Abstract
Objective: Histone deacetylase (HDAC) is a tumor suppressor gene in various carcinomas; however, the effect of HDAC10 on human renal cell carcinoma (RCC) remains unknown. In the current study we analyzed the expression and function of HDAC10 in human clear cell RCC. Methods: RCC tissues from 145 patients who underwent radical nephrectomies were evaluated. HDAC10 protein and mRNA expression was examined by immunohistochemistry and quantitative RT-PCR, respectively. HDAC10 expression was increased by stable transfection with a vector containing full-length cDNA of HDAC10, and HDAC10 expression was decreased by siRNA in two RCC cell lines. Proliferation analysis of RCC cells in vitro was investigated using the WST-1 assay, and the invasion assay was performed using a 24-well Transwell chamber. The phosphorylation of β-catenin induced by HDAC10 was evaluated by Western blot. Results: HDAC10 expression in RCC tissues was significantly down-regulated compared to normal kidney tissues. Moreover, the low level of HDAC10 expression was uniformly associated with advanced clinical stage, larger tumor diameter, higher pathologic grade, and metastatic RCC. In addition, decreased expression of HDAC10 significantly prompted the proliferation and invasion of RCC cells in vitro. Although HDAC10 did not regulate the expression of β-catenin, HDAC10 suppressed the phosphorylation of β-catenin in RCC cells. Conclusions: HDAC10 expression is suppressed in human clear cell RCC and is involved in development and metastasis of RCC. The findings herein suggest that HDAC10 is an independent predictive factor for RCC prognosis, and restoring HDAC10 expression may be a new therapeutic strategy for advanced RCC.
Keywords: Renal cell carcinoma, HDAC10, proliferation, invasion, prognosis
Introduction
Human renal cell carcinoma (RCC) is the most lethal genitourinary malignancy involving the adult kidney. With an incidence of 5-10 per 100,000, RCC accounts for 2%-3% of all tumors [1]. Clear cell RCC is the most common sub-type of human RCC and is responsible for 75% of all cases [2]. Although localized RCC is regarded as a surgical disease, 40% of patients who present with localized RCC will develop advanced RCC [3]. Because RCC is highly resistant to chemotherapy and radiotherapy, no treatment has been shown to be effective for advanced RCC [4]. Recently, a number of genes have been investigated as predictors of survival and as therapeutic targets for human RCC [5,6]. Nevertheless, the detailed molecular mechanism underlying the metastasis and development of RCC remains unknown.
Histone deacetylase (HDAC) can remove the acetyl group from N-acetyllysine on histones [7], and plays an important role in modifying transcription of the chromatin structural genes [8,9]. The HDAC family consists of 18 proteins, which are divided into classes I-IV based on homology and structure. Eleven of the HDAC family members belong to classes I, II, and IV, and are referred to as classic HDACs; 7 HDAC family members belong to class III and are referred to as sirtuins [10]. Aberrant expression of HDACs has been reported to be involved in carcinogenesis and progression of various tumors [11-14]. HDAC10 is a member of the class II HDACs; HDAC10 has one deacetylase domain and one additional catalytically-inactive, leucine-rich domain [15]. HDAC10 has been reported to suppress the accumulation of reactive oxygen species [16] and relieve repression on the melanogenic program [12]. HDAC10 also plays an important role in homologous recombination [17]. A recent study indicated that expression of HDAC10 is significantly decreased in gastric cancer tissues as compared with adjacent tissues in gastric cancer, and HDAC10 is an independent prognostic factor for gastric cancer patients [18]. Another study showed that the level of HDAC10 expression was significantly lower in patients with cervical squamous cell carcinoma and lymph node metastasis compared to patients without lymph node metastasis, and that HDAC10 plays a critical role in suppression of cervical cancer metastasis [19]. Indeed, no study has analyzed the correlation between HDAC10 expression and prognosis of human RCC, and the function of HDAC10 in RCC is still largely unknown.
In the current study we determined the expression of HDAC10 and analyzed its effect on proliferation, invasion, and prognosis in human RCC. Our re-sults indicated that HDAC10 expression is significantly down-regulated in RCC tissue compared with normal kidney tissues, and decreased HDAC10 expression suppresses the proliferation and invasion of RCC cells by inhibiting phosphorylation of β-catenin. Moreover, overexpression of HDAC10 was shown to be associated with a poor prognosis in patients with RCC. These findings suggest that loss of HDAC10 activity may affect the carcinogenesis and development of RCC, and that HDAC10 could serve as a prognostic factor for human RCC. In addition, restoring HDAC10 activity may be a novel therapeutic strategy by which to treat RCC.
Materials and methods
Patients and tissue samples
One hundred forty-five primary RCC tissues and adjacent normal kidney tissues were obtained from patients who underwent radical nephrectomies in the Department of Urology of the First Affiliated Hospital of Kunming Medical University between 2001 and 2008. The current study was approved by the Ethics Committee of the First Affiliated Hospital of Kunming Medical University and RCC patients were followed clinically until November 2013. None of the RCC patients received chemotherapy or radiotherapy before surgery. The histologic cell type of the RCC specimens was evaluated by experienced pathologists and all specimens used in this study were confirmed to be clear cell renal carcinoma. The clinical tumor stages were classified according to the TNM classification system and the pathologic grades were classified according to the Fuhrman grading system. The clinicopathologic characteristics of the tumors are presented in Table 1.
Table 1.
Characteristics of RCC patients and HDAC10 expression detected by immunohistochemistry and quantitative RT-PCR
| Parameters | Total | HDAC10 mRNA | p | HDAC10 | protein | p | ||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| - | + | ++ | +++ | |||||
| RCC | 145 | 2.69±0.24 | 45 | 66 | 23 | 11 | ||
| Normal kidney | 145 | 6.78±0.61 | < 0.05 | 6 | 13 | 74 | 52 | < 0.05 |
| Gender | ||||||||
| Male | 85 | 2.70±0.21 | 25 | 41 | 13 | 6 | ||
| Female | 60 | 2.66±0.22 | > 0.05 | 20 | 25 | 10 | 5 | > 0.05 |
| Age (years) | ||||||||
| < 60 | 74 | 2.73±0.23 | 24 | 33 | 12 | 5 | ||
| ≥ 60 | 71 | 2.67±0.22 | > 0.05 | 21 | 33 | 11 | 6 | > 0.05 |
| Tumor size | ||||||||
| < 7 cm | 81 | 3.56±0.32 | 21 | 32 | 19 | 9 | ||
| ≥ 7 cm | 64 | 1.58±0.13 | < 0.05 | 24 | 34 | 4 | 2 | < 0.05 |
| Histological grade | ||||||||
| G1 | 65 | 3.71±0.32 | 14 | 28 | 14 | 9 | ||
| G2 | 44 | 2.09±0.17 | 16 | 20 | 6 | 2 | ||
| G3 | 36 | 1.58±0.13 | < 0.05 | 15 | 18 | 3 | 0 | < 0.05 |
| Tumor stage | ||||||||
| I | 82 | 3.56±0.32 | 21 | 33 | 19 | 9 | ||
| II | 29 | 1.93±0.15 | 11 | 14 | 3 | 1 | ||
| III | 18 | 1.45±0.11 | 5 | 11 | 1 | 1 | ||
| IV | 16 | 0.96±0.08 | < 0.05 | 8 | 8 | 0 | 0 | < 0.05 |
| Metastasis | ||||||||
| M0 | 115 | 2.99±0.23 | 34 | 48 | 22 | 11 | ||
| M1 | 30 | 1.34±0.11 | < 0.05 | 11 | 18 | 1 | 0 | < 0.05 |
All RCC tissues and corresponding normal kidney tissues were formalin-fixed and paraffin-embedded. Tissue samples were also kept in liquid nitrogen until mRNA or protein extraction.
Cell culture
Two RCC cell lines (ACHN and Caki-2) were purchased from the American Type Culture Collection (ATCC; USA) and used in this study. All RCC cell lines were cultured in RPMI-1640 medium with 10% fetal bovine serum (Gibco, Glasgow, Scotland), 50 μg/ml of streptomycin, and 50 U/ml of penicillin.
All RCC cell lines were cultured as monolayers in 10-cm plastic dishes and incubated in a sterile incubator maintained at 37°C with 5% CO2.
Immunohistochemistry
Paraffin slices (4 μm) of RCC and normal kidney tissues were deparaffinized with xylene and rehydrated with graded alcohol, then all slices were blocked with 10% rabbit serum for 30 min and endogenous peroxidase activity was suppressed with 0.3% hydrogen peroxide for 30 min. Subsequently, the tissue slices were incubated with anti-HDAC10 (H3413) for 4 h, triple-washed with Tris-buffered saline, and the slices were incubated continuously with biotinylated anti-rabbit antibody (DAKO, Glostrup, Denmark) overnight. Determinations of the immunohistochemistry were carried out using a streptavidin-biotin complex system and the staining was examined with a light microscope. Immunostaining of HDAC10 was semi-quantitatively evaluated for positive intensity as follows: negative, -; weak, +; moderate, ++; and strong, +++.
Fractionation and Western blot
Protein from RCC cells was extracted with lysis buffer containing 0.2 mmol/L Na3VO4, 1 mmol/L dithiothreitol, 50 mmol/L NaF, and 5.7 µg/mL of aprotinin. The protein concentration was determined using the Bradford dye-binding protein assay (Bio-Rad, Richmond, CA, USA), and SDS-PAGE was performed. Antibodies to β-catenin and phospho-β-catenin (Ser33/37) were purchased from Cell Signaling Technology and anti-β-actin monoclonal antibody (Abcam, Cambridge, UK) was used as an internal control. The immune complexes were visible using an enhanced chemiluminescence (ECL) system (Amersham, Aylesbury, UK).
Quantitative RT-PCR
The primer sequences of human HDAC10 were designed using Primer Premier 5.0 and GAPDH was used as an internal control. The total RNA of the cells was extracted using a Quick PrepmRNA purification kit (GE Healthcare, Buckinghamshire, UK). Total RNA was reverse-transcribed with a synthesis kit (Amersham Biosciences, Little Chalfont, UK) according to the manufacturer’s instruction. Quantitative RT-PCR was performed using LC FastStart DNA Master SYBR Green (Roche, Indianapolis, IN, USA) and data was collected and analyzed with Light Cycler (Roche).
siRNAi and vector transfection
Oligonucleotide sequences for HDAC10 were designed using siDirect software, and the sequences of two siRNAs are as follows: CGGAGUCAGUGUGCAUGACAGUAC; and UCACUGCACUUGGGAAGCUCCUGU. Briefly, ACHN and Caki-1 cells were cultured with complete medium until cell confluence reached 50%, then RCC cells were transiently transfected with siRNA oligonucleotides with lipofectamine 2000 (Invitrogen, CA, USA). After 48 h, the cells transfected with siRNA were harvested for Western blot analysis to determine the transfection efficiency. RCC cells were also stably transfected with a pFLAG-CMV vector containing full-length cDNA of HDAC10 by lipofectamine 2000. The monoclonal RCC cell line expressing a high level of HDAC10 was selected with G418 and the transfection efficiency with this vector was determined by Western blot analysis.
Proliferation assay of RCC cells
The proliferative ability of RCC cells was assessed using the WST-1 assay, according to the manufacturer’s instructions. ACHN and Caki-1 cells were plated into 96-well plates after being transfected with siHDAC10 or HDAC10 vector; 4×103/well RCC cells were cultured with 3 replicates. The cells were treated with 10 μl WST-1 (Roche, Penzberg, Germany) after incubation for 24, 48, and 72 h, then the incubation was continued for another 2 h. Finally, the absorbance was measured at 450 nm using a microculture plate reader (Immunoreader, Tokyo, Japan); 3 independent experiments were performed.
Invasion assay of RCC cells
A RCC cell invasion assay was performed using a 24-well Transwell chamber, which was pre-coated with 100 μg of Matrigel. ACHN and Caki-1 cells transfected with siHDAC10 or HDAC10 vector were collected and re-suspended in serum-free medium at a concentration of 5×104 cells/ml, then 400-μl cell suspensions were added to the upper chamber and the bottom chamber was filled with 500 μl of culture medium containing 10% FBS. After incubation for 48 h in a sterile incubator maintained at 37°C with 5% CO2, the non-invaded RCC cells on the upper membrane surface were removed with a cotton tip and the cells that passed through the filter were fixed and stained with Hoechst 33342. The number of invaded cells was counted in 10 randomly selected high power fields under a fluorescence microscope.
Statistical analysis
Statistical analyses were performed using SPSS (version 19.0; SPSS, Inc., Chicago, IL, USA). All experiments were performed in triplicate. The data were analyzed using an independent two-tailed t test and represented as the mean ± standard deviation. Categorical data were analyzed using the two-sided chi-square test to determine the correlation between HDAC10 expression and clinical characteristics. Overall survival was analyzed using the Kaplan-Meier method. Values of P < 0.05 were considered statistically significant.
Results
Clinical characteristics of RCC patients
Data were obtained from 85 males and 60 females. The patient ages ranged from 47-78 y (median, 63 y). The tumor sizes ranged from 1.4-15.3 cm (median, 3.7 cm). Among 145 RCC patients, 82, 29, 18, and 16 were stage I-IV, respectively. Sixty-five, 44, and 36 patients had Fuhrman grade 1-3, respectively. One hundred fifteen patients had localized RCC and 30 patients had lymphatic or distant metastases.
HDAC10 expression in RCC
To elucidate the role of HDAC10 in RCC, qRT-PCR and immunohistochemistry were performed to determine HDAC10 levels in 145 pairs of RCC tissues and adjacent normal kidney tissues. The expression of HDAC10 protein was determined by immunohistochemistry; HDAC10 was shown to be highly localized in the cytoplasm and nucleus. Specifically, HDAC10 expression was significantly decreased in RCC compared to normal kidney tissues; HDAC10 expression was detected in 100 of 145 (69%) RCC tissue specimens, 45.5% of which stained weakly. In contrast, the expression of HDAC10 protein expression was detected in 139 of 145 (95.9%) normal kidney tissue specimens, 86.9% of exhibited median or strong HDAC10 expression. We also analyzed the correlation between HDAC10 expression and clinical characteristics. The expression of HDAC10 protein was significantly reduced in patients with large tumor sizes, high tumor stages and pathologic grades, and metastatic tumors. In contrast, gender and age did not have a significant correlation with HDAC10 expression (Table 1). In addition, HDAC10 expression in human normal kidney tissues and RCC tissues was also detected by quantitative RT-PCR and similar findings were found. These results suggested that decreased HDAC10 expression may be involved in the carcinogenesis and development of human RCC.
HDAC10 suppresses the proliferation of RCC cells
The effect of HDAC10 on the proliferation of RCC cells was analyzed using the WST-1 assay. A vector containing the full length cDNA for HDAC10 was transfected into ACHN and Caki-1 cell lines, and HDAC10 expression was also decreased using siRNA. After all transfections, HDAC10 expression was detected by Western blot analysis. HDAC10 expression was significantly increased by the HDAC10 vector insert and decreased by siRNA in two RCC cell lines (Figure 1). Thus, RCC cells expressing a high level of HDAC10 have significantly decreased proliferative ability compared to untreated cells. In contrast, RCC cells expressing a low level of HDAC10 have a higher proliferative ability (Figure 2). These findings suggest that HDAC10 can suppress the proliferation of RCC cells.
Figure 1.
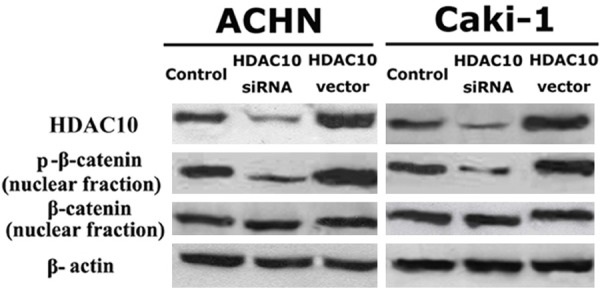
HDAC10 suppresses the phosphorylation of β-catenin in RCC cells. HDAC10 vector was stably transfected into RCC cell lines and HDAC10 expression was also decreased using siRNA; all transfections were confirmed by Western blot analysis.
Figure 2.
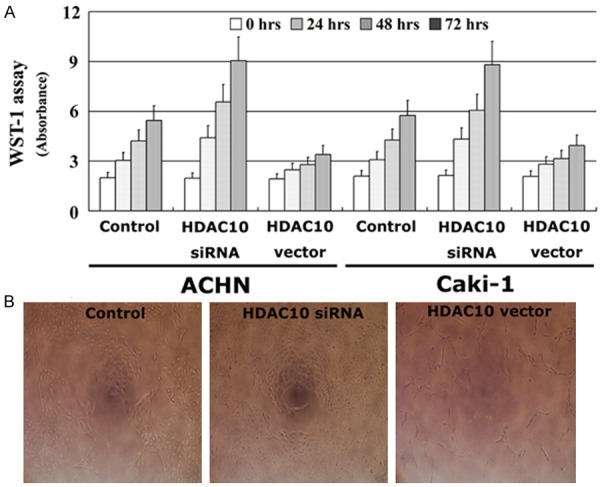
HDAC10 suppresses the proliferation of RCC cells. The proliferative ability in vitro was detected using the WST-1 assay (A), the results of ACHN were also shown (B, ×200). All experiments were performed in triplicate and the error bar represents the SD.
HDAC10 suppresses the invasion of RCC cells
Metastasis is a major cause of mortality in patients with RCC, and cell invasion plays an important role in this process. The effects of HDAC10 on the invasion of RCC cells were also investigated in the current study. The expression of HDAC10 in two TCC cell lines was decreased by siRNA technology, and HDAC10 expression was stably increased by transfection with a vector containing the full length cDNA of HDAC10. HDAC10 expression was evaluated using Western blot analysis (Figure 1). As shown in Figure 3, RCC cells with a high of level of HDAC10 expression had a decreased ability of invasion compared to untreated controls. In contrast, RCC cells with a low level of HDAC10 expression due to siRNA treatment have a significantly increased ability of invasion. These findings indicate that decreased HDAC10 expression can increase the invasion of RCC cells, and that HDAC10 plays an important role in the metastasis of RCC.
Figure 3.
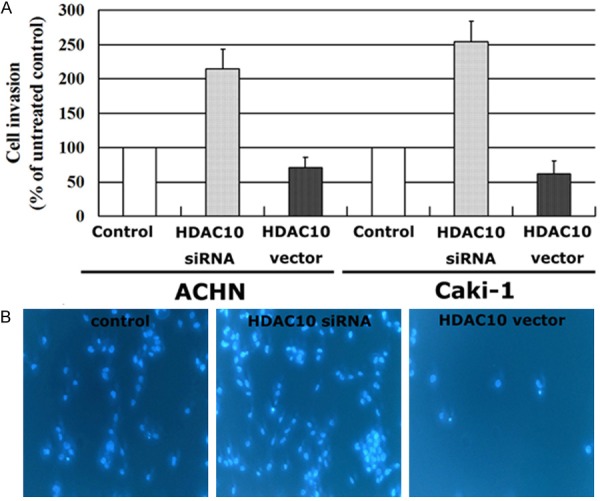
HDAC10 suppresses the invasion of RCC cells. The invasive ability in vitro was detected using a 24-well Transwell chamber (A), the results of ACHN were also shown by Hoechst 33342 staining (B, ×200). All experiments were performed in triplicate and the error bar represents the SD.
HDAC10 decreased β-catenin activity in RCC cells
To clarify how the β-catenin pathway is involved in HDAC10-induced biological function in RCC cells, the phosphorylation of β-catenin was evaluated. HDAC10 expression was increased by transfection with a pcDEF3 vector containing the full length cDNA for HDAC10, and HDAC10 expression was also decreased by siRNA in ACHN and Caki-1 cell lines. Although HDAC10 expression did not regulate the expression of nuclear β-catenin protein in two RCC cell lines, increased expression of HDAC10 suppressed the phosphorylation of nuclear β-catenin based on Western blot analysis. In contrast, decreased expression of HDAC10 enhanced the phosphorylation of nuclear β-catenin (Figure 1). These findings suggest that phosphorylation of nuclear β-catenin is negatively regulated by HDAC10 activity and the β-catenin pathway plays an important role in HDAC10-induced anti-proliferation and anti-invasion in RCC cells.
Prognostic significance of HDAC10 expression in RCC
Because a correlation between HDAC10 expression and clinical stage and histologic grade was demonstrated in this study, we also investigated whether or not HDAC10 could be a prognostic factor for human RCC. The correlation between HDAC10 expression and overall survival in RCC patients was calculated by Kaplan-Meier analysis. Our results suggest that overall survival is significantly different between the high and low HDAC10 expression groups. After 5 years of clinical follow-up, 18 of 57 (31.6%) RCC patients in whom immunohistochemical staining displayed a low level of HDAC10 expression (- and +) were alive and disease-free. In contrast, 20 of 31 (64.5%) RCC patients in whom staining displayed a high level of HDAC10 expression (++ and +++) were alive and disease-free. These findings suggest that HDAC10 expression may be an independent factor in predicting the prognosis of human RCC.
Discussion
Although RCC at early stage is usually regarded as localized disease, some patients can develop metastatic RCC and the prognosis of advanced RCC is extremely poor. Indeed, the current treatments available for advanced RCC are not effective [20]. Because advanced RCC is insensitive to chemotherapy, and no agents are available to eliminate this disease, novel gene targets for the diagnosis, prognosis, and treatment of patients with advanced RCC are needed.
In recent years it has been well-established that histone acetylation in the promoter regions of some tumor suppressor genes is aberrant in various malignancies and some members of the HDAC family play an important role in carcinogenesis [21]. HDACs can serve as a signal to regulate the expression of downstream genes by modifying the structure of chromatin and binding of transcription factors to the DNA region [22]. HDAC members are divided into 4 classes according to enzymatic activity, molecular structure, expression, and localization, as follows: class I (HDAC1, 2, 3, and 8); class IIa (HDAC4, 5, 7, and 9); class IIb (HDAC6 and 10); and class IV (HDAC11) [23]. Class I HDACs have been reported to enhance cell cycles and cancer cell proliferation [24]. A high level of HDAC2 expression predicts an unfavorable prognosis in gallbladder cancer [25]. HDAC2 and HDAC3 are also significantly up-regulated in sub-groups of malignancies with aggressive features [26]. It has been reported that expression of HDAC10 is significantly decreased in gastric cancer and HDAC10 is an independent prognostic factor for this disease [18]. Moreover, another study indicated that HDAC10 suppresses the expression of matrix metalloproteinase 2 and 9, thus inhibiting invasion and metastasis of cervical cancer cells [19]. Although several studies have confirmed that HDAC10 acts as a cancer suppressor gene in human tumors, the role of HDAC10 in human RCC remains unknown. Thus, the effect of HDAC10 expression on the growth, metastasis, and prognosis in RCC needs further evaluation.
This is the first study involving the functions of HDAC10 in human RCC. We demonstrated that HDAC10 expression is significantly decreased in RCC tissues compared to normal kidney tissues by immunohistochemistry and quantitative RT-PCR. In addition, the level of HDAC10 expression was significantly associated with tumor size, clinical stage, pathologic grade, and tumor metastasis. We further analyzed the effect of HDAC10 on the proliferation and invasion of RCC cells, and showed that decreased expression of HDAC10 could significantly prompt the proliferation and invasion of RCC cells in vitro. These findings suggest that decreased HDAC10 expression is involved in the carcinogenesis and development of human RCC. In addition, the correlation between HDAC10 expression and overall survival of human RCC was also investigated by the Kaplan-Meier analysis in this study, which indicated that a decreased level of HDAC10 expression could be regarded as a valuable factor for prediction of poor prognosis in patients with RCC, and that HDAC10 could serve as an independent prognostic factor and guide the follow-up schedule in RCC patients (Figure 4).
Figure 4.
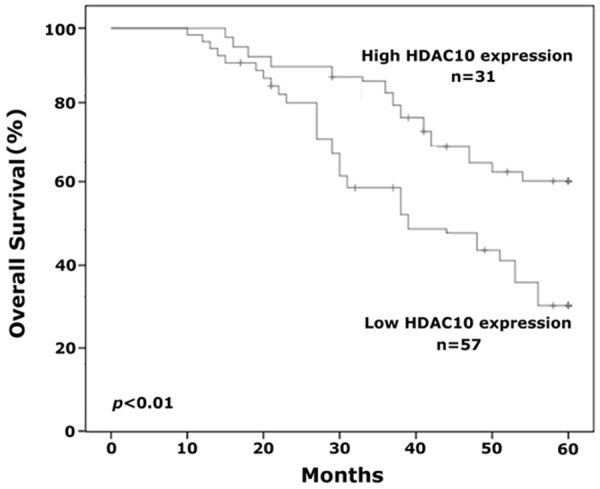
Kaplan-Meier analysis was performed to analyze the correlation between HDAC10 expression and overall survival of patients with RCC. Decreased expression of HDAC10 was associated with a poor prognosis and HDAC10 is an independent factor for predicting the prognosis of human RCC.
Although the expression and functions of HDAC10 in RCC have been investigated in our study, the underlying molecular mechanism of HDAC10 in RCC remains unclear and should be further assessed. β-catenin serves as a co-activator for lymphoid enhancer-binding factor 1 (LEF1) and may be a key transcription activator in the Wnt signaling pathway [27]. LEF1 contains a DNA binding domain near its C terminus and a domain at the N terminus that binds β-catenin [28]. Upon Wnt stimulation, β-catenin could activate Wnt-responsive target genes. Alterations in the β-catenin signaling pathway have been reported in several tumors [29], and dysregulation of β-catenin is an important phenomenon in carcinogenesis and an independent predictor in patients with RCC [30-32]. Our study suggests that although HDAC10 did not regulate the expression of β-catenin in the nucleus, HDAC10 decreased the phosphorylation of nuclear β-catenin in RCC cells. Thus, the β-catenin pathway is a potential target for HDAC10, and β-catenin plays an important role in HDAC10-induced anti-proliferation and anti-invasion of RCC cells.
In summary, our study revealed that HDAC10 expression is significantly suppressed in human RCC and may be involved in the development and metastasis of this disease. These findings suggest that RCC patients with a low level of HDAC10 expression may be vulnerable to develop metastases of RCC, and HDAC10 may be an independent factor to predict the prognosis of patients with RCC. Moreover, our study also suggested that restoring HDAC10 expression may prove to be a novel therapeutic treatment for patients with advanced RCC.
Acknowledgements
This study don’t have any grant supported. WenXing Fan is responsible for data collection, Jie Huang is responsible for data analysis and Hua Xiao is responsible for manuscript preparation.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI, Campbell SC, Escudier B. Renal cell carcin. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 3.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–852. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 4.Janowitz T, Welsh SJ, Zaki K, Mulders P, Eisen T. Adjuvant therapy in renal cell carcinoma-past, present, and future. Semin Oncol. 2013;40:482–491. doi: 10.1053/j.seminoncol.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Z, Ni L, Chen D, Su Z, Yu W, Zhang Q, Wang Y, Li C, Gui Y, Lai Y. Expression and clinical significance of RCDG1 in renal cell carcinoma: A novel renal cancer‑associated gene. Mol Med Rep. 2014;10:1583–1589. doi: 10.3892/mmr.2014.2388. [DOI] [PubMed] [Google Scholar]
- 6.Yang YQ, Chen J. Predictive role of vascular endothelial growth factor polymorphisms in the survival of renal cell carcinoma patients. Genet Mol Res. 2014;13:5011–5017. doi: 10.4238/2014.July.4.16. [DOI] [PubMed] [Google Scholar]
- 7.Sengupta N, Seto E. Regulation of histone deacetylase activities. J Cell Biochem. 2004;93:57–67. doi: 10.1002/jcb.20179. [DOI] [PubMed] [Google Scholar]
- 8.Kumar P, Tripathi S, Pandey KN. Histone deacetylase inhibitors modulate the transcriptional regulation of guanylyl cyclase/natriuretic peptide receptor-a gene: interactive roles of modified histones, histone acetyltransferase, p300, AND Sp1. J Biol Chem. 2014;289:6991–7002. doi: 10.1074/jbc.M113.511444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slaw CH. TNM Classification of Malignant Tumors. 6th edition. New York: John Wiley-Liss; 2002. International Union Against Cancer. [Google Scholar]
- 10.Witt O, Deubzer HE, Milde T, Oehme I. HDAC family: what are the cancer relevant targets? Cancer Lett. 2009;277:8–21. doi: 10.1016/j.canlet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Wilson AJ, Byun DS, Popova N, Murray LB, L’Italien K, Sowa Y, Arango D, Velcich A, Augenlicht LH, Mariadason JM. Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J Biol Chem. 2006;281:13548–13558. doi: 10.1074/jbc.M510023200. [DOI] [PubMed] [Google Scholar]
- 12.Lai IL, Lin TP, Yao YL, Lin CY, Hsieh MJ, Yang WM. Histone deacetylase 10 relieves repression on the melanogenic program by maintaining the deacetylation status of repressors. J Biol Chem. 2010;285:7187–7196. doi: 10.1074/jbc.M109.061861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JH, Kwon HJ, Yoon BI, Kim JH, Han SU, Joo HJ, Kim DY. Expression profile of histone deacetylase 1 in gastric cancer tissues. Jpn J Cancer Res. 2001;92:1300–1304. doi: 10.1111/j.1349-7006.2001.tb02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osada H, Tatematsu Y, Saito H, Yatabe Y, Mitsudomi T, Takahashi T. Reduced expression of class II histone deacetylase genes is associated with poor prognosis in lung cancer patients. Int J Cancer. 2004;112:26–32. doi: 10.1002/ijc.20395. [DOI] [PubMed] [Google Scholar]
- 15.Tong JJ, Liu J, Bertos NR, Yang XJ. Identification of HDAC10, a novel class II human histone deacetylase containing a leucinerich domain. Nucleic Acids Res. 2002;30:1114–1123. doi: 10.1093/nar/30.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Jeong EG, Choi MC, Kim SH, Park JH, Song SH, Park J, Bang YJ, Kim TY. Inhibition of histone deacetylase 10 induces thioredoxin-interacting protein and causes accumulation of reactive oxygen species in SNU-620 human gastric cancer cells. Mol Cells. 2010;30:107–112. doi: 10.1007/s10059-010-0094-z. [DOI] [PubMed] [Google Scholar]
- 17.Kotian S, Liyanarachchi S, Zelent A, Parvin JD. Histone deacetylases 9 and 10 are required for homologous recombination. J Biol Chem. 2011;286:7722–7726. doi: 10.1074/jbc.C110.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin ZL, Jiang WH, Jiao F, Guo Z, Hu H, Wang L, Wang LW. Decreased expression of histone deacetylase 10predicts poor prognosis of gastric cancer patients. Int J Clin Exp Pathol. 2014;7:5872–5879. [PMC free article] [PubMed] [Google Scholar]
- 19.Song C, Zhu S, Wu C, Kang J. Histone deacetylase (HDAC) 10 suppresses cervical cancer metastasis through inhibition of matrix metalloproteinase (MMP) 2 and 9 expression. J Biol Chem. 2013;288:28021–28033. doi: 10.1074/jbc.M113.498758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escudier B, Eisen T, Porta C, Patard JJ, Khoo V, Algaba F, Mulders P, Kataja V ESMO Guidelines Working Group. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii65–71. doi: 10.1093/annonc/mds227. [DOI] [PubMed] [Google Scholar]
- 21.Hellebrekers DM, Griffioen AW, van Engeland M. Dual targeting of epigenetic therapy in cancer. Biochim Biophys Acta. 2007;1775:76–91. doi: 10.1016/j.bbcan.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Wisnieski F, Calcagno DQ, Leal MF, Chen ES, Gigek CO, Santos LC, Pontes TB, Rasmussen LT, Payao SL, Assumpcao PP, Lourenco LG, Demachki S, Artigiani R, Burbano RR, Cardoso Smith M. Differential expression of histone deacetylase and acetyltransferase genes in gastric cancer and their modulation by trichostatin A. Tumour Biol. 2014;35:6373–6381. doi: 10.1007/s13277-014-1841-0. [DOI] [PubMed] [Google Scholar]
- 23.Yasui W, Oue N, Ono S, Mitani Y, Ito R, Nakayama H. Histone acetylation and gastrointestinal carcinogenesis. Ann N Y Acad Sci. 2003;983:220–231. doi: 10.1111/j.1749-6632.2003.tb05977.x. [DOI] [PubMed] [Google Scholar]
- 24.Feng L, Pan M, Sun J, Lu H, Shen Q, Zhang S, Jiang T, Liu L, Jin W, Chen Y, Wang X, Jin H. Histone deacetylase 3 inhibits expression of PUMA in gastric cancer cells. J Mol Med (Berl) 2013;91:49–58. doi: 10.1007/s00109-012-0932-x. [DOI] [PubMed] [Google Scholar]
- 25.Du X, Zhao H, Zang L, Song N, Yang T, Dong R, Yin J, Wang C, Lu J. Overexpression of histone deacetylase 2 predicts unfavorable prognosis in human gallbladder carcinoma. Pathol Oncol Res. 2013;19:397–403. doi: 10.1007/s12253-012-9592-y. [DOI] [PubMed] [Google Scholar]
- 26.Muller BM, Jana L, Kasajima A, Lehmann A, Prinzler J, Budczies J, Winzer KJ, Dietel M, Weichert W, Denkert C. Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer--overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer. 2013;13:215. doi: 10.1186/1471-2407-13-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feigin ME, Malbon CC. OSTM1 regulates beta-catenin/Lef1 interaction and is required for Wnt/beta-catenin signaling. Cell Signal. 2008;20:949–957. doi: 10.1016/j.cellsig.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li TW, Ting JT, Yokoyama N, Bernstein A, van de Wetering M, Waterman M. Wnt Activation and Alternative Promoter Repression of LEF1 in Colon Cancer. Mol Cell Biol. 2006;26:5284–5299. doi: 10.1128/MCB.00105-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watermann ML. Lymphoid enhance factor/T cell factor expression in colorectal cancer. Cancer Metastasis Rev. 2004;23:41–52. doi: 10.1023/a:1025858928620. [DOI] [PubMed] [Google Scholar]
- 30.VON Schulz-Hausmann SA, Schmeel LC, Schmeel FC, Schmidt-Wolf IG. Targeting the Wnt/beta-catenin pathway in renal cell carcinoma. Anticancer Res. 2014;34:4101–4108. [PubMed] [Google Scholar]
- 31.Krabbe LM, Westerman ME, Bagrodia A, Gayed BA, Darwish OM, Haddad AQ, Khalil D, Kapur P, Sagalowsky AI, Lotan Y, Margulis V. Dysregulation of β-catenin is an independent predictor of oncologic outcomes in patients with clear cell renal cell carcinoma. J Urol. 2014;191:1671–1677. doi: 10.1016/j.juro.2013.11.052. [DOI] [PubMed] [Google Scholar]
- 32.Kruck S, Eyrich C, Scharpf M, Sievert KD, Fend F, Stenzl A, Bedke J. Impact of an Altered Wnt1/β-Catenin Expression on Clinicopathology and Prognosis in Clear Cell Renal Cell Carcinoma. Int J Mol Sci. 2013;14:10944–10957. doi: 10.3390/ijms140610944. [DOI] [PMC free article] [PubMed] [Google Scholar]


