ± 16.27*,# |
| Salvia | 8 | 112.35 ± 13.88*,# |
| Compatibility of Astragalus and Salvia | 8 | 60.08 ± 9.97* |
P < 0.05 vs. control group;
P < 0.05 vs. compatibility of Astragalus and Salvia group.
To further verify the specificity of immunohistochemical staining, real-time quantitative RTPCR was employed to confirm the above data. The resulting transcriptional ratio from real-time RT-PCR analysis was logarithm-transformed and then p-lotted against the average log2 ratio values obtained by immunohistochemical staining analysis. As shown in Figure 4, there was a strong positive correlation (R2 = 0.999) between the two techniques. There sult above confirmed the reliability of immunohistochemical staining data.
Figure 4.
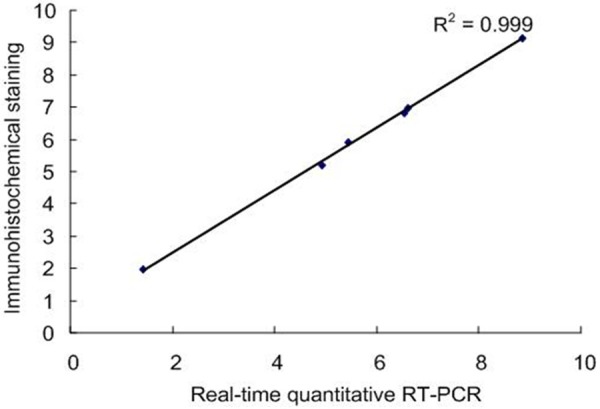
Validation of immunohistochemical staining data by real-time RT-PCR assays. The relative transcriptional levels for PKD1 gene was determined by real-time RT-PCR. The real-time RT-PCR log2 values were plotted against the immunohistochemical staining data log2 values. There sult showed a strong positive correlation (R2 = 0.999) between the two techniques.
Discussion
Salvia and Astragalus extract are commonly used in Chinese medicine to treat MI disease [5,6,15,16]. In this study, the effect of compatibility of Salvia and Astragalus extract on cardiac remodeling was studied in a rat model of MI. Furthermore, the expression of PKD1 in cardiomyocytes treated with Salvia and Astragalus extract was determined. Cardiac function results showed that in the sham-operated group, the Astragalus group, the Salvia group, and the compatibility of Astragalus and Salvia group, LVSP and the maximum rate of left ventricular pressure were significantly increased while LVEDP were significantly decreased (P < 0.05). These results indicate that the systolic and diastolic functions can be improved by treatment with Astragalus and Salvia extract in rats of MI. Hematoxylin-eosin staining results indicate that the normal morphology and arrangement of cardiomyocytes can be maintained in the compatibility of Astragalus and Salvia group. By treatment with Astragalus and Salvia extract, cell dysfunction, myocardial necrosis, and inflammation caused by MI may be reduced. Collagen fibers and myocardial hypertrophy in myocardial tissues are decreased.
Immunohistochemical staining results showed that expression levels of PKD1 are significantly decreased by treatment of Astragalus and Salvia extract, especially in the cardiomyocytes of compatibility of Astragalus and Salvia group. Previous study showed that PKD1 regulated the transcriptional activity of cardiomyocytes enhancer by inducing the phosphorylation of histone deacetylases IIa, resulting in cardiac hypertrophy. In the meanwhile, PKD1 was activated by angiotensin II, which eventually leaded to vascular smooth muscle cell hypertrophy [17]. PKD1 reduces the Ca2+ sensitivity of muscle filaments by the phosphorylation of troponin I [13]. Cardiac hypertrphy can be significantly reduced in the PKD1 knockout mouse, in which the cardiac function is significantly improved. In conclusion, Astragalus and Salvia extract may play an important role in inhibiting myocardial fibrosis and ventricular remodeling by regulation of PKD1 protein.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants No. 81473438 and No. 81173372) and the National Natural Science Foundation of China for Young Scholars (Grant No. 81202791).
Disclosure of conflict of interest
None.
References
- 1.Ringenberg J, Deo M, Filgueiras-Rama D, Pizarro G, Ibañez B, Peinado R, Merino JL, Berenfeld O, Devabhaktuni V. Effects of fibrosis morphology on reentrant ventricular tachycardia inducibility and simulation fidelity in patient-derived models. Clin Med Insights Cardiol. 2014;8:1–13. doi: 10.4137/CMC.S15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayyas FA, Al-Jarrah MI, Ibrahim KS, Alzoubi KH. Level and significance of plasma myeloperoxidase and the neutrophil to lymphocyte ratio in patients with coronary artery disease. Exp Ther Med. 2014;8:1951–1957. doi: 10.3892/etm.2014.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigo R, Libuy M, Feliú F, Hasson D. Molecular basis of cardioprotective effect of antioxidant vitamins in myocardial infarction. Biomed Res Int. 2013;2013:437613. doi: 10.1155/2013/437613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–64. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Mao BY, Xu GC, Ye SS, Bian H, Zeng XT. Effect of astragalus extract on the levels of PKD1 protein in rats with myocardial infarction. Chinese Pharmacol Bull. 2013;29:535–539. [Google Scholar]
- 6.Yang L, Mao BY, Xu GC, Ye SS, Bian H, Zeng XT. Effect of Compatibility of Astragalus membranaceus and Salvia miltiorrhiza Extract on Pathological Changes of Myocardium in Rats after Myocardial Infarction. Chinese J Exp Tradit Med Formulae. 2013;19:175–179. [Google Scholar]
- 7.Yu C, Qi D, Lian W, Li QZ, Li HJ, Fan HY. Effects of danshensu on platelet aggregation and thrombosis: in vivo arteriovenous shunt and venous thrombosis models in rats. PLoS One. 2014;9:e110124. doi: 10.1371/journal.pone.0110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang SG, Xu Y, Chen JD, Yang CH, Chen XH. Astragaloside IV stimulates angiogenesis and increases nitric oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules. 2013;18:12809–19. doi: 10.3390/molecules181012809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Wu L, Liu W, Jin Y, Chen Q, Wang L, Fan X, Li Z, Cheng Y. A network pharmacology study of Chinese medicine QiShenYiQi to reveal its underlying multi-compound, multi-target, multi-pathway mode of action. PLoS One. 2014;9:e95004. doi: 10.1371/journal.pone.0095004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spindler MJ, Burmeister BT, Huang Y, Hsiao EC, Salomonis N, Scott MJ, Srivastava D, Carnegie GK, Conklin BR. AKAP13 Rho-GEF and PKD-binding domain deficient mice develop normally but have an abnormal response to β-adrenergic-induced cardiac hypertrophy. PLoS One. 2013;8:e62705. doi: 10.1371/journal.pone.0062705. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Zhang L, Malik S, Pang J, Wang H, Park KM, Yule DI, Blaxall BC, Smrcka AV. Phospholipase Cε hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell. 2013;153:216–27. doi: 10.1016/j.cell.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozengurt E. Protein kinase D signaling: multiple biological functions in health and disease. Physiology (Bethesda) 2011;26:23–33. doi: 10.1152/physiol.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuello F, Bardswell SC, Haworth RS, Yin X, Lutz S, Wieland T, Mayr M, Kentish JC, Avkiran M. Protein kinase D selectively targets cardiac troponin I and regulates myofilament Ca2+ sensitivity in ventricular myocytes. Circ Res. 2007;100:864–73. doi: 10.1161/01.RES.0000260809.15393.fa. [DOI] [PubMed] [Google Scholar]
- 14.Fielitz J, Kim MS, Shelton JM, Qi X, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. Requirement of protein kinase D1 for pathological cardiac remodeling. Proc Natl Acad Sci U S A. 2008;105:3059–63. doi: 10.1073/pnas.0712265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Mao BY. Protection of Qisheng Yiqi Pills on Rats with Myocardial Infarction. China J Exp Tradit Med Formulae. 2012;18:167–171. [Google Scholar]
- 16.Wu L, Wang Y, Li Z, Zhang B, Cheng Y, Fan X. Identifying roles of “Jun-Chen-Zuo-Shi” component herbs of Qishenyiqi formula in treating acute myocardial ischemia by network pharmacology. Chin Med. 2014;9:24. doi: 10.1186/1749-8546-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waldron RT, Whitelegge JP, Faull KF, Rozengurt E. Identification of a novel phosphorylation site in c-jun directly targeted in vitro by protein kinase D. Biochem Biophys Res Commun. 2007;356:361–7. doi: 10.1016/j.bbrc.2007.02.142. [DOI] [PMC free article] [PubMed] [Google Scholar]


