Abstract
T cell Ig and mucin domain-containing molecule-3 (Tim-3) is a negative regulator preferentially expressed on Th1 cells. Allergic asthma is a clinical syndrome well characterized by Th1/Th2 imbalance. To investigate the role of Tim-3 in the pathogenesis of asthma and its relationship with Th1/Th2 imbalance, a total of 40 patients with allergic asthma and 40 healthy controls were enrolled. Expression of Tim-3 and Th1/Th2 imbalance as well as the relationship between them was analyzed by flow cytometry and real-time PCR. Peripheral blood mononuclear cells (PBMCs) were cultured in vitro and anti-Tim-3 was used to block Tim-3 signaling; Th1/Th2 cytokines in the culture supernatant were detected by enzyme linked immunosorbent assay (ELISA). CD4+ T cells and B cells were sorted and co-cultured in vitro, and anti-Tim-3 was used to block Tim-3 signaling; Total IgG/IgE in the culture supernatant was detected by ELISA. The mRNA level of T-bet and IFN-γ were significantly decreased in allergic asthma patients, while GATA-3 and IL-4 were significantly increased. Expression of Tim-3 on CD4+ T cells was much higher in allergic asthma patients and it was negatively correlated with T-bet/GATA-3 ratio or IFN-γ/IL-4 ratio. Blocking of Tim-3 significantly increased Th1 cytokines (TNF-α and IFN-γ) and decreased Th2 cytokines (IL-4, IL-5, IL-13) in the culture supernatant of PBMCs. Blocking of Tim-3 dramatically reduced the production of IgG and IgE in the co-culture supernatant of CD4+ T cells and B cells. In conclusion, Tim-3 was up-regulated in allergic asthma patients and related with the Th1/Th2 imbalance. Blocking of Tim-3 may be of therapeutic benefit by enhancing the Th1 cytokines response, down-regulating the Th2 cytokines response, and reducing IgG/IgE production.
Keywords: Tim-3, Th1 cell, Th2 cell, asthma
Introduction
Allergic asthma is a well defined Th2 polarized chronic inflammatory disease which is characterized by reversible airflow limitations and airway remodeling [1]. Allergens such as house dust mite (HDM) initiate this allergic response and activate Th2 cells, and Th2 cytokines such as IL-4, IL-5 and IL-13 are produced to facilitate IgE class switching, goblet cell hyperplasia and eosinophils recruitment [2]. A lot of strategies were designed to adjust the imbalance toward Th2 polarization, for instance, blocking of Th2 cells specific transcription factor GATA-3 or Th2 cytokines IL-4 or IL-13 leads to inhibition of Th2 cells differentiation [3]; Elevated level of in situ IL-12 or IFN-γ accomplished by administration of CpG oligodeoxynucleotides may favor Th1 induction and thus inhibit Th2 cells [4]; Treatment of glucocorticoids may lead to nonselective immunosuppression and reduce Th2 cells number and inhibit Th2 cell activation [5].
T cell Ig and mucin domain-containing molecule-3 (Tim-3) is a transmembrane molecular expressed dominantly on terminally differentiated Th1 cells but not on Th2 cells [6]. The function of Tim-3 was initially studied in an exacerbated experimental autoimmune encephalomyelitis (EAE) mouse model, and treated EAE mice with anti-Tim-3 antibody caused increased macrophage activation and aggravated disease activity [7]. The subsequent study revealed that blocking Tim-3 signaling pathway resulted in enhanced Th1 cell proliferation and Th1 cytokines secretion [8]. Galectin-9, a member of the galectin family, was identified as a ligand for Tim-3 recently, and in contrast to blocking Tim-1, triggering of galection-9 selectively induced Th1 cell death and in vivo treatment of galection-9 reduced Th1 cells and ameliorated the disease activity of EAE mice [9]. So the studies above indicate that Tim-3 plays a negative role on Th1 cell response and induces immune tolerance protecting mice from Th1-mediated disease such as EAE.
Since Th1 and Th2 cells are mutual antagonism and kept a balance during CD4+ T cells differentiation, blocking Tim-3 may augment Th1 response and down-regulate Th2 cell response, and thus be therapeutically beneficial to Th2-mediated disease such as allergic asthma. So in the present study, the correlation between Th1/Th2 imbalance and expression level of Tim-3 was analyzed, and Th1/Th2 cytokines as well as B-cell help function were further investigated after blocking Tim-3 signal in vitro by specific anti-Tim-3 antibody.
Materials and methods
Patients and healthy controls
A total of 40 patients with allergic asthma and 40 healthy controls were enrolled in this study. The diagnostic criteria were based on the Global Initiative for Asthma (GINA) and Asthma Control Questionnaire (ACQ) was also taken. Lung function tests and routine lab tests were performed. All the patients were confirmed without taking systemic glucocorticoids or other immunosuppressive drugs within 1 month before this study. Healthy controls were individuals with normal pulmonary function and negative allergy tests. Written informed consent was obtained from every individual in this study. This study was approved by the Ethical Committee of Bethune International Peace Hospital of PLA (HeBei, People’s Republic of China).
Cells isolation
Heparinized venous blood was collected and peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation on Ficoll-Histopaque (Sigma-Aldrich, Saint-Louis, MO, USA). PBMCs were washed twice and part of them were sorted into CD4+ T cells and B cells according to the manufacturer’s protocol (Miltenyi Biotec, German). The sorted cells were stained using specific antibodies and determined by FACS to ensure a purity of ≥ 95%.
Real-time PCR
A total of 5×105 CD4+ T cells were dissolved in 1ml Trizol (Invitrogen, US) and total RNA was extracted by phenol-chloroform based method according to the manufacturer’s protocol. Oligo (dT) primer was used to synthesize cDNA before quantitative PCR was done using SYBR Green PCR Mastermix kit (ABI, US). The primers for PCR were used according to the published data [10] and the sequences were listed as follows: T-bet: 5’-CAAGCAGGGACGGCGGATGT-3’ and 5’-TTGGACGCCCCCTTGTTGTTTG-3’; GATA-3: 5’-GTGCCCGAGTACAGCTCCGGA-3’ and 5’-GAGCCCACAGGCATTGCAGACA-3’; IFN-g: 5’-CTCTGCATCGTTTTGGGTTCTCTTGG-3’ and 5’-GCGACAGTTCAGCCATCACTTGGAT-3’; IL-4: 5’-CGAGTTGACCGTAACAGACAT-3’ and 5’-GTCTTTAGCCTTTCCAAGAAG-3’. The cycle time (Ct) values were converted to relative expression using the ΔΔCt method and normalized to housekeeper gene β-actin. All reactions were performed in 96-well plates on ABI 7300 device and each gene was done by 3 replicates.
Surface staining and flow cytometry analysis
For analysis of the expression level of Tim-3 on CD4+ T cells, PBMCs were incubated with antibodies cocktail (FITC-anti-hCD4, PE-anti-hTim-3, Percp-anti-hCD3, BD, USA) at 4°C for 30 minutes. The cells were then washed twice by phosphate buffered saline (PBS) before analyzed with flow cytometer (FACS Calibur, BD, USA).
Cell stimulations and cell culture
For analysis of the in vitro cytokines production, PBMCs were suspended at a density of 1×106 cells/ml in 48-well plates. Purified anti-human CD3 (clone 145-2C11, BD, USA) was used as a T cell stimuli at a final concentration of 2 µg/ml. For the co-culture of T cells and B cells, 5×104 T cells and 5×104 B cells were cultured together in one well of 96-well U-bottomed plates. Purified anti-human Tim-3 (clone F38-2E2, Biolegend, USA) at a final concentration of 10 µg/ml was used to block Tim-3 signaling, and purified mouse IgG1 (clone MOPC-21, Biolegend, USA) at the same concentration was used as an iso-type control. All the cells were cultured in Roswell Park Memorial Institute (RPMI) media 1640 supplemented with 100 U/ml of penicillin, 100 μg/ml of streptomycin, 10% heat inactivated fetal calf serum (Gibco, USA) in an incubator of 37°C and 5% CO2. The supernatant on the indicated days were collected and stored at -80°C for analysis.
Enzyme linked immunosorbent assays (ELISA)
The concentrations of IFN-γ, TNF-α, IL-12, IL-4, IL-5, IL-13, IL-17 and total IgE, IgG in the culture supernatant were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (eBioscience, USA). All samples were measured in duplicate.
Statistical analyses
Data were analyzed using Graph Prism version 5.0 (Graphpad software Inc, San Diego, CA, USA). Differences between two groups were determined with a two-tailed unpaired Student’s test. Pearson’s correlation analysis was used to analyze the co-relationship, and the significance was evaluated using the t statistic. Differences at P < 0.05 or less were defined to be statistically significant.
Results
General characteristics of patients and controls
The characteristics of the studied subjects are summarized in Table 1. There are no significant difference in age, gender, body mass index (BMI) and C-reactive protein (CRP) between asthma patients and healthy controls. FEV1 (% predicted) in asthma patients was significantly lower than healthy controls, while the eosinophils count and serum total IgE were significantly higher in asthma patients.
Table 1.
Characterization of asthma patients and healthy controls
| Characteristics | Control | Asthma | P value |
|---|---|---|---|
| N | 40 | 40 | |
| Age (years) | 31.36±5.71 | 32.53±6.48 | 0.394 |
| Gender (male/female) | 22/18 | 23/17 | 0.823 |
| BMI (kg/m2) | 21.89±5.37 | 22.14±6.23 | 0.848 |
| FEV1 (%predicted) | 93.12±5.45 | 76.38±10.19 | < 0.0001 |
| CRP (ug/ml) | 3.32±2.18 | 3.78±2.62 | 0.396 |
| Eosinophils count (106/L) | 157±43 | 546±203 | < 0.0001 |
| Total IgE (ng/ml) | 212.66±72.39 | 984.43±278.61 | < 0.0001 |
Th1/Th2 imbalance in asthma patients
Specific amplification and quantitation of different genes associated to Th1/Th2 transcription factors and cytokines were showed in Figure 1. T-bet and GATA-3 are specific transcription factors of Th1 and Th2 cells respectively [11], and IFN-γ and IL-4 are iconic cytokines of Th1 and Th2 cells respectively [12]. Asthma patients had much higher expression level of GATA-3 and IL-4, and much lower T-bet and IFN-γ. And the Th1/Th2 ratio, which can be represented by T-bet/GATA-3 or IFN-γ/IL-4, was dramatically declined in asthma patients.
Figure 1.
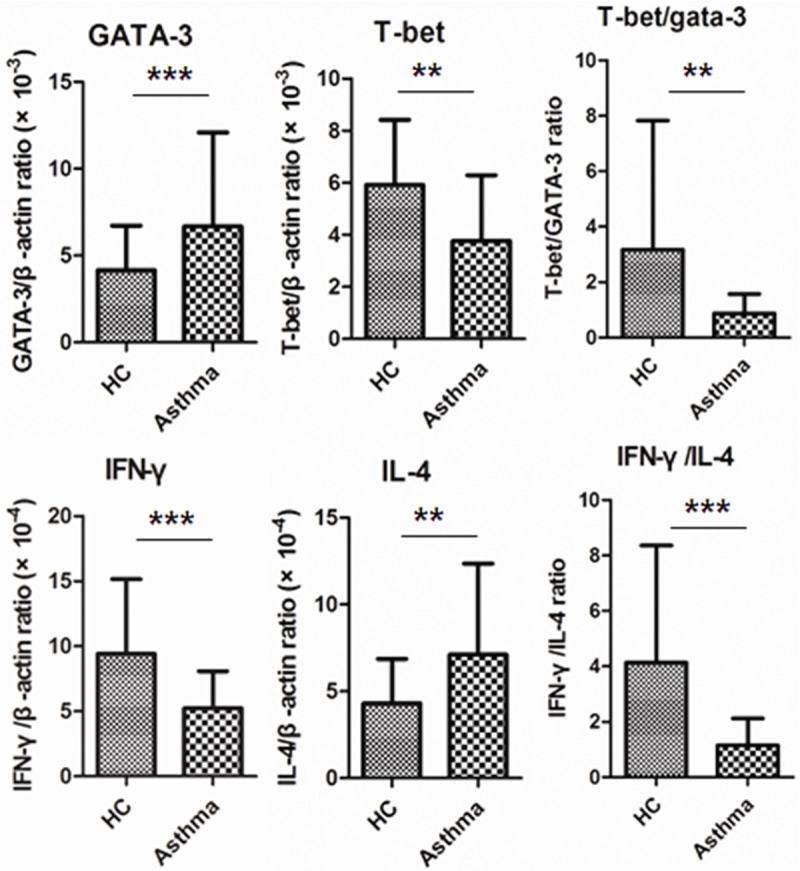
Th1/Th2 imbalance in peripheral blood of patients with asthma. The purified CD4+ T cells from Asthma patients and healthy controls were determined for mRNA level of transcriptional factors and cytokines by quantitative PCR. The expression of mRNA of specific genes were normalized to housekeeper gene β-actin (**P < 0.01; ***P < 0.0001).
Elevated expression of Tim-3 on CD4+ T cells of asthma patients
The expression of Tim-3 on CD4+ T cells was investigated in this study by flow cytometry. Cells were gated within CD4+ T cells and the percentage of Tim-3+ cells/CD4+ T cells was showed in Figure 2A. A significantly elevated expression of Tim-3 was observed in asthma patients comparing to healthy control (Figure 2B).
Figure 2.
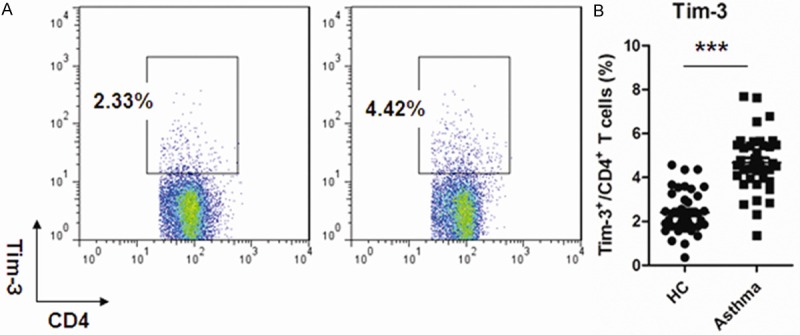
Up-regulation of Tim-3 expression on peripheral CD4+ cells of patients with asthma. A: Representative expression pattern of Tim-3 expression on CD4+ T cells in Asthma patients and healthy controls. Cells were gated on CD4+ T cells; B: Percentage of Tim-3+ T cells in asthma patients and healthy controls (***P < 0.0001). Each data point represents an individual subject, and horizontal lines show the median and SEM.
Correlation between Tim-3 on CD4+ cells and Th1/Th2 imbalance in asthma patients
To investigate the relationship between Tim-3 and Th1/Th2 imbalance, the correlation between Tim-3 and T-bet/GATA-3 ratio or IFN-γ/IL-4 ratio were done. There were significantly negative correlation between Tim-3 on CD4+ cells and T-bet/GATA-3 (r=-0.5201, P=0.0006) as well as between Tim-3 on CD4+ cells and IFN-γ/IL-4 ratio (r=-0.4939, P=0.0012) (Figure 3).
Figure 3.
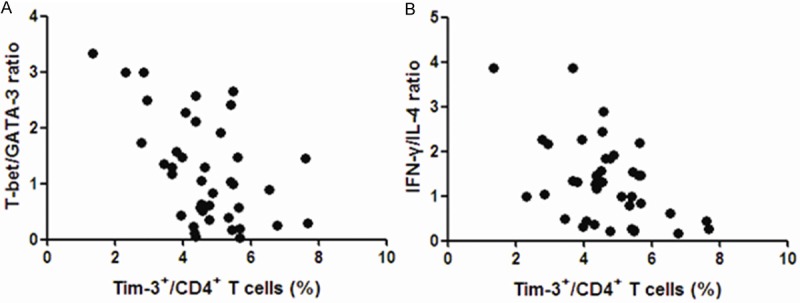
Tim-3 on CD4+ T cells correlated with Th1/Th2 ratio. A: Relationship between the frequency of circulating Tim-3+CD4+ T cells and the T-bet/GATA-3 ratio (r=-0.5201, P=0.0006); B: Relationship between the frequency of circulating Tim-3+CD4+ T cells and IFN-γ/IL-4 ratio (r=-0.4939, P=0.0012).
Tim-3 blockage enhanced Th1 response and decreased Th2 response in asthma patients
The negative correlation between Tim-3 and Th1/Th2 imbalance as well as elevated Tim-3 expression in asthma patients indicate that Tim-3 might be targeted to adjust the Th1/Th2 imbalance in asthma patients. An in vitro study was performed by using anti-CD3 to activate T cells and using anti-Tim-3 to blocking signaling through Tim-3. Th1 and Th2 cytokines in the culture supernatant were detected and summarized in Table 2. Blocking with anti-Tim-3 dramatically increased the production of Th1 cytokines such as IFN-γ and TNF-α, while decreased the production of Th2 cytokines such as IL-4, IL-5 and IL-13. IL-17 in the culture supernatant was comparable between Tim-3 blocking group and control group.
Table 2.
Th1/Th2 cytokines response after Tim-3 blocking by anti-Tim-3
| Cytokines | No stimulation | Stimulation + Ig control | Stimulation + Anti-Tim3 |
|---|---|---|---|
| IFN-γ (pg/ml) | 9.35±5.45 | 34.29±11.47 | 68.62±17.41*** |
| TNF-a (pg/ml) | 10.27±3.73 | 28.11±12.36 | 42.85±15.39** |
| IL-12 (pg/ml) | 466.43±121.18 | 571.38±220.14 | 583.27±241.36 |
| IL-4 (fg/ml) | 218.43±114.75 | 841.45±265.50 | 415.32±138.56*** |
| IL-5 (pg/ml) | 12.53±5.49 | 89.69±32.93 | 53.27±20.31** |
| IL-13 (pg/ml) | < 12 | 23.54±9.51 | 15.42±5.73** |
| IL-17 (pg/ml) | 23.71±20.28 | 48.73±18.54 | 47.19±21.38 |
P < 0.01;
P < 0.0001, comparing with “Stimulation + Ig control” group.
Tim-3 blockage reduced T-cell helped B cell response
Over-activated B cell response and high level of IgE were considered as one of the mechanisms for asthma. Th2 cells were responsible for helping B cells mediated humoral immunity. To investigate the affection of decreased Th2 cell response by blocking Tim-3 to humoral immunity, CD4+ T cells and B cells were sorted and co-cultured in vitro. Total IgE and IgG in the culture supernatant were detected and showed in Figure 4: both IgG and IgE production were enhanced if CD4+ T cells were added in the culture system, and blockage of Tim-3 significantly decreased the production of both IgG and IgE.
Figure 4.
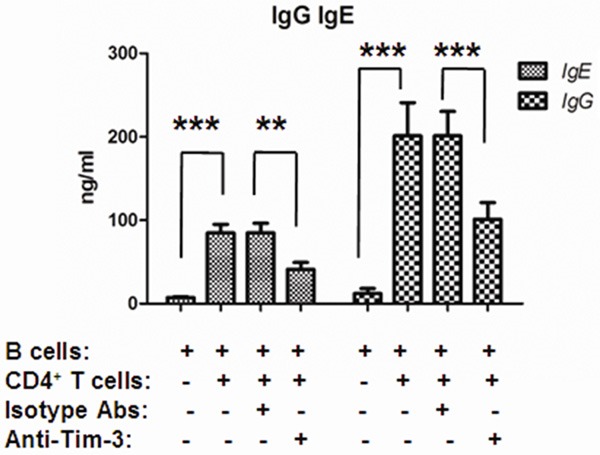
Anti-Tim3 reduced IgG and IgE production by T-cell helped B cells. CD4+ T cells and B cells were sorted and co-cultured in vitro (5×104 T cells and 5×104 B cells) for 12 days. Anti-human Tim-3 antibodies or isotype control antibodies were added in the co-culture system. The supernatant were collected at Day 12 and determined for IgG or IgE by ELISA (**P < 0.01; ***P < 0.0001).
Discussion
Upon activation, CD4+ T cells differentiate into distinct effector subpopulations which produce distinct patterns of cytokines and mediate different types of immune response. Type 1 helper T cells (Th1) cells produced interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin-12 (IL-12) and mediate cellular immune response to intracellular bacteria or viruses, whereas type 2 helper T cells (Th2) produced IL-4, IL-5, IL-13 and mediate humoral immune response against extracellular bacteria or helminthes [13]. The pathological consequences of uncontrolled Th1/Th2 response are related to autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) and allergic asthma. In this study, CD4+ T cells were sorted and analyzed for the transcription factors and signature cytokines of Th1/Th2 cells. An imbalance towards Th2 cells was found in patients with allergic asthma. It was consistent with reported data [14,15], and suggested that Th2 polarized immune response was responsible for the pathogenesis of allergic asthma.
T cell Ig and mucin domain-containing molecule-3 (Tim-3) is a negative regulatory molecule on T cells. A decreased level of Tim-3 mRNA and protein was observed in patients with sarcoidosis, resulting in over-activated Th1 immune response and high level of IL-2 and IFN-γ production [16]. Moreover, Polymorphisms in human Tim-3 had been reported significantly associated to atopy and eczema [17,18]. It suggested that Tim-3 may functionally affect T cell response and influence susceptibility to allergic diseases. In this study, we found that expression of Tim-3 on CD4+ T cells was much higher in patients with allergic asthma, demonstrating that CD4+ T cells of asthma patients were in a more suppressed state than healthy controls. Since Tim-3 was selectively expressed on Th1 cells but not Th2 cells, high expression level of Tim-3 on CD4+ T cells of asthma patients may result in suppression of Th1 cells but not Th2 cells and thus lead to Th1/Th2 imbalance. To investigate the relationship between Tim-3 and Th1/Th2 imbalance, correlation between T-bet/GATA-3 ratio or IFN-γ/IL-4 ratio with percentage of Tim-3 on CD4+ T cells were made. We demonstrated that both T-bet/GATA-3 ratio and IFN-γ/IL-4 ratio were negatively correlated to the percentage of Tim-3, suggesting that Tim-3 functionally related to Th1/Th2 imbalance in allergic asthma patients.
Since elevated Tim-3 expression was related to the Th1/Th2 imbalance in asthma patients, blocking Tim-3 signaling may adjust this imbalance and be of therapeutic benefit to patients. In this study, anti-CD3 was used to mimic stimulation of peptide-MHC through T cell receptor (TCR) signaling [19], and both Th1 and Th2 cytokines were enhanced a lot after activation by anti-CD3. Blocking Tim-3 signaling significantly increased the production of Th1 cytokines such as IFN-γ and TNF-α, which is consistent with the published data [20], suggesting the negative role of Tim-3 signaling on T cells. Interestingly, Th2 cytokines such as IL-4, IL-5 and IL-13 were dramatically decreased after Tim-3 blockage. Because there is no expression of Tim-3 on Th2 cells, the decreased Th2 cytokines response may be an indirect outcome caused by the increased Th1 response, for instance, IFN-γ was able to promote naïve T cells differentiating into Th1 cells and suppress Th2 cell response [21]. By the way, it has been reported that Tim-3 was also expressed on Th17 cells [22], which plays a critical role in inflammatory and pathogenesis of severe asthma [23]. However, in this study, no difference of IL-17 production was found after blocking of Tim-3. This may be owing to the much lower expression of Tim-3 on Th17 cells comparing to Th1 cells [24], and the low frequency of Th17 cells in the mild-to-moderate asthma cohorts [25].
Besides of cytokines, T cells are also responsible for helping B cells differentiation and antibodies production [26]. What’s more, over-activated B cell response, specially the production of high titer of IgE, are involved in the pathogenesis of allergic asthma. In this study, CD4+ T cells and B cells from peripheral blood were isolated and cultured in vitro. Both the IgE and IgG in the supernatant were increased after T cells were added in the culture system, and anti-Tim-3 significantly reduced the production of both IgG and IgE. It suggested that blocking of Tim-3 signaling was beneficial for attenuation of T-cell helped B cell response. This could be partially because of the decreased Th2 cytokines produced after Tim-3 blockage, since IL-4 has been reported to help IgE class switching and promote antibody production [27]. The mechanism under this need to be further investigated.
In summary, this study demonstrated that up-regulated Tim-3 on T cells was responsible for Th1/Th2 imbalance in allergic asthma patients. Blocking of Tim-3 signaling enhanced Th1 cytokines response, decreased Th2 cytokines response and reduced antibody response. It may be therapeutically beneficial for adjustment of Th2 polarized immune response in allergic asthma patients.
Acknowledgements
This study was supported by Science and Technology Department of Hebei province (Mandatory subject, Grant No. ZL20140227).
Disclosure of conflict of interest
None.
References
- 1.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;34:509–520. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 3.Bice JB, Leechawengwongs E, Montanaro A. Biologic targeted therapy in allergic asthma. Ann Allergy Asthma Immunol. 2014;112:108–115. doi: 10.1016/j.anai.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Aryan Z, Holgate ST, Radzioch D, Rezaei N. A new era of targeting the ancient gatekeepers of the immune system: toll-like agonists in the treatment of allergic rhinitis and asthma. Int Arch Allergy Immunol. 2014;164:46–63. doi: 10.1159/000362553. [DOI] [PubMed] [Google Scholar]
- 5.Heijink IH, Van Oosterhout AJ. Strategies for targeting T-cells in allergic diseases and asthma. Pharmacol Ther. 2006;112:489–500. doi: 10.1016/j.pharmthera.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 8.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 9.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 10.Das M, Tomar N, Sreenivas V, Gupta N, Goswami R. Effect of vitamin D supplementation on cathelicidin, IFN-γ, IL-4 and Th1/Th2 transcription factors in young healthy females. Eur J Clin Nutr. 2014;68:338–343. doi: 10.1038/ejcn.2013.268. [DOI] [PubMed] [Google Scholar]
- 11.Jenner RG, Townsend MJ, Jackson I, Sun K, Bouwman RD, Young RA, Glimcher LH, Lord GM. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc Natl Acad Sci U S A. 2009;106:17876–17881. doi: 10.1073/pnas.0909357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elser B, Lohoff M, Kock S, Giaisi M, Kirchhoff S, Krammer PH, Li-Weber M. IFN-gamma represses IL-4 expression via IRF-1 and IRF-2. Immunity. 2002;17:703–712. doi: 10.1016/s1074-7613(02)00471-5. [DOI] [PubMed] [Google Scholar]
- 13.Barnes AG, Cerovic V, Hobson PS, Klavinskis LS. Bacillus subtilis spores: a novel microparticle adjuvant which can instruct a balanced Th1 and Th2 immune response to specific antigen. Eur J Immunol. 2007;37:1538–1547. doi: 10.1002/eji.200636875. [DOI] [PubMed] [Google Scholar]
- 14.Seumois G, Chavez L, Gerasimova A, Lienhard M, Omran N, Kalinke L, Vedanayagam M, Ganesan AP, Chawla A, Djukanović R, Ansel KM, Peters B, Rao A, Vijayanand P. Epigenomic analysis of primary human T cells reveals enhancers associated with TH2 memory cell differentiation and asthma susceptibility. Nat Immunol. 2014;15:777–788. doi: 10.1038/ni.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Daghri NM, Alokail MS, Draz HM, Abd-Alrahman SH, Yakout SM, Clerici M. Th1/Th2 cytokine pattern in Arab children with severe asthma. Int J Clin Exp Med. 2014;7:2286–2291. [PMC free article] [PubMed] [Google Scholar]
- 16.Idali F, Wahlström J, Dahlberg B, Khademi M, Olsson T, Eklund A, Grunewald J. Altered expression of T cell immunoglobulin-mucin (TIM) molecules in bronchoalveolar lavage CD4+ T cells in sarcoidosis. Respir Res. 2009;10:42. doi: 10.1186/1465-9921-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graves PE, Siroux V, Guerra S, Klimecki WT, Martinez FD. Association of atopy and eczema with polymorphisms in T-cell immunoglobulin domain and mucin domain-IL-2-inducible T-cell kinase gene cluster in chromosome 5 q 33. J Allergy Clin Immunol. 2005;116:650–656. doi: 10.1016/j.jaci.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Ju Z, Frieri M. The T-cell immunoglobulin and mucin domain (Tim) gene family in asthma, allergy, and autoimmunity. Allergy Asthma Proc. 2013;34:e21–26. doi: 10.2500/aap.2013.34.3646. [DOI] [PubMed] [Google Scholar]
- 19.Boding L, Nielsen MW, Bonefeld CM, von Essen MR, Nielsen BL, Lauritsen JP, Hansen AK, Nielsen MM, Kongsbak M, Rubin M, Vennegaard MT, Odum N, Geisler C. Polymorphisms of the T cell receptor CD3delta and CD3epsilon chains affect anti-CD3 antibody binding and T cell activation. Mol Immunol. 2010;47:2450–2457. doi: 10.1016/j.molimm.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 21.Tang C, Inman MD, van Rooijen N, Yang P, Shen H, Matsumoto K, O’Byrne PM. Th type 1-stimulating activity of lung macrophages inhibits Th2-mediated allergic airway inflammation by an IFN-gamma-dependent mechanism. J Immunol. 2001;166:1471–1481. doi: 10.4049/jimmunol.166.3.1471. [DOI] [PubMed] [Google Scholar]
- 22.Kanai Y, Satoh T, Igawa K, Yokozeki H. Impaired expression of Tim-3 on Th17 and Th1 cells in psoriasis. Acta Derm Venereol. 2012;92:367–371. doi: 10.2340/00015555-1285. [DOI] [PubMed] [Google Scholar]
- 23.Song L, Guo Y, Deng Q, Li J. TH17 functional study in severe asthma using agent based model. J Theor Biol. 2012;309:29–33. doi: 10.1016/j.jtbi.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chesné J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in Severe Asthma. Where Do We Stand? Am J Respir Crit Care Med. 2014;190:1094–1101. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- 26.Alam S, Knowlden ZA, Sangster MY, Sant AJ. CD4 T cell help is limiting and selective during the primary B cell response to influenza virus infection. J Virol. 2014;88:314–324. doi: 10.1128/JVI.02077-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashiwada M, Levy DM, McKeag L, Murray K, Schröder AJ, Canfield SM, Traver G, Rothman PB. IL-4-induced transcription factor NFIL3/E4BP4 controls IgE class switching. Proc Natl Acad Sci U S A. 2010;107:821–826. doi: 10.1073/pnas.0909235107. [DOI] [PMC free article] [PubMed] [Google Scholar]


