Abstract
The current meta-analysis incorporating 15 case-control studies involving 4,138 cases and 4,269 controls was performed on the basis of a systematical search in electronic databases for a more precise estimation on the associations of three common polymorphisms -765 G>C (rs20417), -1195G>A (rs689466) and +8473 C>T (rs5275) in Cyclooxygenase-2 (Cox-2) gene with the susceptibility to bladder cancer. The results showed that there was a significant association between rs5275 polymorphism and bladder cancer risk (C vs. T; OR=0.84; CC vs. TT: OR=0.76), especially among Chinese (CC vs. TC+TT: OR=0.48) and American (C vs. T; OR=0.83; TC vs. TT: OR=0.73; CC+TC vs. TT: OR=0.73). and the rs20417 polymorphism was significantly associated with an increased risk of bladder cancer among Chinese (C vs. G: OR=1.46; GC vs. GG: OR=1.49; CC+GC vs. GG: OR=1.51) and Indian (GC vs. GG: OR=1.63; CC+GC vs. GG: OR=1.46), but a reduced risk among American (C vs. G: OR=0.81; GC vs. GG: OR=0.76; CC+GC vs. GG: OR=0.76). Additionally, we found that the rs689466 polymorphism was associated with a decreased risk of bladder cancer in Indian (GA vs. GG: OR=0.68; AA vs. GG: OR=0.39).The present meta-analysis suggests that Cox-2 rs5275 polymorphism may contribute to the risk of bladder cancer, particularly among Chinese and American. The rs20417 polymorphism may play a protective role in the development of bladder cancer in Indian and Chinese but act as a risk factor among American, while the rs689466 polymorphism was more likely to be associated with a decreased risk of bladder cancer in Indian.
Keywords: Bladder cancer, cyclooxygenase-2, polymorphism, meta-analysis
Introduction
Bladder cancer, as the most serious urinary neoplasm around the world, is the fourth most common cancer among males, accounting for 7% of the total malignancies [1]. The incidence of bladder cancer greatly varies regionally, with the lowest median bladder cancer incidence rate reported in Asia (5.9) and the highest in Europe (23.9) [2]. Although the etiopathogenesis remains enigmatic, it has been generally accepted that environmental factors such as tobacco smoking and occupational exposures may contribute to the risk of bladder cancer [3,4]. However, most individuals exposing to those known risk factors never develop bladder cancer, whereas many bladder cancer cases develop from individuals without those risk factors, suggesting the important role of genetic variation in the development of bladder cancer [5]. Allele variants in oncogenes are candidate genetic risk factors that may influence the onset and outcome of bladder cancer. Accumulative evidences have indicated that genetic polymorphisms in pathways controlling essential cellular activities including mediation of inflammation response, carcinogen metabolism, cell cycle regulation, DNA damage/repair and apoptosis, may alter the susceptibility to bladder cancer [6-8].
Cyclooxygenase-2 (Cox-2), an inducible and immediate-early gene encoding a key enzyme that converts arachidonic acid to prostaglandins [9], is able to be rapidly induced by a variety of mitogenic and inflammatory stimuli and elevate the production of prostaglandins, which contribute to tumor occurrence and progression by modulating cell proliferation, apoptosis, and angiogenesis [10-12]. The human Cox-2 gene (also known as PTGS2) consisting of 10 exons and 9 introns approximately spanning 8.3 kb is mapped on the chromosome 1q25.2-q25.3 [13]. A large volume of research data has demonstrated that Cox-2 is normally absent in bladder tissue, while overexpression of Cox-2 is often observed in bladder cancer, indicating a potential role in bladder carcinogenesis [14,15], however, the exact mechanism have mostly remained elusive. With the emerging evidence regarding the possible mechanism involved in the carcinogenesis for Cox-2 gene, genetic variants in Cox-2 have been frequently shown to exert profound effects on gene transcriptional activity by altering the binding capacity of certain nuclear proteins, thereby affecting expression of Cox-2 enzyme and influence the susceptibility to various carcinomas including bladder cancer [16-19].
In recent years, several potentially functional single-nucleotide polymorphisms (SNP) related to bladder cancer risk have been identified, of which three functional SNPs, -765 G>C (rs20417), -1195 G>A (rs689466) in the promoter region, and the +8473 C>T (rs5275) in the 3’UTR of Cox-2, have been widely studied [2]. Although numerous epidemiologic investigations assessing the associations of the three common SNPs in Cox-2 gene with bladder cancer risk have been carried out [2,19-23], the results remain inconsistent even conflicting, which may partially be due to the different sample sizes and different ethnicities of the populations investigated. To better address the concerned associations, we performed a meta-analysis of all eligible studies to evaluate the association between the three common polymorphisms in Cox-2 and bladder cancer risk and to quantify the potential influencing factors.
Materials and methods
Search strategy
A systematic literature search were conducted in the electronic databases of PubMed, Web of Knowledge, Embase, Cochrane Library, Wan Fang, China National Knowledge Infrastructure, and the Chinese Biomedicine Database to retrieve relevant studies regarding the associations of Cox-2 polymorphisms (rs20417, rs689466, rs5275) with bladder cancer risk, using the following limits: Humans, and article in English or Chinese. We developed a search strategy using the following query: [“Cox-2” or “cyclooxygenase 2” or “prostaglandin synthase-2” or “PTGS2”] and [“bladder cancer” or “bladder carcinoma”] and [“SNP” or “polymorphisms” or “polymorphism” or “variant” or “genotype”]. Additional eligible studies were identified by individually and manually reviewing reference lists of major textbooks, review articles on this topic. Furthermore, in case of overlapping publications, only the one with the most recent and/or the latest sample size was selected for the analysis.
Selection criteria
In the current meta-analysis, eligible studies for inclusion were as follows: (1) case-control studies evaluated the association of Cox-2 polymorphisms and bladder cancer risk. (2) Identification of bladder cancer patient was confirmed histologically or pathologically. (3) Studies with sufficient information on the frequencies of alleles or genotypes in both cases and controls were available to estimate an odds ratio (OR) with a 95 % confidence interval (CI). The major exclusion criteria were: (1) the design was based on family or sibling pairs or not case-control study; (2) the outcomes of the study were not reported or were difficult to determine; (3) the extraction of detailed frequencies of alleles or genotypes was unavailable; (4) studies duplicated the results of previous publications; (5) they were conference abstracts, case reports, editorials, review articles, and letter articles.
Data extraction
Employing standardized abstraction sheets, two reviewers extracted data independently from individual studies, and any disagreements between the two reviewers were resolved by discussion until a consensus was reached on all the items. For each enrolled study, the following information was collected: first author, year of publication, country of origin, ethnicity, source of control, genotyping methods and genotype frequency in cases and controls, respectively. Ethnicity descents were categorized as Chinese, Indian and American, because all the studied populations were from the three countries in the included studies.
Statistical analysis
Individual or pooled OR and 95% CIs were calculated for the strength of the association between the Cox-2 polymorphisms and the risk of bladder cancer using Review Manager Version 5.2 software (provided by The Cochrane Collaboration, Oxford, UK; http://www.cochrane.org/software/revman.htm). The significance of the pooled OR was determined by Z test and P<0.05 was considered significant. The Cochran’s Q-test was used to assess the statistical heterogeneity among studies [24], and if the Ph>0.1 indicated the absence of heterogeneity, then the fixed-effects model (the Mantel-Haenszel method) was used to calculate the pooled Ors [25]; otherwise, the random-effects model (the DerSimonian and Laird method) was applied [26]. To evaluate the ethnic-specific effects, subgroup analyses were conducted according to ethnicity descents (Chinese, Indian and American). Sensitivity analysis was performed by sequential omission of individual studies under various contrasts to assess the stability of results. The Begg’s funnel plot was applied to detect potential publication bias [27], which was further assessed by the method of Egger’s linear regression test (P<0.05 indicated the presence of publication bias) [28]. The Begg’s funnel plot and Egger’s linear regression test were performed using Stata 12.0 software (Stata Corp., College Station, USA).
Results
Study characteristics
Initially, 99 potentially relevant studies were retrieved based on our search strategy. According to the established inclusion and exclusion criteria for eligible studies, 6 publications were ultimately included in the meta-analysis [2,19-23]. The flow chart summarizing study selection is shown in Figure 1. Since more than one case-control study was included in five articles [2,19-22], they were considered as separate studies in the meta-analysis. Totally, there were 15 case-control studies from 6 articles involving 4,138 bladder cancer cases and 4,269 controls were included in the final meta-analysis, among which there were four articles in English and two in Chinese. The study populations in the 6 included articles consisted of 3 Chinese, 2 Indian and 1 American studies. The distribution of genotypes in the controls of all included case-control studies was in agreement with Hard-Weinberg equilibrium except for the studies conducted by Chang et al. [19] regarding rs5275, Gangwar et al. [2], Srivastava et al. [20], and Yang et al. [21] regarding rs20417. Detailed characteristics of the included studies are summarized in Table 1.
Figure 1.
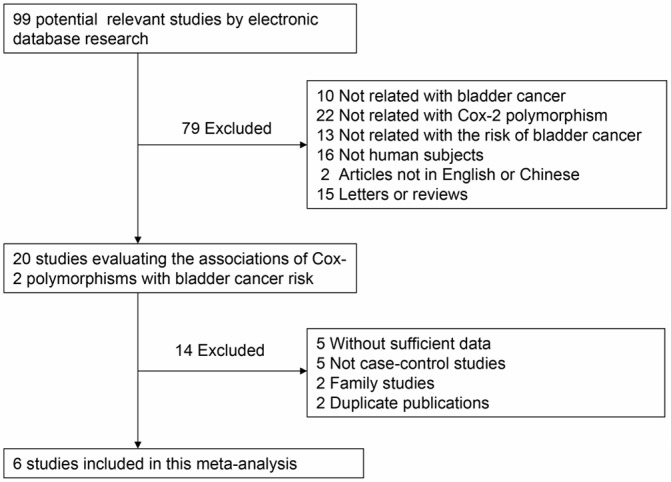
Flow chart showing eligible studies selection procedure.
Table 1.
The characteristics of included studies in the meta-analysis
| First author | Year | Country | Ethnicity | Genotype-case | Genotype-control | Source of control | Genotype method | Cox-2 polymorphism | HWE test |
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| VR Ho/Ht/WT Ho* | VR Ho/Ht/WT Ho* | ||||||||
| Chang | 2013 | China | Chinese | 105/181/89 | 107/171/97 | hospital | PCR-RFLP | rs689466 | Y |
| 2013 | China | Chinese | 0/89/286 | 0/60/315 | hospital | PCR-RFLP | rs20417 | Y | |
| 2013 | China | Chinese | 0/127/248 | 0/117/258 | hospital | PCR-RFLP | rs5275 | N | |
| Gangwar | 2011 | India | Indian | 2/48/162 | 4/64/182 | hospital | PCR-RFLP | rs689466 | Y |
| 2011 | India | Indian | 4/80/128 | 12/61/177 | hospital | PCR-RFLP | rs20417 | N | |
| 2011 | India | Indian | 24/106/82 | 34/119/97 | hospital | PCR-RFLP | rs5275 | Y | |
| Srivastava | 2009 | India | Indian | 5/37/142 | 11/52/104 | population | PCR-RFLP | rs689466 | Y |
| 2009 | India | Indian | 8/45/131 | 8/32/127 | population | PCR-RFLP | rs20417 | N | |
| 2009 | India | Indian | 29/88/67 | 25/91/51 | population | PCR-RFLP | rs5275 | Y | |
| Song | 2008 | China | Chinese | 51/99/30 | 65/86/29 | hospital | PCR-RFLP | rs689466 | Y |
| 2008 | China | Chinese | 1/19/154 | 0/18/159 | hospital | PCR-RFLP | rs20417 | Y | |
| 2008 | China | Chinese | 4/39/132 | 5/61/113 | hospital | PCR-RFLP | rs5275 | Y | |
| Qin | 2014 | China | Chinese | 24/26/4 | 64/32/1 | hospital | Taqman | rs5275 | Y |
| Yang | 2008 | USA | American | 10/163/446 | 11/200/416 | population | SNPlex | rs20417 | N |
| 2008 | USA | American | 76/268/279 | 85/312/236 | population | SNPlex | rs5275 | Y |
VR, variant; WT, wild-type; Ht, heterozygote; VR Ho, variant homozygote; WT Ho, wide-type homozygote;
Y, in agreement with HWE; (Hardy-Weinberg equilibrium); N, in disagreement with HWE.
Quantitative synthesis
Cox-2 rs689466
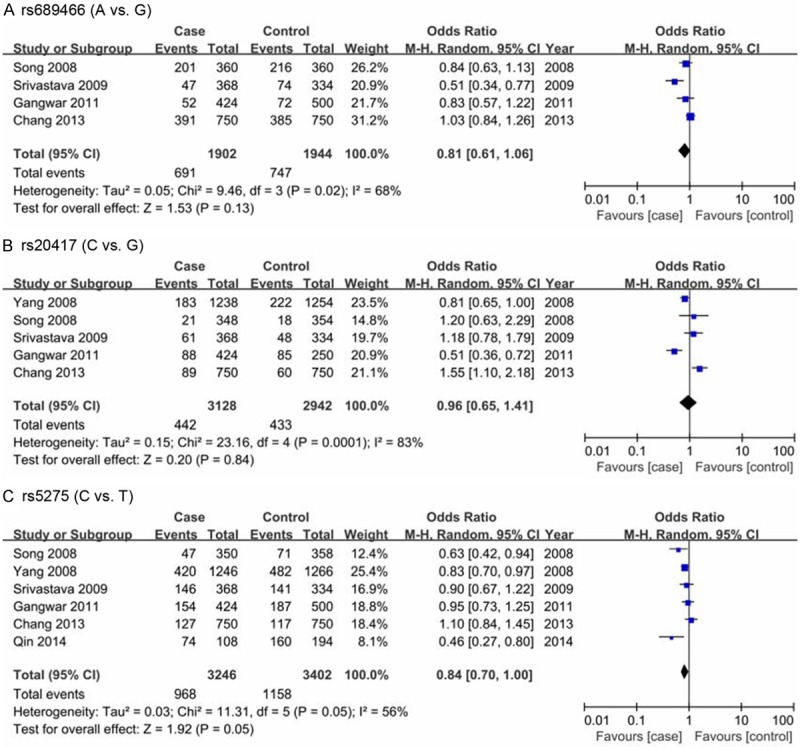
The aggregated ORs and heterogeneity test results for the association between the Cox-2 polymorphisms and bladder cancer risk were listed in Table 2. Four case-control studies with 951 cases and 972 controls have investigated the association between bladder cancer risk and Cox-2 rs689466 polymorphism. As shown in Table 2 and Figure 2, there was no evidence of significant association between bladder cancer risk and Cox-2 rs689466 polymorphism in the overall analyses under any genetic contrasts. In the subgroup analysis by ethnicity descents, a significantly decreased risk of bladder cancer for rs689466 polymorphism was observed in the homozygote contrast and heterozygote contrast among Indian population (GA vs. GG: OR=0.68, 95% CI=0.50-0.95; AA vs. GG: OR=0.39, 95% CI=0.15-0.97), whereas no significant association was observed in any genetic model among Chinese.
Table 2.
Meta-analysis results of the associations between Cox-2 polymorphisms and the risk of bladder cancer
| Cox-2 polymorphisms | Study group | Sample size (case/control) | Allele contrast | Ht vs. WT Ho* | VR Ho vs. WT Ho* | Dominant model | Recessive model | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| OR [95% CI] | Ph | OR [95% CI] | Ph | OR [95% CI] | Ph | OR [95% CI] | Ph | OR [95% CI] | Ph | |||
| rs689466 | overall | 951/972 | 0.81 [0.61, 1.06] | 0.02 | 0.87 [0.61, 1.24] | 0.07 | 0.86 [0.63, 1.18] | 0.21 | 0.82 [0.57, 1.18] | 0.04 | 0.82 [0.64, 1.06] | 0.32 |
| Chinese | 555/555 | 0.97 [0.82, 1.14] | 0.27 | 1.14 [0.84, 1.55] | 0.92 | 0.97 [0.70, 1.35] | 0.36 | 1.08 [0.81, 1.43] | 0.64 | 0.87 [0.67, 1.13] | 0.23 | |
| Indian | 396/417 | 0.66 [0.41, 1.05] | 0.09 | 0.68 [0.50, 0.95] | 0.15 | 0.39 [0.15, 0.97] | 0.61 | 0.64 [0.38, 1.07] | 0.1 | 0.44 [0.18, 1.10] | 0.7 | |
| rs20417 | overall | 1564/1596 | 0.96 [0.65, 1.41] | <0.01 | 1.26 [0.84, 1.89] | <0.01 | 0.79 [0.46, 1.37] | 0.63 | 1.23 [0.85, 1.77] | 0.002 | 0.76 [0.44, 1.30] | 0.49 |
| Chinese | 549/552 | 1.46 [1.08, 1.98] | 0.49 | 1.49 [1.08, 2.06] | 0.3 | 3.10 [0.13, 76.61] | NA | 1.51 [1.10, 2.08] | 0.37 | 3.07 [0.12, 75.86] | NA | |
| Indian | 396/417 | 0.77 [0.34, 1.76] | 0.002 | 1.63 [1.18, 2.23] | 0.39 | 0.69 [0.33, 1.44] | 0.34 | 1.46 [1.08, 1.97] | 0.5 | 0.60 [0.29, 1.26] | 0.27 | |
| American | 619/627 | 0.81 [0.65, 1.00] | NA | 0.76 [0.59, 0.97] | NA | 0.85 [0.36, 2.02] | NA | 0.76 [0.59, 0.97] | NA | 0.92 [0.39, 2.18] | NA | |
| rs5275 | overall | 1623/1701 | 0.84 [0.70, 1.00] | 0.05 | 0.81 [0.63, 1.05] | 0.05 | 0.76 [0.58, 0.99] | 0.45 | 0.81 [0.63, 1.04] | 0.04 | 0.82 [0.65, 1.04] | 0.29 |
| Chinese | 604/651 | 0.71 [0.42, 1.20] | 0.006 | 0.72 [0.36, 1.45] | 0.02 | 0.38 [0.13, 1.13] | 0.13 | 0.68 [0.33, 1.43] | 0.01 | 0.48 [0.26, 0.87] | 0.37 | |
| Indian | 396/417 | 0.93 [0.76, 1.14] | 0.78 | 0.91 [0.67, 1.23] | 0.25 | 0.86 [0.55, 1.33] | 0.9 | 0.90 [0.67, 1.20] | 0.37 | 0.92 [0.62, 1.38] | 0.51 | |
| American | 623/633 | 0.83 [0.70, 0.97] | NA | 0.73 [0.57, 0.92] | NA | 0.76 [0.53, 1.08] | NA | 0.73 [0.57, 0.92] | NA | 0.90 [0.64, 1.25] | NA | |
VR, variant; WT, wild-type; Ht, heterozygote; VR Ho, variant homozygote; WT Ho, wide-type homozygote.
The results were in bold, if the 95% CI excluded 1 or P<0.05; Ph, P value of Q-test for heterogeneity test, and Random effects model was used when P value for heterogeneity test <0.1; otherwise, fixed effects model was used in the analysis.
Figure 2.
Forest plots of bladder cancer risk associated with variants of Cox-2 in the allele contrast.
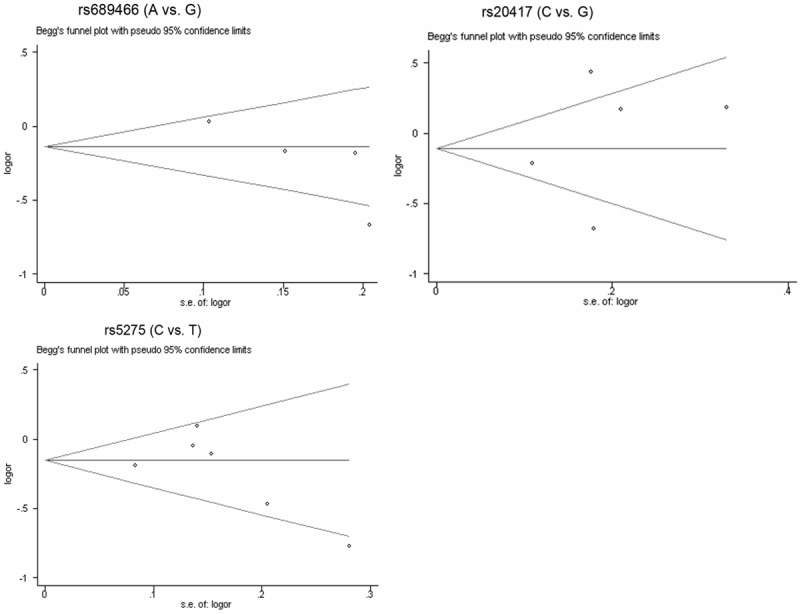
Sensitivity analysis was performed by sequential omission of individual studies to investigate the influence of each study on the overall OR. As a result, the significance of pooled ORs in the analyses for rs689466 polymorphism was excessively influenced by omitting the study of Chang et al. [19] under several contrasts (AA+GA vs. GG: OR=0.71, 95% CI=0.54-0.94; A vs. G: OR=0.74, 95% CI=0.60-0.90; AA vs. GA+GG: OR=0.64, 95% CI=0.43-0.95), meanwhile, we observed that the between-study heterogeneity was significantly reduced after excluding the study of Chang et al. [19], which was likely to partially interpret the obvious heterogeneity. In addition, the Begg’s funnel plot and Egger’s linear regression test were both used to detect the potential publication bias. As a result, as shown in Figure 3, the funnel plots failed to detect any obvious asymmetry, and the Egger’s test did not provide any evidence of publication bias (allele contrast: P=0.136), indicating the robustness of the results in the meta-analysis.
Figure 3.
Begg’s funnel plots for publication bias test on the associations of Cox-2 polymorphisms with bladder cancer risk in the allele contrast.
Cox-2 rs20417
The Cox-2 rs20417 polymorphism was investigated in five case-control studies involving 1564 cases and 1596 controls. As shown in Table 2 and Figure 2, the results of overall analyses did not suggest any significant association between Cox-2 rs20417 polymorphism and bladder cancer in any genetic contrasts. In the stratified analysis, a significantly increased risk of bladder cancer for the rs20417 polymorphism was revealed among Chinese population (C vs. G: OR=1.46, 95% CI=1.08-1.98; GC vs. GG: OR=1.49, 95% CI=1.08-2.06; CC+GC vs. GG: OR=1.51, 95% CI=1.10-2.08). Moreover, similar association was also found among Indian population (GC vs. GG: OR=1.63, 95% CI=1.18-2.23; CC+GC vs. GG: OR=1.46, 95% CI=1.08-1.97). However, the rs20417 polymorphism presented a significantly reduced risk of bladder cancer (C vs. G: OR=0.81, 95% CI=0.65-1.00; GC vs. GG: OR =0.76, 95% CI=0.59-0.97; CC+GC vs. GG: OR=0.76, 95% CI=0.59-0.97) among American population.
Similarly, the pooled OR in the sensitivity analysis was significantly affected for rs20417 polymorphism by omitting the study by Yang et al. [21] under the dominant contrast (GC+CC vs. GG: OR=1.48, OR=1.19-1.85) and heterozygote contrast (GC vs. GG: OR=1.56, OR=1.24-1.95), with significant decrease of heterogeneity, suggesting that the study by Yang et al. [21] may be mainly responsible for the observed heterogeneity. Moreover, as shown in Figure 3, the Begg’s funnel plot seemed basically symmetry and the results of Egger’s test revealed no publication bias (allele contrast: P=0.597), suggesting no significant publication bias in the meta-analysis.
Cox-2 rs5275
A total of six case-control studies with 1623 cases and 1701 controls assessing the relationship between Cox-2 rs5275 polymorphism and bladder cancer susceptibility were pooled onto this meta-analysis. As shown in Table 2 and Figure 2, the results of overall analyses indicated that the Cox-2 rs5275 polymorphism was significantly associated with a decreased risk of bladder cancer (C vs. T; OR=0.84, 95% CI=0.70-1.00; CC vs. TT: OR=0.76, 95% CI=0.58-0.99). Similarly, in terms of the stratified analysis by ethnicity descents, we observed a significant positive association between the rs5275 polymorphism and bladder cancer risk in Chinese population (CC vs. TC+TT: OR=0.48, 95% CI=0.26-0.87) and American population (C vs. T; OR=0.83, 95% CI=0.70-0.97; TC vs. TT: OR=0.73, 95% CI=0.57-0.92; CC+TC vs. TT: OR=0.73, 95% CI=0.57-0.92), while no such association was found in Indian population.
In the sensitivity analysis, we found that the combined OR for rs5275 was substantially influenced by omitting the individual studies under dominant (Chang et al. [19]), allele (Yang et al. [21] and Gangwar et al. [2]), heterozygote (Chang et al. [19]) and homozygote contrasts (Qin et al. [23] and Yang et al. [21]) (data not shown), suggesting the instability of the results in the meta-analysis, which may be due to limited number of eligible studies included. Hence, more studies with large sample size were greatly needed and of extreme importance in further exterminating the association between Cox-2 rs5275 polymorphism and bladder cancer risk. Additionally, no evident publication bias was suggested by the Begg’s funnel plot (Figure 3) and Egger’s linear regression test (allele contrast: P=0.388).
Discussion
The current meta-analysis involving 4,138 bladder cancer cases and 4,269 controls from 15 case-control studies was conducted to investigate the association between the common polymorphisms (rs689466, rs2017 and rs5275) in Cox-2 gene and bladder cancer risk. To the best of our knowledge, this is the first meta-analysis focusing on the association of the common Cox-2 polymorphisms with the risk of bladder cancer to date. Overall, the results showed that the Cox-2 rs5275 polymorphism was associated with a decreased risk of bladder cancer. However, no significant association of rs689466 as well as rs20417 polymorphisms with bladder cancer risk was revealed.
The rs689466 polymorphism located in 10th exon, 1195 bp upstream of the promoter region of Cox-2 gene was frequently found to be associated with the development of carcinoma in various organs including bladder [20], oesophagus [29] and colorectal [30]. These findings may be biologically plausible. Recently, studies have shown that the rs689466 variant was likely implicated in the regulation of the function of the c-MYB-binding site, resulting in lower transcriptional activity of the Cox-2 gene, which may regulate the exquisite balance between cell division, differentiation and survival of cells, modulating the individual’s susceptibility to cancer [31]. However, the current reports regarding the Cox-2 rs689466 polymorphism and bladder cancer risk obtained inconsistent results. In the present meta-analysis, four case-control studies with a total of 951 cases and 972 controls concerning the association between Cox-2 rs689466 polymorphism and bladder cancer risk was included. As a result, we failed to show any significant association in the overall analyses. In the subgroup analysis by ethnicity descents, the Cox-2 rs689466 polymorphism, however, presented a protective role in the development of bladder cancer in the Indian population, but not in Chinese population, suggesting the importance of the influence of ethnicity variation, and the environment in which they live on the bladder cancer risk.
For the rs20417 polymorphism which is at position -765 bp of the promoter region of Cox-2, previous functional studies have exhibited its role in the alteration of Cox-2 expression [32]. The rs20417 polymorphism was shown to disrupt a stimulatory protein1 binding site but create an E2 promoter factor (E2F) binding site, leading to high transcription activity and increased COX-2 expressions which might be involved in the development of cancers [33].The role of Cox-2 rs20417 polymorphism has been widely studied with diverse results in various carcinomas, including bladder cancer [19], gastric cancer [34] and breast cancer [13], while in our meta-analysis, we enrolled five case-control studies with 1564 cases and 1596 controls to investigate the association of rs20417 polymorphism and bladder cancer risk. Unfortunately, no significant association was observed in the overall population. However, in the stratified analysis based on ethnicity descents, the rs20417 polymorphism was significantly associated with an increased risk of bladder cancer among Chinese and Indian population, but a reduced risk of bladder cancer in American population, indicating that the Cox-2 rs20417 polymorphism may have different effects on bladder cancer risk in different ethnic genetic backgrounds. Nevertheless, owing to the single study included among American population in this meta-analysis, the observed positive association is likely to be caused by chance because a single study may have insufficient statistical power to detect a slight effect or may raise the likehood to have a fluctuated risk estimate. In addition, gene- environmental factors may also explain the discrepancies. Consequently, this association should be further confirmed by large-scale case-control studies in the future researches.
With respect to the rs5275 polymorphism mapped on the 3’-untranslated region which contains highly-conserved adenine-uracil-rich elements, this polymorphism was shown to be associated with the alteration of mRNA level of the Cox-2 gene through the regulation of message stability and translational efficiency [35]. As the rs20417 and rs689466 polymorphisms, much attention has been paid to the association between Cox-2 rs5275 polymorphism and cancer risk, whereas no significant association was reported by the most of previous pooled analyses regarding different cancer types [13,36,37]. However, the current meta-analysis including six case-control studies involving 1623 cases and 1701 controls suggested a decreased risk of bladder cancer for Cox-2 rs5275 polymorphism. In subgroup analysis by ethnicity descents, similar association was also found in Chinese population although modest, and in American population, while not in Indian population. In short, the inconsistence may be interpreted by different ethnic groups. Additionally, interactions with other genetic variants are possible reasons as well. In spite of this, considering the instability of the result of sensitivity analysis and the limitation of the single sample in investigations (only one included study conducted in American population), the result should be explained with caution, and more studies are exceedingly required.
There were several limitations in the meta-analysis, which should be acknowledged. First, this meta-analysis was based on a limited number of studies on the association between COX-2 polymorphisms and bladder cancer risk and only one relevant study conducted in American population was included, reminding us that the results of the meta-analysis should be cautiously interpreted. Second, due to the lack of necessary information, our results were based on unadjusted estimates; some potentially suspected factors such as age, sex, smoking and environmental factors should be considered for a more precise estimation. Third, there is a lack of available studies regarding these associations in different ethnicities, which would limit the comprehensiveness and veracity of the results. Therefore, more case-control studies with large sample size from different ethnicities are urgently needed. In addition, since bladder cancer is a multifactor and complex disease, the impact of the COX-2 variants may be masked by the presence of other as-yet-unidentified genes involved in carcinogenesis under various environments, potential interactions between gene-gene and gene-environment were usually neglected in the original articles.
In summary, the evidence of our meta-analysis supported an association between Cox-2 rs5275 polymorphism and decreased risk of bladder cancer, especially in Chinese and Indian populations. Similarly, the rs689466 polymorphism was associated with a reduced risk of bladder cancer in Indian population. Additionally, our results also suggested that the rs20417 polymorphism may have an increased bladder cancer risk among Chinese and Indian population, but a reduced bladder cancer risk in American population. Nevertheless, the results should be, herein, explained with great caution in consideration of the limitations in the meta-analysis, and more multicentre well-designed studies with larger sample sizes are warranted to verify our findings in future investigations.
Disclosure of conflict of interest
None.
References
- 1.Franekova M, Halasova E, Bukovska E, Luptak J, Dobrota D. Gene polymorphisms in bladder cancer. Urol Oncol. 2008;26:1–8. doi: 10.1016/j.urolonc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Gangwar R, Mandhani A, Mittal RD. Functional polymorphisms of cyclooxygenase-2 (COX-2) gene and risk for urinary bladder cancer in North India. Surgery. 2011;149:126–134. doi: 10.1016/j.surg.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Burin GJ, Gibb HJ, Hill RN. Human bladder cancer: evidence for a potential irritation-induced mechanism. Food Chem Toxicol. 1995;33:785–795. doi: 10.1016/0278-6915(95)00045-4. [DOI] [PubMed] [Google Scholar]
- 4.Kiriluk KJ, Prasad SM, Patel AR, Steinberg GD, Smith ND. Bladder cancer risk from occupational and environmental exposures. Urol Oncol. 2012;30:199–211. doi: 10.1016/j.urolonc.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo MT. Cyclooxygenase-2 in oncogenesis. Clin Chim Acta. 2011;412:671–687. doi: 10.1016/j.cca.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Gu J, Grossman HB, Amos CI, Etzel C, Huang M, Zhang Q, Millikan RE, Lerner S, Dinney CP, Spitz MR. Bladder cancer predisposition: a multigenic approach to DNA-repair and cell-cycle-control genes. Am J Hum Genet. 2006;78:464–479. doi: 10.1086/500848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Closas M, Malats N, Silverman D, Dosemeci M, Kogevinas M, Hein DW, Tardon A, Serra C, Carrato A, Garcia-Closas R, Lloreta J, Castano-Vinyals G, Yeager M, Welch R, Chanock S, Chatterjee N, Wacholder S, Samanic C, Tora M, Fernandez F, Real FX, Rothman N. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet. 2005;366:649–659. doi: 10.1016/S0140-6736(05)67137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 9.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 10.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 11.Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol. 2010;2010:215158. doi: 10.1155/2010/215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai ZJ, Shao YP, Ma XB, Xu D, Tang W, Kang HF, Lin S, Wang M, Ren HT, Wang XJ. Association of the three common SNPs of cyclooxygenase-2 gene (rs20417, rs689466, and rs5275) with the susceptibility of breast cancer: An updated Meta-analysis involving 34,590 subjects. Dis Markers. 2014;2014:484729. doi: 10.1155/2014/484729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang HP, Yu B, Zheng XD, Hu HJ, Gao ZB, Li L, Zhou LF. [Expression of MMP-2 and COX-2 mRNA in bladder transitional cell carcinoma and their correlation] . Zhonghua Nan Ke Xue. 2008;14:1011–1014. [PubMed] [Google Scholar]
- 15.Yu L, Chen XJ, Shi PQ, Ding Q, Sun CH, Liu GB, Shi DM, Huang YG. [Expression of cyclooxygenase-2 in bladder transitional cell carcinoma and the significance thereof] . Zhonghua Yi Xue Za Zhi. 2008;88:2683–2684. [PubMed] [Google Scholar]
- 16.Papafili A, Hill MR, Brull DJ, McAnulty RJ, Marshall RP, Humphries SE, Laurent GJ. Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler Thromb Vasc Biol. 2002;22:1631–1636. doi: 10.1161/01.atv.0000030340.80207.c5. [DOI] [PubMed] [Google Scholar]
- 17.Tang Z, Nie ZL, Pan Y, Zhang L, Gao L, Zhang Q, Qu L, He B, Song G, Zhang Y, Shukui W. The Cox-2-1195 G>A polymorphism and cancer risk: a meta-analysis of 25 case-control studies. Mutagenesis. 2011;26:729–734. doi: 10.1093/mutage/ger040. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Xu Y, Zhang Z, Liu R, Ma B. Association between COX-2 rs2745557 polymorphism and prostate cancer risk: a systematic review and meta-analysis. BMC Immunol. 2012;13:14. doi: 10.1186/1471-2172-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang WS, Tsai CW, Ji HX, Wu HC, Chang YT, Lien CS, Liao WL, Shen WC, Tsai CH, Bau DT. Associations of cyclooxygenase 2 polymorphic genotypes with bladder cancer risk in Taiwan. Anticancer Res. 2013;33:5401–5405. [PubMed] [Google Scholar]
- 20.Srivastava K, Srivastava A, Pandey SN, Kumar A, Mittal B. Functional polymorphisms of the cyclooxygenase (PTGS2) gene and risk for gallbladder cancer in a North Indian population. J Gastroenterol. 2009;44:774–780. doi: 10.1007/s00535-009-0071-5. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Gu J, Lin X, Grossman HB, Ye Y, Dinney CP, Wu X. Profiling of genetic variations in inflammation pathway genes in relation to bladder cancer predisposition. Clin Cancer Res. 2008;14:2236–2244. doi: 10.1158/1078-0432.CCR-07-1670. [DOI] [PubMed] [Google Scholar]
- 22.Song D, Chen K, Li Z, Li L, Yang X, Liu J, Zhang L. Association study of cyclooxygenase 2 polymorphisms and bladder cancer. Chin J Urol. 2008;29:704–709. [Google Scholar]
- 23.Qin Q, Qin J, Bai X, Meng Q, Cheng J, Lu H. Relationship between polymorphism of COX-2 and susceptibility of bladder cancer. J Pract Med. 2014;30:1076–1079. [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 28.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Upadhyay R, Jain M, Kumar S, Ghoshal UC, Mittal B. Functional polymorphisms of cyclooxygenase-2 (COX-2) gene and risk for esophageal squmaous cell carcinoma. Mutat Res. 2009;663:52–59. doi: 10.1016/j.mrfmmm.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Ueda N, Maehara Y, Tajima O, Tabata S, Wakabayashi K, Kono S. Genetic polymorphisms of cyclooxygenase-2 and colorectal adenoma risk: the Self Defense Forces Health Study. Cancer Sci. 2008;99:576–581. doi: 10.1111/j.1349-7006.2007.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsay RG, Barton AL, Gonda TJ. Targeting c-Myb expression in human disease. Expert Opin Ther Targets. 2003;7:235–248. doi: 10.1517/14728222.7.2.235. [DOI] [PubMed] [Google Scholar]
- 32.Papafili A, Hill MR, Brull DJ, McAnulty RJ, Marshall RP, Humphries SE, Laurent GJ. Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler Thromb Vasc Biol. 2002;22:1631–1636. doi: 10.1161/01.atv.0000030340.80207.c5. [DOI] [PubMed] [Google Scholar]
- 33.Szczeklik W, Sanak M, Szczeklik A. Functional effects and gender association of COX-2 gene polymorphism G-765C in bronchial asthma. J Allergy Clin Immunol. 2004;114:248–253. doi: 10.1016/j.jaci.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 34.Xu YS, Zhao B, Long CY, Li H, Lu X, Liu G, Tang XZ, Tang WZ. Cyclooxygenase-2 promoter 765C increase of digestive tract cancer risk in the Chinese population: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:4563–4566. doi: 10.7314/apjcp.2014.15.11.4563. [DOI] [PubMed] [Google Scholar]
- 35.Cok SJ, Morrison AR. The 3’-untranslated region of murine cyclooxygenase-2 contains multiple regulatory elements that alter message stability and translational efficiency. J Biol Chem. 2001;276:23179–23185. doi: 10.1074/jbc.M008461200. [DOI] [PubMed] [Google Scholar]
- 36.Peng Q, Yang S, Lao X, Tang W, Chen Z, Lai H, Wang J, Sui J, Qin X, Li S. Meta-analysis of the association between COX-2 polymorphisms and risk of colorectal cancer based on case-control studies. PLoS One. 2014;9:e94790. doi: 10.1371/journal.pone.0094790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan F, Tian J, Pan Y, Zhang Y. Lack of association of the cyclooxygenase 8473 T>C polymorphism with lung cancer: evidence from 9841 subjects. Asian Pac J Cancer Prev. 2011;12:1941–1945. [PubMed] [Google Scholar]


