Abstract
Background: Osteoporosis is a significant cause of morbidity and mortality in the elderly and an important public health issue. Bisphosphonates are the primary treatment options for osteoporosis. The oral administration of bisphosphonates may result in poor patient compliance and thence reduced treatment efficacy. Intravenously administered bisphosphonates may therefore show better treatment efficacy. We have carried out a meta-analysis to evaluate the efficacy of zoledronic acid treatment for osteoporosis in both men and women with either vertebral or non-vertebral fracture. Material and methods: Randomized controlled trials with zoledronic acid treatment for osteoporosis were retrieved from PubMed, EMBASE and clinicaltrials.gov. The risk ratio with 95% confidence interval (RR, 95% CI) was calculated to evaluate the effect of zoledronic acid treatment on incidence of fracture. Data on changes in bone mineral density (BMD) following zoledronic acid (ZOL) treatment was also extracted. STATA software was used for all the statistical analyses. Results:Significant reduction in the incidence of both vertebral and nonvertebral fracture was observed following ZOL treatment, as seen from the values for RR with 95% CI (RR 0.24 and 95% CI 0.15 to 0.40 for vertebral fractures; RR 0.76 and 95% CI 0.67 to 0.86 for nonvertebral fractures). BMD was also seen to be increased after ZOL treatment.Conclusion: Ourmeta-analysis showed that zoledronic acid was effective in reducing the incidence of vertebral fractures as well as nonvertebral fractures, including hip fractures. Significant increase in bone mineral density (BMD) was also observed in patients administered ZOL as compared to placebo.
Keywords: Bisphosphonates, hip fracture, bone mineral density, osteoporosis
Introduction
Osteoporosis is a skeletal disease characterized by bone fragility and increased susceptibility to fractures. This medical condition is strongly associated with age and is a significant cause of morbidity and mortality in the elderly, affecting both men and women. According to World Health Organization (WHO) data, 75 million people throughout US, Europe and Japan are affected by osteoporosis [1]. Between 1990 and 2050, the incidence of hip fracture will increase up to 240% in women and 310% in men [2]. Thus osteoporosis is an important global public health issue with both societal and economic implications.
Osteoporotic fractures can occur anywhere in the skeleton; amongst these fractures, vertebral, hip and wrist fractures are more common. Vertebral fractures, while painless to a large extent, can potentially result in height loss and respiratory dysfunction. An associated drawback is that this also results in reduced quality of life and social withdrawal, all of which result in significant morbidity [3,4]. Fractures of the hip are associated with increased mortality rates, with most deaths occurring in the first 3-6 months after the event [5].
The lifetime fracture risk is higher in women, compared to men as evidenced by the overall 10-year fracture risk at 50 years of age (9.8% in women, 7.1% in men). At 80 years, this increases to 21.7% in women and 8% in men [6]. This can be attributed to the different microstructural changes occurring in men and women. Trabecular thinning with increasing age is seen in both sexes, while trabecular dropout is observed in women. These factors, together with the larger bone size and lesser degree of cortical thinning in men with age, may contribute to the lower fracture risk in men compared to women [7]. Though the risk is lower, osteoporotic fractures constitute an important cause of morbidity and mortality in men as well [8].
The therapeutic options for osteoporotic fractures include bisphosphonates, PTH analogues, selective estrogen receptor modulators (SERMS), denosumab (a monoclonal antibody) and strontium ranelate [7]. Amongst these, bisphosphonates, which are most common, reduce bone resorption, a process mediated by osteoclasts [9]. This class of compounds is further classified as non-nitrogen containing and nitrogen-containing bisphosphonates. The latter group (which includes alendronate, ibandronate, risedronate) are more effective than the former (clodronate and etidronate) [5]. An inherent drawback is that oral absorption of bisphosphonates is poor (less than 1%). Oral administration requires strict adherence to guidelines: fasting prior to the administration is required and the patient must not lie down for 30 minutes following administration, as the drugs cause oesophageal irritation [10]. This leads to poor patient compliance and lower treatment efficacy. Less frequent dosing regimens have not had a major impact on patient compliance [11]. However, there are reports stating the use of enteric coated risedronate, delayed release oral formulation that ensures adequate bioavailability of risedronate when taken with food [21]. Intravenous bisphosphonates, such as zoledronic acid, a nitrogen containing bisphosphonate, present an alternative option to oral bisphosphonates, avoiding gastrointestinal adverse effects and offering regimens with less frequent dosing and thus improving adherence to therapy.A single intravenous infusion of zoledronic acid significantly improves the bone mineral density and the risk of bone fracture is decreased [12]. Zoledronic acid, after i.v infusion, rapidly localizes to the bone and inhibits osteoclastic bone resorption by inhibiting farnesyl pyrophosphate synthase action in mevalonate pathway. Zoledronic acid has high binding affinity for hydroxyapatite and is efficacious in increasing bone mineral density [13]. However, long term treatment of zoledronic acid has certain side effects, though rare, such as musculoskeletal pain, osteonecrosis of jaw, esophageal cancer and more, thus the potential side effects of this bisphosphonate are to be mentioned a prior to the patient before administering [22].
There are multiple reviews which state the significance of Zoledronic acid treatment in osteoporotic patients, however, we found lacunae with respect to its treatment in vertebral fracture, non-vertebral fracture and hip fracture in both the sexes and thus this meta-analysis was derived on. Here we report the Meta-analysis depicting the significance of Zoledronic acid in treating both men and women who are suffering from vertebral and non-vertebral fractures.
Materials and methods
Literature search
PubMed, EMBASE and clinicaltrials.gov were searched for articles published between 2003- 2014. The search terms used were ‘bisphosphonate and osteoporosis’, ‘zoledronic acid and osteoporosis’, ‘zoledronic acid and vertebral fracture’; ‘zoledronic acid and non-vertebral fracture’.
Inclusion and exclusion criteria
Inclusion criteria: Studies were included, if they met all the following criteria: a) The study must be a randomized, controlled trial. b) Osteoporosis was the primary diagnosis. c) It included postmenopausal women or individuals aged >50 years. d) Fracture was the primary outcome. e) Data for changes in bone mineral density (BMD) over the treatment period was available. The exclusion criteria included: a) Studies in patients with cancer related condition or any other disease. b) Studies in patients with a history of prolonged glucocorticoid use.
Data extraction
Two independent authors reviewed the title, abstracts and keywords of all the articles retrieved to determine whether the articles are relevant or irrelevant. The authors were contacted for any other additional information. The references list was also scrutinized for relevant trials by using author names, location and study type. Out of 52 articles retrieved, 36 were excluded based on the exclusion criteria. A detailed review of the 16 selected articles was carried out and ultimately, 3 articles [12,14,15], which met the inclusion criteria, were included in the study (Figure 1).
Figure 1.
Prisma Flow chart depicting selection and retrieval of studies for meta-analysis.
Statistical analyses
Statistical software STATA Intercooled Version 8.2 was used for statistical analyses. Risk ratio (RR) with 95% confidence interval (95% CI) was used to estimate the efficacy of zoledronic acid on the risk of fracture. The heterogeneity assumption was calculated using chi-square test (P<0.1). Data was combined according to random effects (DerSimonian and Laird’s method) or fixed effects model, depending on the significance of the τ2 statistic. If the heterogeneity was significant, random effects model was used; otherwise the fixed effects model was used.
Results
Literature search
The initial search retrieved 52 articles, from which 16 were selected for further detailed review. Among these, one was found to be a perspective article; another was a review while a third had been conducted in men undergoing therapy for prostate cancer. One study was not included as it did not include fracture as a primary outcome. The other studies were not considered due to duplication, and various publications were derived from the same data set. Three randomized, controlled trials were finally included for our meta-analysis.
Study characteristics
The characteristics of the selected studies are summarized in Table 1. The data related to a total of 10,974 patients, with 5,482 randomized to receive 5 mg ZOL as an annual infusion while 5,492 received a placebo. The patients were aged above 50 years and had either a diagnosis of osteoporosis or had undergone repair for a hip fracture. All patients received calcium and vitamin D supplements; in two of the trials, other therapy for osteoporosis was allowed at the discretion of the investigator.
Table 1.
Characteristics of the selected studies
| Sl. No. | Source (PMID) | No. of patients ZOL/placebo | Age (years) | Sex | Inclusion criteria | Double-blinding | Follow-up period |
|---|---|---|---|---|---|---|---|
| 1 | Boonen et.al., 2012 [14] (23113482) | 553/574 | 50-85 | M | Primary osteoporosis or osteoporosis associated with low testosterone levels | Yes | 2 years |
| 2 | Lyles et.al., 2007 [15] (17878149) | 1054/1057 | 74.6±9.86 (placebo), 74.4±9.48 (ZOL) | F, M | Recent hip fracture | Yes | Upto 5 years (Median time 1.9 years) |
| 3 | Black et.al., 2007 [12] (17476007) | 3875/3861 | 73 (65-89) | F | BMD T score , vertebral fracture | Yes | 24 months |
Vertebral fractures
Incidence of vertebral fractures was reported in all three trials. The estimated RR for vertebral fractures was 0.24 [95% CI, (0.15, 0.40), P=0.000]. The heterogeneity statistic τ2 =0.1322 was significant at the 5% CI, and hence the random effects model was used to arrive at the pooled D-L estimate. A significant reduction in the incidence of fracture was observed following treatment with ZOL (Figure 2).
Figure 2.
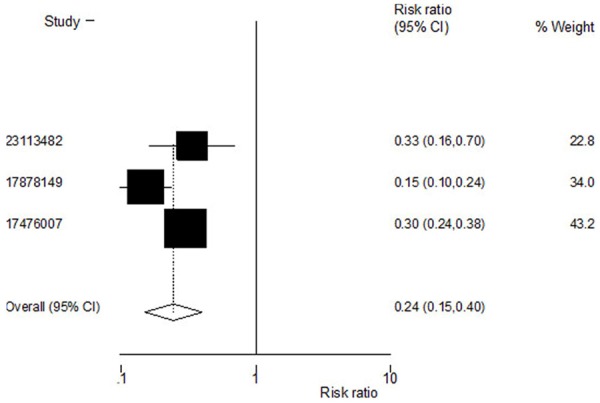
Effect of zoledronic acid on incidence of vertebral fractures.Pooled estimate for the risk of vertebral fractures shows that zoledronic acid reduces the risk of vertebral fractures. Heterogeneity chi-squared = 7.43 (d. f. = 2) P = 0.024. Estimate between-study variance Tau-squared = 0.1322; Test of RR = 1: z = 5.63 P = 0.000.
Nonvertebralfractures
Incidence of nonvertebral fractures was reported in two of the trials. The estimated RR for nonvertebral fractures was 0. 76 [95% CI, (0.67, 0.86), P=0.000]. The heterogeneity statistic τ2 was not significant at the 5% CI and therefore the fixed effects model was used to arrive at the pooled estimate. Zoledronic acid was observed to reduce the incidence of nonvertebral fractures (Figure 3).
Figure 3.
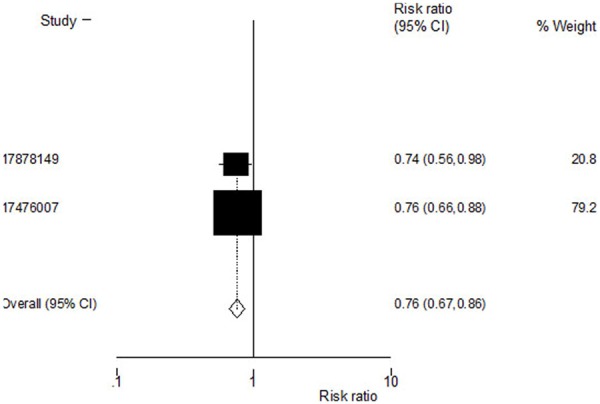
Effect of zoledronic acid on incidence of nonvertebral fractures.Pooled estimate for the risk of nonvertebral fractures showing a reduction in vertebral fractures with zoledronic acid.Heterogeneity chi-squared = 0.03 (d.f. = 1) P = 0.864; Test of RR = 1: z = 4.31 P = 0.000.
Hip Fractures
Incidences of hip fractures were reported in two out of three trials. The estimated RR for hip fractures was 0.73 [95% CI, (0.63, 0.84), P=0.000]. The fixed effects model was used to arrive at the pooled estimate since the heterogeneity statistic τ2 was not significant at the 5% CI. Treatment with ZOL contributed to a reduced incidence of hip fractures (Figure 4).
Figure 4.
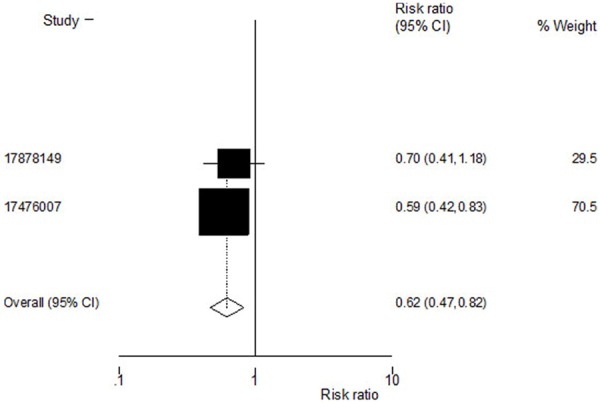
Effect of zoledronic acid on incidence of hip fractures. Pooled estimate for the risk of hip fractures shows that zoledronic acid reduces the risk of vertebral fractures. Heterogeneity chi-squared = 0.29 (d. f. = 1) P = 0.591; Test of RR = 1: z = 3.29 P = 0.001.
Change in BMD
The BMD changes at three sites, the total hip, femoral neck and lumbar spine; these were followed for a period of 24 months in the treatment and placebo group in the three trials included in the meta-analysis. Significant increases in BMD were observed on treatment with zoledronic acid at all three sites (Table 2), indicating its efficacy.
Table 2.
Difference in BMD between patients administered zoledronic acid and those administered placebo
| Sl. No. | Source (PMID) | Follow-up period | Difference in BMD between zoledronic acid and placebo (%) | P value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Total hip | Femoral neck | Lumbar spine | ||||
| 1 | Boonen et.al., 2012 (23113482) | 24 months | 2.10 | 3.30 | 6.10 | <0.001 |
| 2 | Lyles et.al., 2007 (17878149) | 24 months | 6.40 | 4.30 | NA | <0.001 |
| 3 | Black et.al., 2007 [12] (17476007) | 24 months | 6.02 | 5.06 | 6.71 | <0.001 |
NA: Not assessed.
Discussion
Bisphosphonates, which are the drugs of choice for the treatment of osteoporosis act by inhibiting osteoclastic activity [16] and can, therefore, reduce the incidence of fracture [17]. However, oral administration of bisphosphonates has limited efficacy due to poor patient compliance [10,11]. Zoledronic acid, which can be administered as an annual intravenous infusion, may thus improve treatment outcomes. The meta-analysis carried out showed that zoledronic acid was effective in reducing the incidence of fracture. It also shows that it has a wide spectrum of efficacy, reducing the incidence of both vertebral and nonvertebral fractures. Among nonvertebral fractures, its effect on reducing the incidence of new hip fractures is of significance, as hip fractures contribute to increased morbidity in the elderly [5].
The three trials included in the meta-analysis also provide evidence of an increase in BMD, as a significant increase was observed in the patients on zoledronic acid, compared to placebo, in all the trials. The baseline BMD values were available for both groups; however, for subsequent time points, the per cent change from the baseline value was reported, due to which a pooled estimate could not be calculated. However, the increase in BMD was found to be significant in all three trials (P<0.001), which supports an inference of an increase in BMD following zoledronic acid treatmentthe levels of markers of bone turnover(fasting serum β-C-terminaltelopeptide of type 1 collagen [β-CTX], bonespecificalkaline phosphatase [BSAP], and procollagentype I N-terminal propeptide [PINP]). This was carried out in two of the trials. Levels of Serum β-CTX, PINP, and BSAP were observed to have decreased, indicating an inhibition ofbone resorption [12,14].
Though osteoporosis is a disease affecting both sexes, not many studies are available about the efficacy of ZOL in treating osteoporosis in men. The trials included in our meta-analysis included one conducted on post-menopausal women [12] and another conducted on men [14]. Comparison of the RR values for these two trials clearly indicates that zoledronic acid is effective in reducing the incidence of vertebral fractures in men and women. The third trial [15] had participants of both sexes and it can be inferred that the treatment was effective in both sexes. Therefore, we conclude that an annual 5 mg intravenous infusion of ZOL is equally effective in preventing osteoporosis in men and women.
One disadvantage of bisphosphonates is their safety profile. The incidence of arthralgia, myalgia, flu-like symptoms, headache was significantly higher for the ZOL group in comparison to the placebo group in all three trial [12,14,15] None of the trials included here reported renal adverse events. However, two out of the three trials reported cardiovascular adverse events. Increased incidence of myocardial infarction (MI) [14] and serious atrial fibrillation [12] were reported. The incidence of cardiovascular adverse events following administration of ZOL has been reported in the literature. Increased arrhythmia was reported in a study carried out in patients with bone metastasis [18], while absence of association between serious atrial fibrillation and bisphosphonate or ZOL usage was reported in two other studies [19,20]. The conflicting nature of the available evidence points to a requirement for studies with longer follow-up periods for better gauging the safety profile of zoledronic acid.
Conclusions
Our meta-analysis assessed the efficacy of zoledronic acid in reducing the incidence of fractures and increasing BMD in osteoporotic individuals. Our analysis shows that zoledronic acid is effective in the prevention of vertebral and nonvertebral fractures as well as increasing the BMD, which could make a significant contribution to reducing morbidity and mortality in the elderly.
Acknowledgements
This study was supported by PLA Health Care Foundation (12BJZ42, Effect and vitamin D deficiency on cardiovascular diseases in elderly health care population and intervention studies) and Beijing Science Nova Program (2011116).
Disclosure of conflict of interest
None.
References
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 3.Khosla S, Lufkin EG, Hodgson SF, Fitzpatrick LA, Melton LJ III. Epidemiology and clinical features of osteoporosis in young individuals. Bone. 1994;15:551–5. doi: 10.1016/8756-3282(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 4.Orwoll ES, Klein RF. Osteoporosis in men. Endocr Rev. 1995;16:87–116. doi: 10.1210/edrv-16-1-87. [DOI] [PubMed] [Google Scholar]
- 5.Demontiero O, Duque G. Once-yearly zoledronic acid in hip fracture prevention. Clin Interv Aging. 2009;4:153–164. doi: 10.2147/cia.s5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guggenbuhl P. Osteoporosis in males and females: Is there really a difference? Joint Bone Spine. 2009;76:595–601. doi: 10.1016/j.jbspin.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Goncalves MJ, Rodrigues AM, Canhao H, Fonseca JE. Osteoporosis: From bone biology to individual treatment decision. Acta Med Port. 2013;26:445–455. [PubMed] [Google Scholar]
- 8.Piper PK, Gruntmanis U. Management of osteoporosis in the aging male: Focus on zoledronic acid. Clin Interv Aging. 2009;4:289–303. doi: 10.2147/cia.s4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClung M, Harris ST, Paul D, Miller PD, Bauer DC, Davison KS, Dian L, Hanley DA, Kendler DL, Yuen CK, Lewiecki EM. Bisphosphonate therapy for osteoporosis: Benefits,risks, and drug holiday. Am J Med. 2013;126:13–20. doi: 10.1016/j.amjmed.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Rakel A, Boucher A, Ste-Marie LG. Role of zoledronic acid in the preventionand treatment of osteoporosis. Clin Interv Aging. 2011;6:89–99. doi: 10.2147/CIA.S7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzoli R. Zoledronic acid for the treatment and prevention of primary and secondary osteoporosis. Ther Adv Musculoskelet Dis. 2010;2:3–16. doi: 10.1177/1759720X09352920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR HORIZON Pivotal Fracture Trial. Once yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 13.Kimmel DB. Mechanism of action, pharmacokinetic, and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res. 2007;86:1022–1033. doi: 10.1177/154405910708601102. [DOI] [PubMed] [Google Scholar]
- 14.Boonen S, Jean-Yves Reginster JY, Kaufman JM, Lippuner K, Zanchetta J, Langdahl B, Rizzoli R, Lipschitz S, Dimai HP, Witvrouw R, Eriksen E, Brixen K, Russo L, Claessens F, Papanastasiou P, Antunez O, Su G, Bucci-Rechtweg C, Hruska J, Incera E, Vanderschueren D, Orwoll E. Fracture risk and zoledronic acid therapyin men with osteoporosis. N Engl J Med. 2012;367:1714–23. doi: 10.1056/NEJMoa1204061. [DOI] [PubMed] [Google Scholar]
- 15.Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fracturesand mortality after hip fracture. N Engl J Med. 2007;357:1799–809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morabito N, Lasco A, Gaudio A, Crisafulli A, Di Pietro C, Meo A, Frisina N. Bisphosphonates in the treatment of thalassemia-induced osteoporosis. Osteoporosis Int. 2002;13:644–649. doi: 10.1007/s001980200087. [DOI] [PubMed] [Google Scholar]
- 17.Boonen S, Orwoll E, Magaziner J, Colón-Emeric CS, Adachi JD, Bucci-Rechtweg C, Haentjens P, Kaufman JM, Rizzoli R, Vanderschueren D, Claessens F, Sermon A, Witvrouw R, Milisen K, Su G, Lyles KW HORIZON Recurrent Fracture Trial. Once-yearly zoledronic acid in older men compared with women with recent hip fracture. J Am Geriatr Soc. 2011;59:2084–2090. doi: 10.1111/j.1532-5415.2011.03666.x. [DOI] [PubMed] [Google Scholar]
- 18.Yazici O, Aksoy S, Ucar O, Ozdemir N, Demir M, Sendur MA, Arik Z, Yaman S, Eren T, Uncu D, Zengin N. Arrhythmias during and after zoledronic acid infusion patients with bone metastasis. Med Oncol. 2013;30:609. doi: 10.1007/s12032-013-0609-5. [DOI] [PubMed] [Google Scholar]
- 19.Arslan C, Aksoy S, Dizdar O, Dede DS, Harpu-tluoglu H, Altundag K. Zoledronic acid and atrial fibrillation in cancer patients. Support Care Cancer. 2011;19:425–30. doi: 10.1007/s00520-010-0868-z. [DOI] [PubMed] [Google Scholar]
- 20.Grosso A, Douglas I, Hingorani A, MacAllister R, Smeeth L. Oral bisphosphonates and risk of atrial fibrillation and flutter in women: a self-controlled case-series safety analysis. PLoS One. 2009;4:e4720. doi: 10.1371/journal.pone.0004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClung MR, Miller PD, Brown JP, Zanchetta J, Bolognese MA, Benhamou CL, Balske A, Burgio DE, Sarley J, McCullough LK, Recker RR. Efficacy and safety of a novel delayed-release risedronate 35 mg once-a-week tablet. Osteoporos Int. 2012;23:267–76. doi: 10.1007/s00198-011-1791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watts NB. Long-term risks of bisphosphonate therapy. Arq Bras Endocrinol Metabol. 2014;58:523–9. doi: 10.1590/0004-2730000003308. [DOI] [PubMed] [Google Scholar]


