Abstract
Systemic scleroderma is an autoimmune disease characterized by fibrotic changes in skin and other organs involving excessive collagen deposition. The transforming growth factor-β (TGF-β) signaling pathway plays a key role in the fibrotic process in systemic scleroderma (SSc). Astragalus polysaccharides (APS) isolated from one of the Chinese herbs, Astragalus mongholicus, are known to have a variety of immunomodulatory activities. The present study aimed to investigate the effect of APS on TGF-β signaling and its potential mechanism using a murine model of bleomycin-induced scleroderma. Scleroderma was induced in C3H/He N mice by subcutaneous bleomycin injections daily for 21 days. Skin samples were obtained 7, 14, and 21 days and TGF-β1, Smad2, Smad3 mRNA expression was observed by real time PCR. The hydroxyproline content which consistent with the collagen content in skin samples from the BLM-injected group was significantly higher than the PBS group, and corresponded with dermal thickening at the injection site. In contrast, mice treated with APS after initiating BLM injection showed obviously lesser collagen content. Increased TGF-β1, Smad2, Smad3 mRNA expression were also observed in the BLM group. TGF-β1, Smad2, Smad3 expression was significantly lesser for the APS group than for the BLM group. In contrast, TGF-β1 mRNA expression was remarkably inhibited by APS. These results suggest that APS treatment may inhibit TGF-β1 production, and thus could be a potential drug for managing fibrotic disorders such as SSc.
Keywords: Bleomycin-induced fibrosis, APS, transforming growth factor-β1
Introduction
Systemic sclerosis (SSc) is an autoimmune disease characterized by severe alterations in the microvasculature, prominent inflammatory and immunological changes, and excessive accumulation of extracellular matrix in the skin and internal organs [1,2]. Despite research efforts, no standard protocol for treating SSc has been established to date. Several cytokines, chemokines, and growth factors have been shown to be strongly associated with the pathogenesis of SSc, including the transforming growth factor-beta (TGF-beta), interleukin-4 (IL-4), monocyte chemoattractant protein-1 (MCP-1/CCL2) [3-5].
The dried root of Astragalus membranaceus (Huangqi) has a long history of medicinal use in traditional Chinese medicine. It is now commonly used as an immunomodulating agent in mixed herbal decoctions to treat common cold, diarrhea, fatigue and anorexia, and it is also prescribed to patients with cardiac diseases [6-8]. In recent years, radix Astragalus membranaceus has also been used to ameliorate the side effects of cytotoxic anti-neoplastic drugs [9]. The active pharmacological constituents of radix Astragalus membranaceus include various polysaccharides, saponins and flavonoids. Among these, Astragalus polysaccharides (APS) have been most widely studied, mainly on their immunopotentiating properties like stimulation of murine B-cell proliferation and cytokine production [10]. Apart from these actions, clinical studies also showed that APS could counteract the side effects of chemotherapeutic drugs, such as a significant amelioration in the degree of myelosuppression in cancer patients [11].
In the present study, we investigated the influence of APS administration on inducing dermal sclerosis using the BLM-based murine model, and identified that APS attenuated excessive collagen accumulation by inhibiting the expression of transforming growth factor beta (TGF-β) in this model.
Materials and methods
Drugs
APS for clinical application is composed of a-1,4 (1,6) glucan, arabinose-galactose polysaccharides, rhamnose-galacturonic acid polysaccharides, and arabinose galactose protein polysaccharide with molecular weights of 20000-60000, and an endotoxin content less than 0.1 EU/mg.
Mice
Specific pathogen-free, female C3H/He N mice 6 weeks old, weighing 20 g, were purchased from Vital River Laboratories, Beijing, China. All animals were housed in separate cages in a temperature-controlled room with 12 hour (h) light and 12 h darkness to acclimatize for at least 7 days before being used. All experimental manipulations were undertaken in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of the General Hospital of Beijing region of PLA, Beijing, China.
Model establishment and treatment
The BLM-induced murine fibrosis model was performed using a previously described method. Briefly, 100 uL BLM solutions at the concentration of 600 ug/ml were injected subcutaneously into the shaved back of mice using 27-gauge needles daily for 21 days. Mice in control group were administered with 100 ul PBS. 54 mice were randomly divided into three groups as follows: BLM group (18 mice), control group (18 mice), BLM with APS (200 mg/kg) treatment group (18 mice). All of these groups were further divided into three subgroups of six mice each, and they were sacrificed on 1, 2, 3 weeks after the start of the experiment, respectively. Animals of all groups were sacrificed at designated time points, and peripheral blood samples were collected to procure ELISA immediately. The skin tissue around the injection site of all the mice of PBS, BLM group, and APS treatment groups were employed to evaluate the therapeutic effect of APS.
Measurement of collagen content
Punch biopsies (6 mm) were obtained from the shaved back skin of C3H mice after 7, 14 and 21 days of treatment. Collagen deposition was estimated by determining the total content of hydroxyproline in the skin. The stored skin pieces were hydrolyzed with 6 N hydrochloric acid at 110°C for 18 h. After neutralization with sodium hydroxide, the hydrolysates were diluted with distilled water. Hydroxyproline in the hydrolysates was assessed colorimetrically at 560 nm with p-dimethylaminobenzaldehyde. The results are expressed as micrograms of hydroxyproline per 6-mm diameter skin piece.
Histological examination
Skin samples were obtained from shaved backs of mice 7 days/2 weeks/3 weeks after BLM treatment. Skin sections were routinely stained with hematoxylin and eosin. In addition, collagen production was identified by Masson’s trichrome stain. The dermal thickness of BLM- (BLM and APS groups) and PBS-(control group) injected skin were calculated using an image processor Win Roof (Mitani Corp., Tokyo, Japan). To avoid bias, the histologist was single blinded to the treatment groups of mice.
ELISA
Punched skin samples were obtained from shaved backs of mice 7 days after BLM treatment. Each sample homogenized in Dulbecco’s modified Eagle’s medium, centrifuged for 5 min at 200 g, and the supernatants were collected. Concentrations of several inflammatory cytokines and chemokines were measured by commercial ELISA kit (Biosciences, San Diego, CA) following the manufacturer’s instructions.
Immunohistochemical examination
Paraffin-embedded sections (5 μm) were washed with PBS, treated with 3% (v/v) H2O2 for 5 min, blocked with 10% (w/v) normal goat serum for 1 h, and then incubated with primary antibodies, including rabbit antibodies to Iba-1, α-SMA (ABCAM, UK), HGF (Santa Cruz Biotechnology, Santa Cruz, CA, USA), TGF-β1 (Santa Cruz Biotechnology), collagen I, and fibronectin (Boshide Biological Technology Co., Ltd., Wuhan, China), at 4°C overnight as described previously (Xie et al., 2008). Slides then were incubated with appropriate biotin-conjugated secondary immunoglobulin G, and treated with reagents from a Vecta-Elite Burlingame, CA, USA). The scores were determined as previously reported (Fu et al., 2006). Briefly, the percentage of positive stained area in the examined tubulointerstitium was measured and scored in a blind manner on slides.
Real time-PCR
Total RNA was isolated from skin samples of the model mice (7, 14, 21 days after initiating BLM treatment) using the Total RNA kit (Omega Inc., USA). All samples were treated with RNA Stabilization Reagent (RNA later, Qiagen, Valencia, CA) at 37°C overnight and stored at -80°C until use. Total RNA was reverse-transcribed into cDNA according to the manufacturer’s protocol for the Reverse Transcription System (TIAN Script RT Kit, Beijing, China). mRNA expression was analyzed by quantitative RT-PCR according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Sequence-specific primers were designed using Primer Premier 5.0 Software. RT-PCR (1 cycle at 95°C for 3 min, 40 cycles at 95°C for 3 s and 60°C for 20 s, and 1 cycle at 95°C for 15 s, 60°C for 15 s and 97°C for 15 s.) was performed using a real-time PCR System 7900HT (Applied Biosystems). Actin was used to normalize mRNA. To compare EGR1, Smad2, Smad3, TGFβ1 and TGIF1 mRNA and housekeeping Actin mRNA expression, the relative expression of PCR products was determined using the 2-ΔΔCt method.
Western blot analysis
Protein samples were heated at 99°C for 5 min and then transferred to a polyvinylidene difluoride membrane. Nonspecific binding to the membrane was blocked for 1 h at room temperature with 5% (w/v) bovine serum albumin (BSA) in Tris-buffered saline buffer (20 mmol/L Tris-HCl, 150 mmol/L NaCl, and 0.1% (v/v) Tween 20). The membranes were then incubated overnight at 4°C with primary antibodies, including rabbit antibodies to α-SMA, rabbit antibodies to HGF, rabbit antibodies to TGF-β1, and GAPDH (Chemicon, Temecula, CA). After washing, the membranes were incubated with appropriate secondary immunoglobulin G-peroxidase conjugates, and developed using the enhanced chemiluminescence method. Band intensities were analyzed using a software named Quantity one. For quantification, the densities of the signals were normalized to that for GAPDH.
Statistical analysis
All data were expressed as mean ± SD. One-way analysis of variance (ANOVA) was used to perform comparisons between the different groups, and a P value < 0.05 was considered as statistically significant. All analyses were performed with the SPSS statistical software package (SPSS, Chicago, IL, USA).
Results
APS reduced collagen production in BLM-treatment skin
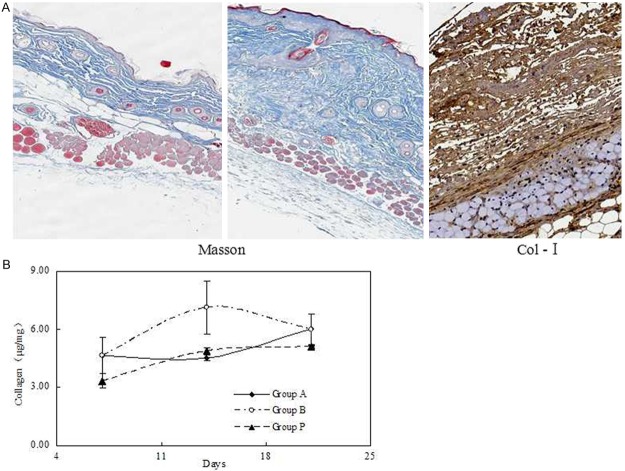
Thickening in the dermal layer was corresponded to accumulation of collagen by Masson’s trichrome stain and immunohistochemistry of type I collagen (Figure 1A). We determined the collagen content in skin samples from mice treated with BLM for 35 days, and found that they had significantly higher collagen content than the PBS group. Supplementary we confirmed that the similar results were obtained when the extent of fibrosis was determined with the contents of hydroxyproline, another index of fibrosis (Figure 1B).
Figure 1.
Collagen content and the effect of APS treatment at different time. A. Collagen in the dermis was observed Masson’s trichrome stains and immunohistochemistry of type I collagen in the BLM-treated mice. B. APS was administered to mice intravenously after initiating BLM treatment and contents of hydroxyproline were detected to determine collagen content.
APS reduced TGF-beta in BLM-treatment skin
To examine the influence of TGF-β1 on APS mechanisms in early SSc, we obtained a skin sample 7 days after initiating the experiment. We subsequently determined mRNA expression levels. We found that TGF-β1 mRNA levels were upregulated in the BLM group compared to the PBS group, and were subsequently suppressed in the APS group (Figure 2).
Figure 2.
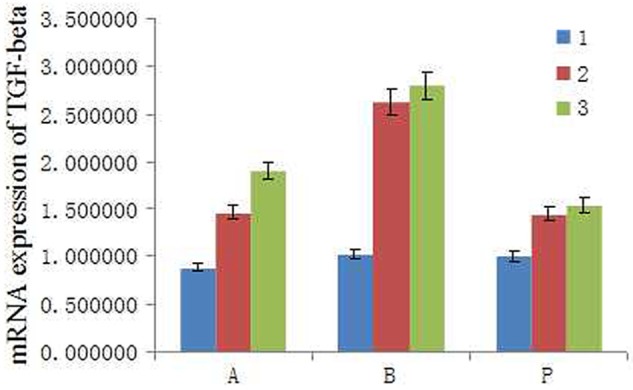
Expression of TGF- β1 mRNA in lesional skin of mice. Mice were locally treated with either PBS or BLM for 5 days and total mRNA was isolated from skin samples. APS was administered intravenously after initiating BLM treatment. Skin samples were obtained after 7 days to determine TGF- β1 mRNA expression. Representative data are shown from 3 independent experiments.
APS suppressed fibrogenic cytokine and chemokine in the skin
We examined MCP-1 expression in this experimental in vivo model, and found that MCP-1 levels were significantly increased in the BLM group compared to the PBS group. APS treatment lowered the expression of MCP-1 (Figure 3A). We also measured mRNA expression levels of INF-γ (Figure 3B), TNF-α (Figure 3C), and IL-4 (Figure 3D).
Figure 3.
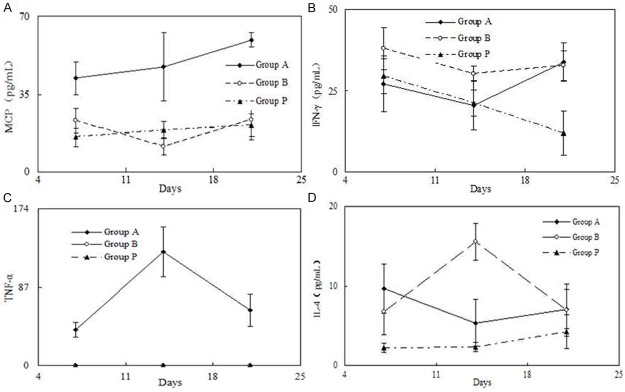
Fibrogenic cytokine and chemokine in the skin. Mice were locally treated with either PBS or BLM for 5 days, and APS was administered intravenously immediately after initiating BLM treatment. Skin samples were homogenized, centrifuged, and supernatants measured by ELISA to detect the expression of MCP-1 (A), INF-γ (B), TNF-α (C), and IL-4 (D).
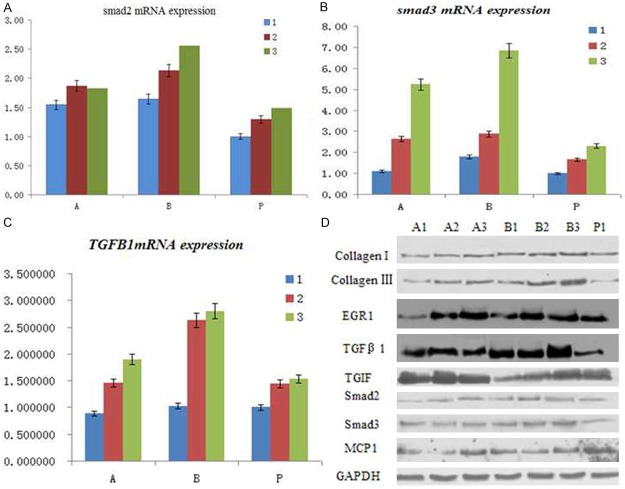
Changes in the protein expression after APS treatment in the skin
We next examined the mRNA expression of smad2 (Figure 4A), smad3 (Figure 4B), TGF-beta1 (Figure 4C) as well as the corresponding protein expression (Figure 4D) in this experimental in vivo model, and results showed that that these protein levels were significantly increased in the BLM group compared to the PBS group. APS treatment lowered the expression of these proteins.
Figure 4.
Changes in the mRNA and protein expression after APS treatment in the skin. Mice were locally treated with either PBS or BLM for 5 days, and APS was administered intravenously immediately after initiating BLM treatment. Real time PCR was used to examine the mRNA expression of smad2 (A), smad3 (B), TGF-beta1 (C) as well as the corresponding protein expression by western blot (D).
Discussion
In this study, we used BLM-induced murine fibrotic models to investigate the efficacy and mechanisms of APS in treating SSc. In our first experiments, we observed dermal thickening and excess collagen production in the lesion skin of BLM-treated mice.
Besides, APS was demonstrated to antagonize fibrosis induced by various profibrotic agents. For example, an earlier in vivo study showed that APS application prevented collagen accumulation in the BLM-injured rat lung [12]. In accordance with these previous findings, our results also support the anti-fibrotic activity of APS.
We also examined how APS affected the lesional skin. APS was found to partially suppress the increase in dermal thickness, as well as collagen content induced by BLM treatment. The concentration of APS and the time schedule used in this study was implemented to match typical clinical.
Upon treatment of APS, these TGF-β-induced effects were potently inhibited. The antifibrotic activity of APS was demonstrated in multiple pathological tissues. TGF-β signaling alterations have been proposed as a key molecular event in the fibrosis of scleroderma [13-15]. Although there is no evidences of significant difference in the levels of total and active TGF-β protein between normal and SSc fibroblast cultures, increased expression of TGF-β receptors have been reported in SSc fibroblasts that contributes to the establishment of autocrine TGF-β signaling [16].
In this study, we carefully analyzed the effect of APS on TGF-β signaling in SSc. Characteristically, TGF-β signaling activation results in Smad2/3 phosphorylation and upregulation of several downstream target genes [17,18]. This is verified by our findings that BLM treatment significantly induced Smad2/3 phosphorylation and upregulation of type I collagen. Besides, enhanced TGF-β signaling also can be via accelerating internalization of TGF-β receptors in SSc fibroblasts [19,20]. Sustained activation of TGF-β signaling will result in enhanced deposition of ECM, which is the hallmark of scleroderma [21].
In conclusion, the findings in the present study confirmed the antifibrotic activity of APS by suppression of TGF-β signaling pathway.
Acknowledgements
This study was supported by the capital health research and development of special of China (No. 2011-5021-01).
Disclosure of conflict of interest
None.
References
- 1.Akamata K, Asano Y, Aozasa N, Noda S, Taniguchi T, Takahashi T, Ichimura Y, Toyama T, Sato S. Bosentan reverses the pro-fibrotic phenotype of systemic sclerosis dermal fibroblasts via increasing DNA binding ability of transcription factor Fli1. Arthritis Res Ther. 2014;16:R86. doi: 10.1186/ar4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levis B, Burri A, Hudson M, Baron M, Thombs BD. Sexual activity and impairment in women with systemic sclerosis compared to women from a general population sample. PLoS One. 2012;7:e52129. doi: 10.1371/journal.pone.0052129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandinelli F, Del RA, Gabrielli A, Giacomelli R, Bartoli F, Guiducci S, Matucci CM. CCL2, CCL3 and CCL5 chemokines in systemic sclerosis: The correlation with SSc clinical features and the effect of prostaglandin E1 treatment. Clin Exp Rheumatol. 2012;30:S44–S49. [PubMed] [Google Scholar]
- 4.Hasegawa M, Takehara K. Potential immunologic targets for treating fibrosis in systemic sclerosis: A review focused on leukocytes and cytokines. Semin Arthritis Rheum. 2012;42:281–296. doi: 10.1016/j.semarthrit.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Liakouli V, Cipriani P, Marrelli A, Alvaro S, Ruscitti P, Giacomelli R. Angiogenic cytokines and growth factors in systemic sclerosis. Autoimmun Rev. 2011;10:590–594. doi: 10.1016/j.autrev.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Li M, Wang W, Xue J, Gu Y, Lin S. Meta-analysis of the clinical value of Astragalus membranaceus in diabetic nephropathy. J Ethnopharmacol. 2011;133:412–419. doi: 10.1016/j.jep.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Wojcikowski K, Wohlmuth H, Johnson DW, Gobe G. Effect of Astragalus membranaceus and Angelica sinensis combined with Enalapril in rats with obstructive uropathy. Phytother Res. 2010;24:875–884. doi: 10.1002/ptr.3038. [DOI] [PubMed] [Google Scholar]
- 8.Gao XH, Xu XX, Pan R, Li Y, Luo YB, Xia YF, Murata K, Matsuda H, Dai Y. Saponin fraction from Astragalus membranaceus roots protects mice against polymicrobial sepsis induced by cecal ligation and puncture by inhibiting inflammation and upregulating protein C pathway. J Nat Med. 2009;63:421–429. doi: 10.1007/s11418-009-0348-2. [DOI] [PubMed] [Google Scholar]
- 9.Yang B, Xiao B, Sun T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2013;62:287–290. doi: 10.1016/j.ijbiomac.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Li XT, Zhang YK, Kuang HX, Jin FX, Liu DW, Gao MB, Liu Z, Xin XJ. Mitochondrial protection and anti-aging activity of astragalus polysaccharides and their potential mechanism. Int J Mol Sci. 2012;13:1747–1761. doi: 10.3390/ijms13021747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang B, Xiao B, Sun T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2013;62:287–290. doi: 10.1016/j.ijbiomac.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Zhou XM, Zhang GC, Li JX, Hou J. Inhibitory effects of Hu-qi-yin on the bleomycin-induced pulmonary fibrosis in rats. J Ethnopharmacol. 2007;111:255–264. doi: 10.1016/j.jep.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Zhu H, Luo H, Li Y, Zhou Y, Jiang Y, Chai J, Xiao X, You Y, Zuo X. MicroRNA-21 in scleroderma fibrosis and its function in TGF-beta-regulated fibrosis-related genes expression. J Clin Immunol. 2013;33:1100–1109. doi: 10.1007/s10875-013-9896-z. [DOI] [PubMed] [Google Scholar]
- 14.Leask A. Signaling in fibrosis: Targeting the TGF beta, endothelin-1 and CCN2 axis in scleroderma. Front Biosci (Elite Ed) 2009;1:115–122. doi: 10.2741/E12. [DOI] [PubMed] [Google Scholar]
- 15.Lakos G, Takagawa S, Chen SJ, Ferreira AM, Han G, Masuda K, Wang XJ, DiPietro LA, Varga J. Targeted disruption of TGF-beta/Smad3 signaling modulates skin fibrosis in a mouse model of scleroderma. Am J Pathol. 2004;165:203–217. doi: 10.1016/s0002-9440(10)63289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honda N, Jinnin M, Kajihara I, Makino T, Makino K, Masuguchi S, Fukushima S, Okamoto Y, Hasegawa M, Fujimoto M, Ihn H. TGF-beta-mediated downregulation of microRNA-196a contributes to the constitutive upregulated type I collagen expression in scleroderma dermal fibroblasts. J Immunol. 2012;188:3323–3331. doi: 10.4049/jimmunol.1100876. [DOI] [PubMed] [Google Scholar]
- 17.Shi S, Yu L, Zhang T, Qi H, Xavier S, Ju W, Bottinger E. Smad2-dependent downregulation of miR-30 is required for TGF-beta-induced apoptosis in podocytes. PLoS One. 2013;8:e75572. doi: 10.1371/journal.pone.0075572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sengupta S, Kundu S, Bhattacharyya A. Attenuation of Smad2 activity shows resistance to TGF-beta signalling in mammary adenocarcinoma (MCF-7) cells. Cell Biol Int. 2013;37:449–457. doi: 10.1002/cbin.10061. [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Zhang B, Pat BK, Wei MQ, Gobe GC. Lentiviral-mediated RNA interference against TGF-beta receptor type II in renal epithelial and fibroblast cell populations in vitro demonstrates regulated renal fibrogenesis that is more efficient than a nonlentiviral vector. J Biomed Biotechnol. 2010;2010:859240. doi: 10.1155/2010/859240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee EK, Lee YS, Han IO, Park SH. Expression of Caveolin-1 reduces cellular responses to TGF-beta1 through down-regulating the expression of TGF-beta type II receptor gene in NIH3T3 fibroblast cells. Biochem Biophys Res Commun. 2007;359:385–390. doi: 10.1016/j.bbrc.2007.05.121. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Fong CC, Yu WK, Chen Y, Wei F, Koon CM, Lau KM, Leung PC, Lau CB, Fung KP, Yang M. Herbal formula Astragali Radix and Rehmanniae Radix exerted wound healing effect on human skin fibroblast cell line Hs27 via the activation of transformation growth factor (TGF-beta) pathway and promoting extracellular matrix (ECM) deposition. Phytomedicine. 2012;20:9–16. doi: 10.1016/j.phymed.2012.09.006. [DOI] [PubMed] [Google Scholar]