Abstract
Objective: This study aimed to investigate the changes of serum levels of TNF-α, IL-5 and IgE in mycoplasma pneumonia (MP) infection patients with or without bronchial asthma and explore its clinical importance. Methods: ELISA and Western blot assay were performed to detect the serum levels of TNF-α, IL-5 and IgE in 35 healthy subjects, 45 patients with MP infection and 40 bronchial asthma patients with MP infection. Results: The serum levels of TNF-α, IL-5 and IgE in MP infection patients and asthma patients with MP infection were significantly higher than those in healthy subjects (P<0.01). Moreover, the serum levels of TNF-α, IL-5 and IgE in asthma patients with MP infection was markedly higher than those in MP infection patients (P<0.05). Correlation analysis showed serum TNF-α was positively associated with serum IL-5 (r=0.9636, P<0.01), serum TNF-α positively related to IgE (r=0.9841, P<0.01) and serum IgE positively relevant with serum IL-5 (r=0.9572, P<0.01) in asthma patients with MP infection. Conclusion: Our findings indicate that serum levels of TNF-α, IL-5 and IgE may play important roles in the pathogenesis of MP infection, especially in asthma patients.
Keywords: Mycoplasma pneumoniae infection, bronchial asthma, IL-5, TNF-α, IgE, Enzyme-linked immunosorbent assay
Introduction
Mycoplasma pneumoniae (MP) is a major pathogen of respiratory infection and usually causes community-acquired pneumonia. MP causes not only different respiratory diseases including mild upper respiratory tract infection and severe fatal pneumonia, but extrapulmonary diseases including myocarditis, meningitis and hemolytic anemia [1]. Some studies have shown that MP infection is an important factor in stable asthma and acute attack of asthma [2-5]. Wu et al [2] found 42% of patients with stable asthma had concomitant MP infection. However, lack of awareness of MP infection and lack of indicators specific for the diagnosis of MP infection, the specific mechanism underlying the MP infection induced deterioration and progression of asthma is still poorly understood.
Although available findings are not conclusive, they are enough to provide effective evidence for the evaluation of asthma. A variety of studies [5-8] have shown that MP infection patients have increased expression of a large amount of cytokines and some immunoglobulins, especially in those with concomitant stable or acute asthma. This study was undertaken to detect the serum levels of TNF-α, IL-5 and IgE in healthy subjects, MP infection patients and asthma patients with MP infection, aiming to investigate the role of these factors in the pathogenesis of MP infection of asthma patients.
Materials and methods
Clinical information
MP patients and asthma patients with MP infection were recruited from the Department of Respiratory Diseases, Tongji Hospital, from June 2013 to September 2014. MP infection was diagnosed according to the diagnostic criteria, and concomitant asthma was diagnosed on the basis of Chinese guideline for the prevention and management of bronchial asthma (Primary Health Care Version) [9]. 1) Healthy subjects: a total of 35 healthy subjects receiving physical examination were recruited, and there were 18 males and 17 females. The mean age was 33.52±4.8 years (range: 25-46 years). These subjects and their relatives had no respiratory allergic diseases and had no history of infection recently; 2) MP infection patients: A total of 45 patients diagnosed with MP infection were recruited. There were 24 males and 21 females. The mean age was 25.81±4.15 years (range: 18-40 years); 3) asthma patients with MP infection: a total of 40 asthma patients with MP infection were recruited. There were 19 males and 21 females. The mean age was 23.61±3.62 years (range: 14-36 years). There were no marked differences in the age and gender among three groups (P>0.05). Informed consent was obtained before study, and this study was approved by the Ethics Committee of our hospital.
Detection of serum TNF-α, IL-5 and IgE by ELISA
Double-antibody sandwich ELISA was employed to detect the serum TNF-α, IL-5 and IgE in these participants. For healthy subjects and patients, fasting blood (3 ml) was collected and anti-coagulated. After centrifugation, serum was collected and stored at -80°C. Corresponding ELISA kits were purchased from Shanghai Licheng Biotech Co., Ltd, and detection was done according to manufacturer’s instructions.
Detection of TNF-α, IL-5 and IgE by Western blot assay
Western blot assay was performed to detect the serum TNF-α, IL-5 and IgE in healthy subjects and patients. Antibodies against TNF-α, IL-5 and IgE were purchased from Abcam (UK; Lot number: #ab9739, #ab22448 and #ab7382). Internal reference antibody and secondary antibody were purchased from Cell Signaling Technology (USA; Lot number: #5174; #7074).
Statistical analysis
Data are expressed as mean ± standard deviation (X̅±S). Data of three groups were from independent random samples. Homogeneity of variance test was performed before comparisons of means among groups. One way analysis of variance was employed for comparisons among groups. Correlation analysis of serum TNF-α, IL-5 and IgE was done with Sperman’s correlation analysis. Statistical analysis was performed with SPSS version 19.0. A value of P<0.05 was considered statistically significant.
Results
Serum TNF-α, IL-5 and IgE determined by ELISA
When compared with healthy subjects, the serum TNF-α, IL-5 and IgE increased significantly (Figures 1, 2 and 3; **P<0.01). In addition, the serum TNF-α, IL-5 and IgE in asthma patients with MP infection were also markedly higher than those in MP infection patients (*P<0.05; Table 1).
Figure 1.
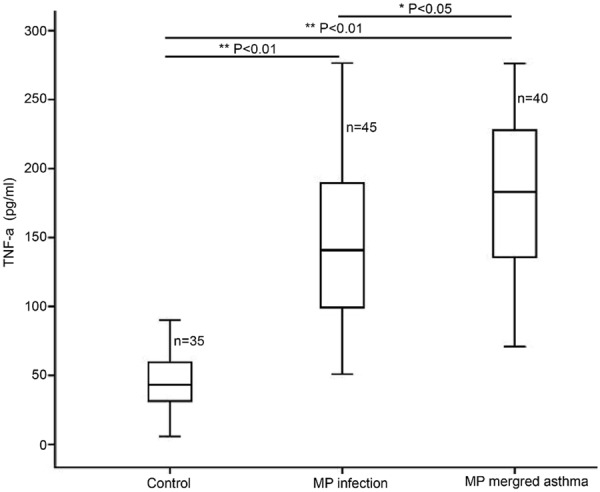
Serum TNF-α determined by ELISA in healthy subjects, MP infection patients and asthma patients with MP infection.
Figure 2.
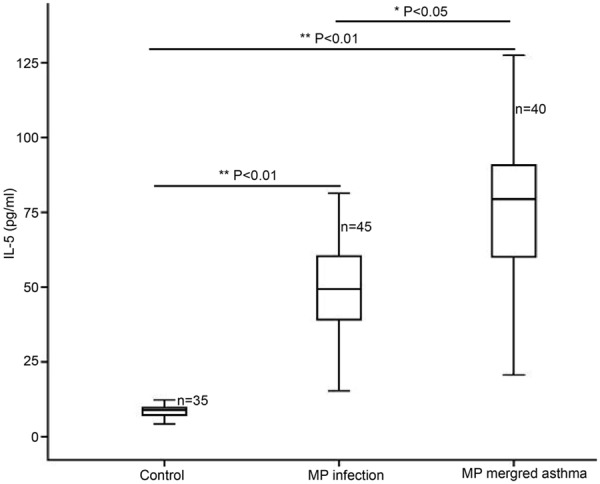
Serum IL-5 determined by ELISA in healthy subjects, MP infection patients and asthma patients with MP infection.
Figure 3.
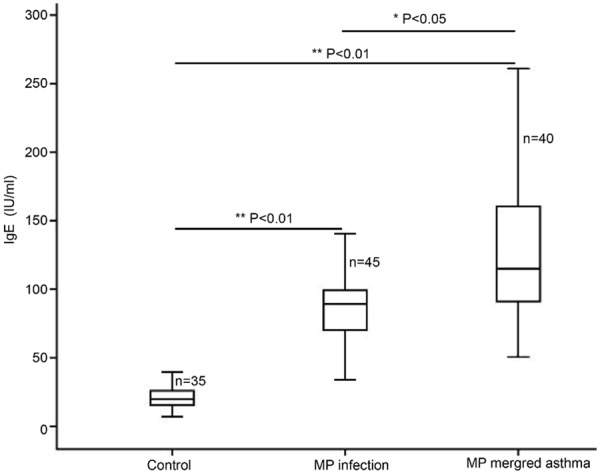
Serum IgE determined by ELISA in healthy subjects, MP infection patients and asthma patients with MP infection.
Table 1.
Serum TNF-α, IL-5 and IgE in healthy subjects, MP infection patients and asthma patients with MP infection
| Variable | Healthy controls (n=35) | MP infection patients (n=45) | Asthma patients with MP infection (n=40) |
|---|---|---|---|
| TNF-α (pg/ml) | 45.46±21.46 | 141.72±52.26* | 182.27±55.45*,** |
| IL-5 (pg/ml) | 8.65±2.04 | 49.02±16.33* | 76.11±22.8A*,** |
| IgE (IU/ml) | 20.38±8.08 | 84.03±23.94* | 126.91±47.68*,** |
P<0.01 vs. healthy controls;
P<0.05 vs. MP infection patients.
Serum TNF-α, IL-5 and IgE determined by Western blot assay
Western blot assay was performed to detect the serum TNF-α, IL-5 and IgE. When compared with healthy controls, the serum TNF-α, IL-5 and IgE increased dramatically in MP infection patients and asthma patients with MP infection (**P<0.01). In addition, the serum TNF-α, IL-5 and IgE in asthma patients with MP infection were markedly higher than those in MP infection patients (*P<0.05; Figure 4A, 4B).
Figure 4.
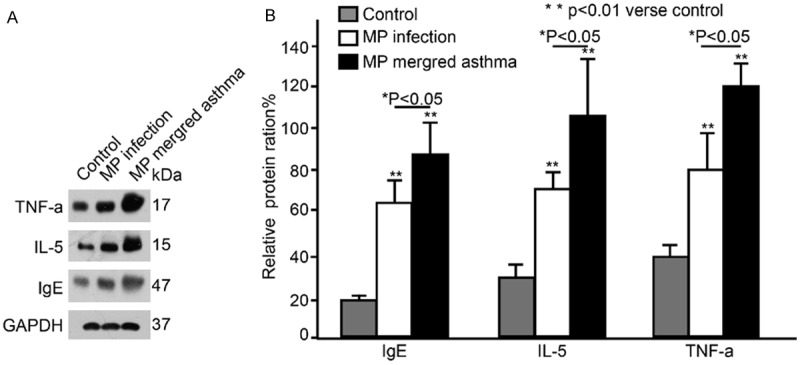
Serum TNF-α, IL-5 and IgE determined by Western blot assay in healthy subjects, MP infection patients and asthma patients with MP infection.
Correlation analysis of serum TNF-α, IL-5 and IgE in asthma patients with MP infection
In asthma patients with MP infection, correlation analysis showed serum TNF-α was positively related to serum IL-5 (r=0.9636, P<0.01) and serum IgE (r=0.9841, P<0.01), and serum IgE was also positively associated with IL-5 (r=0.9572, P<0.01).
Discussion
Combined MP infection and asthma is a common clinical characteristic in adolescents and usually characterized by fever and chronic cough, which are similar to clinical manifestations of common upper respiratory tract infection. Thus, combined MP infection and asthma is often misdiagnosed in clinical practice, which eventually influences the subsequent treatment of this disease [7,10]. MP infection may directly injury the airway epithelial cells, disrupt the its mucosal barrier, dys-regulate mucus secretion of the airway, resulting in production and release of cytokines and inflammatory mediators [11], including platelet activating factor and leukotriene. This may cause airway mucosal edema and increased glandular secretion, finally resulting in lung dysfunction, immune mediated injury and chronic respiratory allergic reaction which usually presents small airway dysfunction characterized by increase in airway resistance and reduction in respiratory peak flow, leading to asthma attack or deterioration of asthma [12,13]. In addition, MP infection may induce IgE production which may also activate neurological mechanism of asthma and airway hyper-responsiveness [14]. Thus, MP infection may not only cause frequent attack of asthma, but induce the occurrence of asthma.
TNF-α is a protein mainly produced by activated macrophages and monocytes, involved in the normal inflammatory reaction and immune response and crucial for the maintenance of homeostasis [15]. TNF-α production increases under a variety of pathological conditions including sepsis, malignancies, heart failure and chronic infection [16]. After MP infection, serum TNF-α level increases, and it may be one of mechanisms inducing or deteriorating asthma [17]. Our results showed patients with respiratory MP infection had significantly increased serum TNF-α when compared with healthy controls (P<0.01). In addition, the serum TNF-α in patients with combined asthma and MP infection was also markedly higher than that in both healthy controls and patients with MP infection alone (P<0.05). This suggests that a large amount of TNF-α is released in patients with MP infection to combat with the inflammatory reaction. On the contrary, the increased TNF-α also aggravate airway hyper-responsiveness, inducing or deteriorating asthma.
IgE is mainly produced by the plasma cells in the lamina propria of airway and also an immunoglobulin with the lowest level among immunoglobulins in the serum. The presence of allergic disease or parasitic infection may specifically cause increase in IgE. After MP infection, a lot of cytokines and inflammatory factors are released in the airway, causing airway hyper-responsiveness. IgE antibody may bind to IgE on the basophils and mast cells, sensitizing these cells, which is also known as primary sensitization [18]. When the same allergen is encountered again, type I immediate hypersensitivity may occur. Under this condition, different inflammatory mediators and cytokines are related by a variety of inflammatory cells, which dilates capillaries, increases their permeability and elevates glandular secretion, resulting in airway edema, increase in secretions and subsequent deterioration of infection and asthma. In MP infection induced asthma, IgE is not only a source of infection but an allergen. Our results showed MP infection patients had significantly increased serum IgE when compared with healthy controls (P<0.01). Moreover, in patients with combined asthma and MP infection, the serum IgE was markedly higher than that in both patients with MP infection alone and healthy controls (P<0.05). Thus, we speculate that IgE plays a crucial role in the pathogenesis of MP infection, especially in patients with concomitant asthma.
IL-5 is a cytokine secreted by Th2 cells and mast cells and mainly promotes the growth and differentiation of eosinophils. Studies [19,20] have showed the serum IL-5 in patients with acute MP infection is significantly higher than that in healthy controls, and it in patients with combined asthma and MP infection further increased. Thus, IL-5 is a major cytokine in patients with acute MP infection and plays an important role in the pathophysiology of asthma.
In asthma patients with MP infection, the serum TNF-α, IgE and IL-5 were markedly higher than those in healthy controls. Further correlation analysis showed serum TNF-α was positively with serum IL-5 (r=0.9636, P<0.01) and IgE (r=0.9841, P<0.01), and positive correlation was also observed between IgE and Il-5 (r=0.9572, P<0.01). This suggests that detection of TNF-α, IgE and IL-5 is helpful for the diagnosis of combined asthma and MP infection. Serum TNF-α, IgE and IL-5 may serve as a reference indicator for the early diagnosis of combined asthma and MP infection, and they not only provide evidence for the determination of disease progression, but may guide the clinical medication of this disease.
Taken together, our findings provide an indicator for the diagnosis of combined asthma and MP infection. Detection of serum TNF-α, IgE and IL-5 is convenient and rapid and has good sensitivity and specificity. Thus, it may be helpful for the early diagnosis and therapy of this disease and reduce the misdiagnosis in clinical practice. However, there are still limitations in the present study (such as small sample size and limited expansion of our results). Thus, more studies with large sample size are required to confirm our findings.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81200065 and 81400071) and the projects of Science and Technology Commission of Shanghai Municipality (No. 12ZR1428300 and 14411971700) and the project of Shanghai Municipal Health Bureau (No. 20134Y004).
Disclosure of conflict of interest
None.
References
- 1.Nilsson AC, Bjorkman P, Welinder-Olsson C, Widell A, Persson K. Clinical severity of Mycoplasma pneumoniae (MP) infection is associated with bacterial load in oropharyngeal secretions but not with MP genotype. BMC Infect Dis. 2010;10:39. doi: 10.1186/1471-2334-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Q, Martin RJ, LaFasto S, Chu HW. A low dose of Mycoplasma pneumoniae infection enhances an established allergic inflammation in mice: the role of the prostaglandin E2 pathway. Clin Exp Allergy. 2009;39:1754–1763. doi: 10.1111/j.1365-2222.2009.03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 4.Chang YT, Yang YH, Chiang BL. The significance of a rapid cold hemagglutination test for detecting mycoplasma infections in children with asthma exacerbation. J Microbiol Immunol Infect. 2006;39:28–32. [PubMed] [Google Scholar]
- 5.Nisar N, Guleria R, Kumar S, Chand Chawla T, Ranjan Biswas N. Mycoplasma pneumoniae and its role in asthma. Postgrad Med J. 2007;83:100–104. doi: 10.1136/pgmj.2006.049023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters J, Singh H, Brooks EG, Diaz J, Kannan TR, Coalson JJ, Baseman JG, Cagle M, Baseman JB. Persistence of community-acquired respiratory distress syndrome toxin-producing Mycoplasma pneumoniae in refractory asthma. Chest. 2011;140:401–407. doi: 10.1378/chest.11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood PR, Hill VL, Burks ML, Peters JI, Singh H, Kannan TR, Vale S, Cagle MP, Principe MF, Baseman JB, Brooks EG. Mycoplasma pneumoniae in children with acute and refractory asthma. Ann Allergy Asthma Immunol. 2013;110:328–334. e321. doi: 10.1016/j.anai.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn DL, Schure A, Patel K, Childs T, Drizik E, Webley W. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945. doi: 10.1371/journal.pone.0035945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asthma Workgroup; Chinese Thoracic Society; Chinese Societ of General Practitioners. Chinese guideline for the prevention and management of bronchial asthma (Primary Health Care Version) J Thorac Dis. 2013;5:667–677. doi: 10.3978/j.issn.2072-1439.2013.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin JE, Cheon BR, Shim JW, Kim DS, Jung HL, Park MS, Shim JY. Increased risk of refractory Mycoplasma pneumoniae pneumonia in children with atopic sensitization and asthma. Korean J Pediatr. 2014;57:271–277. doi: 10.3345/kjp.2014.57.6.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kannan TR, Baseman JB. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc Natl Acad Sci U S A. 2006;103:6724–6729. doi: 10.1073/pnas.0510644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YQ. Relationship between mycoplasma pneumoniae infection and asthma in children. Chin Prac J Rura Doc. 2013:5–5. [Google Scholar]
- 13.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161:694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 14.Wu ZY. Mycoplasma pneumoniae infection and asthma in children. ShiYong ZhenLiao. 2013:28–28. 46. [Google Scholar]
- 15.Yao ZL, Wang B, Qian DM, Song XX, Wang AH. Expression of TNF-α and IgE in peripheral blood of children with mycoplasma pneumonia infection and bronchitis asthma. J Prac Diag Ther. 2008;22:191–192. 195. [Google Scholar]
- 16.Clark IA, Atwood CS. Is TNF a link between aging-related reproductive endocrine dyscrasia and Alzheimer’s disease? J Alzheimers Dis. 2011;27:691–699. doi: 10.3233/JAD-2011-110887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brightling C, Berry M, Amrani Y. Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol. 2008;121:5–10. doi: 10.1016/j.jaci.2007.10.028. quiz 11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang MH, Xue JL, Zhao H, Gong YD, Fang MS. The detection and significance of total serum IgE in allergic rhinitis. Chin J Lab Diag. 2012;16:1063–1065. [Google Scholar]
- 19.Weber-Chrysochoou C, Crisafulli D, Kemp AS, Britton WJ, Marks GB. Allergen-specific IL-5 responses in early childhood predict asthma at age eight. PLoS One. 2014;9:e97995. doi: 10.1371/journal.pone.0097995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panganiban RP, Pinkerton MH, Maru SY, Jefferson SJ, Roff AN, Ishmael FT. Differential microRNA epression in asthma and the role of miR-1248 in regulation of IL-5. Am J Clin Exp Immunol. 2012;1:154–165. [PMC free article] [PubMed] [Google Scholar]


