Abstract
Research and practice of neuro-oncology compiles clinical neuroscience expertise from neurosurgery, radiation oncology, neuroradiology, medical oncology, neuropathology and related disciplines to optimize planning and therapy in central nervous system malignancies. Such an interdisciplinary context prompted health-care providers from all related disciplines to establish the Neuro-Oncology Scientific Club (NOSC) in Iran and let it flourish since 3 years ago. With the advent of advanced technologies and through continued share of experience, NOSC members have tried to provide more integrated diagnoses and therapeutic care to brain tumor patients across the country. NOSC activities revolve around some key tenets including dissemination of education and updates, facilitation of institutional collaborations; data registry and patients’ awareness. By virtue of recent insights on molecular characterization of brain tumors such as codeletion of chromosomes 1p and 19q in anaplastic gliomas and O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in glioblastoma, a range of translational research is being followed within NOSC. The most recent NOSC meeting which was held in Tehran, recapitulated main advances and dealt with the current debates on functional neurosurgery, biological markers and neuroimaging, risk prediction models in high grade gliomas and clinical issues in pediatric neuro-oncology. This article gives an overview of current hotspots in neuro-oncology research and practice which are pursued within NOSC.
Keywords: Interdisciplinary, brain tumors, neuro-oncology, NOSC, Iran
Introduction
As an emerging subspecialty, neuro-oncology has witnessed exciting advances over the past years. Diagnosis and treatment of central nervous system malignancies as well as neurologic complications of systemic cancers are main constituents of the practice of neuro-oncology. To provide individualized and optimal care, an interdisciplinary work becomes indicated in many instances [1-3].
Due to the aggressive nature and lethality, high grade gliomas (HGGs) and glioblastoma multiforme (GBM) in particular, have received more focus for research in neuro-oncology [4]. Untill almost a decade ago, and before the advent of temozolomide and bevacizumab, medical therapy was not able to provide notable survival benefits in GBM [5,6].
Today, with the availability of such options and the cutting-edge technological advances, ad-vanced therapies are being field tested with often encouraging outcomes, and research is on the verge to uncover the hidden sides of brain tumors’ pathogenesis [4,7,8]. Many researchers and clinical professionals in the field, may never find a more exciting time to study and invest in neuro-oncology than today.
Three years with the Neuro-Oncology Scientific Club (NOSC)
Neuro-oncology is perhaps one of the most spectacular fields where cancer crosses disciplines. The benefits of interdisciplinary approach in such clinical conditions has been well-established both for the clinicians and the patients [1,2]. The proven advantages of interdisciplinary care in brain tumor has prompted the related field professionals to form the Neuro-Oncology Scientific Club (NOSC) to foster interdisciplinary care and research in brain tumors in Iran. NOSC members has advocated the working team concept in neuro-oncology care across the country since 2011.
We at NOSC believe that education, frequent field updates and shared initiatives are the main stay to reach our goals. NOSC has attempted the above through holding scientific meetings, neuro-oncology update sessions and round table discussions in different provinces. This scientific club has strived to: 1) disseminate neuro-oncology updates to allied health care providers, 2) facilitate instituational and nationwide collaborations in neuro-oncology reaseach and practice and 3) provide awareness and education to brain tumor patients as well as general public. In addition, NOSC has suceeded to establish a brain tumor collaborative registry (BTCR) and to design and implement multi-centeric neuro-oncology investigations in Iran [9]. Along these lines, NOSC has published several scientific reports, original research findings and consensus statements since establishments [3,10-14]. With over 200 members from all allied disciplines, NOSC’s overall strategies and plans are governed by its steering board and provincial founding panels. NOSC continues to receive endorsement from the related national scientific societies, and believes that such collaboration will allow optimizing the brain tumor care. Over the past 3 years, the emerging concepts which dominated debates in neuro-oncology, encouraged us to design and run clinical investigations within NOSC. We now know that alkylating agent chemotherapy may prolong survival when added to radiotherapy for patients with anaplastic oligodendroglial tumours with 1p19q codeletion, and the progression-free survival (PFS) in patients with newly diagnosed glioblastoma can be improved with some novel approaches [15,16].
In August 2014, the NOSC faculty and members attended quite an interactive meeting themed “interdisciplinary efforts for better outcome in newly diagnosed malignant gliomas”, in Tehran. Participants from various disciplines including radiation oncology, neurosurgery, radiology, hematology-oncology, pediatric hematology-oncology, neurology, medical physics and other related fields actively took part in this NOSC event. Discussions during this meeting were focused on the role of functional surgery in HGG patients’ outcome, peudoprogression vs. pseudoresponseupon evaluating radiologic outcomes following treatments, standard of care in HGG and the emerging trends as well as response prediction models in adult and pediatric brain tumor patients. Here we present an outline of the communicated insights during this interactive scientific forum.
Functional surgery and its predictive role in clinical outcome following maximal safe resection of high grade gliomas
With regard to surgical removal of the central nervous system tumors, recent studies have confirmed that the maximal safe cytoreduction without causing neurological damages leaves a remarkable prognostic impact on patients’ survival and treatment outcome [17]. According to literature, the extent of maximal safe resection of the tumor would enable optimization of adjuvant treatment and consequently enhances patients’ quality of life [18,19].
The challange lies where in many high-grad glioma cases, tumor cells infiltrate into eloquent brain areas which are involved in motor and language functions [18]. Preoperative brain mapping for surgical planning as well as functional intraoperative mapping may assist us to carefully locate these functions in the brain. This would allow surgeons to minimize the risk of damaging such eloquent brain regions while resecting the tumor and nearby infiltrated areas [20]. The mapping process, especially in case of language assessment, requires the patient to remain awake during the procedure. Some selected centeres in Iran have purseued establishing the functional neurosurgery setup allowing awake craniotomy and intraoperative brain mapping using functional magnetic resonance imaging, tractography, comprehensive neuropsychological batteries, electrocorticography and somatosensory evoked potentials [21]. This allows careful survillance of language, motor and sensory functions before, during and after the operation, minimizing the tumor surgery sequellae [21].
During this meeting, some state-of-the-art surgical procedures which are used in management of malignant glioma in functional neurosurgery setting were discussed.
With respect to preoperative confirmation of diagnosis, the neuro-oncology team depends on stereotactic biopsy in many instances as radiologic findings are often inconclusive. Depending on the type of pathology being tested, stereotactic biopsy’s success rate to arrive at a definitive diagnosis can be quite high [22]. In some cases, the sterotactic biopsy has helpedus to avoid surgery since the primary diagnosis of GBM was changed into multiple sclerosis (MS). Figure 1 demonstrate a typical case which turned to be diagnosed as MS rather than glioma. In general, the diagnostic success rate is highest for tumor cases [22]. Stereotactic biopsy plays a crucial role in diagnosis and influence the treatment of brain tumors especially in recurrence cases [23]. It seems the only cases in whom the diagnosis does not depend on the biopsy are patients with diffuse pontine glioma [24].
Figure 1.
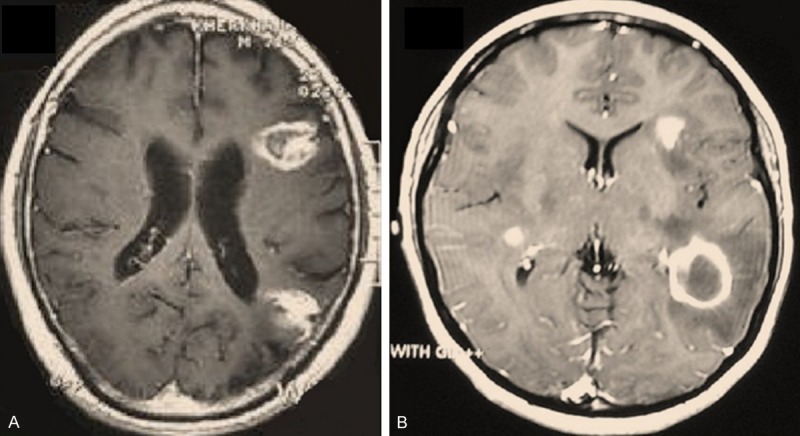
Two cases with similarly looking gadolinium-enhanced lesions in right temporal lobe in brain MRI who turned out to be of totally different diagnoses upon stereotactic needle biopsy. (A) a 24 Y/o male turned out to be a case of GBM, and (B) a 19 y/o female who was diagnosed with multiple sclerosis. Image from NOSC case files, 2014.
New imaging biomarkers for early assessment of brain tumor response to chemoradiotherapy; pseudoprogression vs. pseudoresponse
The most widely used measure to assess the tumor response to treatment is based upon evaluating the enhancing areas on conventional MRI known as the Mcdonald criteria [25]. However, non-tumoral increased enhancement and/or false decreases in enhancement, which are referred to as pseudoprogression and pseudoresponse respectively, may confuse the outcome evaluation [26]. Figures 2 and 3 demonstrate examples of pseudoprogression and pseudoresponse following therapy, respectively.
Figure 2.
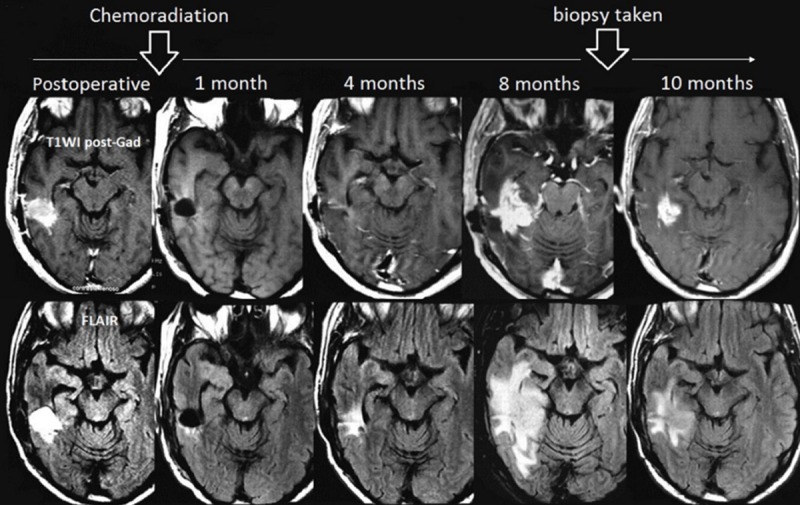
Pseudoprogression in a 54-year-old woman with GBM. Despite an increased lesion size in follow-up imaging 8 months after chemoradiation, biopsy revealed a mixed tissue with treatment-related changes rather than tumor progression. Image from NOSC case files, 2013.
Figure 3.
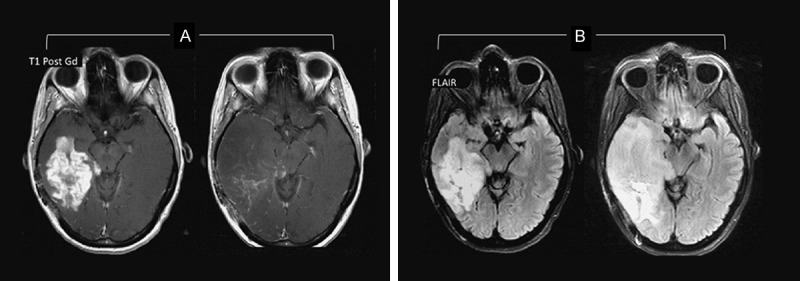
Pseudoresponse. Although imaging showed decreased enhancing portion of the lesion some months after therapy (A), the fluid-attenuated inversion recovery (FLAIR) sequence revealed a notable expansion of the lesion (B).Image from NOSC case files, 2013.
Where several imaging techniques are available for the detection of brain tumors’ outcome, the conventional brain MRI should not be overvalued due to its low negative predictive values [26]. Some advanced imaging technologies include magnetic resonance spectroscopy (MRS), single-photon emission computed tomography (SPECT), diffusion-weighted imaging (DWI), diffusion tensor imaging (DTI) or tractography and arterial spin labeling (ASL) [27]. One of the diagnostic challenges in neuroradiology is differentiating tumor progression from pseudoprogression. Once a brain mass is found to enhance, it does not necessarily mean recurrence of the brain tumor since necrosis would do the same [28]. In such cases, MRS and DWI would assist us to differentiate pseudo-progression from true progression; however, these techniques subject to some limitations [29]. For instance, in MRS, the brain mass should neither be smaller than 1 cm, nor be positioned near the cortex, hemorrhagic and calcified; and the prepared voxel size should not be under 1×1×1 cm. To ensure the accuracy of DWI technique, if the mass is small, the experiment should be repeated at least 5 or 6 times in order to restrict the mass in the apparent diffusion capacity (ADC) map [30].
DWI is a potential biomarker for the initial evaluation of tumor treatment response, however the findings maybe confounded by the surrounding edema and the radiation-induced necrosis. Some recent endeavors have proposed a diffusion abnormality index (DAI) which weighs the abnormal ADC map for edema and cellularity to predict the tumor response [31].
On the other hand, MRS has the potential to demonstrate post-radiotherapy neurochemical and structural changes in brain tissue prior to the development of symptoms or emergence of radiological changes in conventional MRI [32]. Altered brain metabolites such as decreased N-acetyl aspartate (NAA) or increased choline (Cho) may suggest tumor progression in a given voxel. Meanwhile, the extensively peaked lipids/lactate ratio suggests radiation necrosis. In many instances, MRS helps differentiating pseudo- vs. true disease [32]. DWI however, is mostly conclusive in differentiating pseudoresponse from true response of the tumor following therapy [30,31].
Glial brain tumors, response-prediction models and interdisciplinary diagnostic and therapeutic approaches
The primary treatment of glial brain tumors includes surgery, followed by chemoradiation and adjuvant chemotherapy using the standard of care, temozolomide (TMZ) [8]. With the use of intraoperative imaging techniques, surgical advances, biomarker assays and implementation of randomized trial-based protocols, malignant glioma patients have seen marginal increase in survival time over the recent years [1]. Various clinical and molecular variable including age, Karnofsky performance status (KPS), genetic and epigenetic status are shown to bear a significant predictive value with respect to the post-surgical outcome in glioma [33].
A mathematical predictive model as a prognostic tool in cancer treatment known as Recursive Partitioning Analysis or RPA has incorporated such variables and stratified patients into three classes in which class III, IV and V refer to good, intermediate and poor prognosis for outcome, respectively [34]. In this regards, when researchers decide to carry out a specific trial, the selected cases should be homogenous and the obtained data would become further valid when RPA is taken into account. Meanwhile, RPA can be considered a handy prognostic and predictive calculation even in daily practice of brain tumor care especially with HGGs [35,36]. Based on the evidence, providing the standard of care to RPA class III and IV GBMs vs. class V patients is shown to result in more favorable outcome as compared to radiotherapy alone [8].
Glial brain tumors, chemoradiotherapy and beyond
GBM has always been linked with dismal prognosis and despite the standard of care advantages, survival rate at 2 and 5 year-follow up remains 27% and 11%, respectively [8]. The role of angiogenesis in the pathogenesis of the tumor and its progression has drawn much attention recently [37,38]. Targeting vascular endothelial growth factor (VEGF) using bevacizumab (an anti-VEGF agent) has revealed marked pattern of change on MR imaging and such changes yielded an impact on clinical trials of new therapies [37]. Evolving evidence support the use of bevacizumab alone or in combination with TMZ or irinotecan in recurrent GBM setting and on individual patient care basis [39-41].
There are some cumulative evidence on differential approaches in the management of low-grade gliomas. As such, the choice of preferred regimen for the treatment of anaplastic oligodendroglioma (AOD) and anaplastic oligoastrocytoma (AOA) seems to depend on determinant molecular/genetic markers. While procarbazine, lomustine and vincristine regimen (PCV) is efficient in AOD patients who are 1p/19q co-deleted; non-co-deleted patients seem to benefit from TMZ. The investigation trying to prove this is however ongoing [42-44].
AODs account for almost 20% of adult brain tumors [44]. This tumor has generally been classified as WHO grade III and is recognized by its distinct histological appearance (i.e. ‘fried-egg’ for cell morphology and ‘chicken-wire’ for capillary network). Combined allelic loss of heterozygosity (LOH) in 1p/19q is seen in nearly half of patients. Moreover, there are other more recently uncovered prognostic mutations in IDH-1 (Isocitrate Dehydrogenese-1), CIC (homolog of Drosophila gene capicua) and FUBP-1 (Far Upstream Element Binding Protein-1) which are found in some cases of AOD with 1p/19q loss. A series of retrospective studies and molecular assessments has confirmed the impact of 1p/19q LOH prognostic factor in oligodendroglial tumors [45,46].
The large RTOG 9402 (Radiation Therapy-Oncology Group) prospective trial has compared chemo-radiotherapy vs. radiotherapy (RT) alone, in AOD tumors. In fact, RTOG tried to investigate the efficacy and safety of RT vs. PCV then RT in AOD, while EORTC (European Organization for Research and Treatment of Cancer) trial assessed the same for RT vs. RT then PCV [47,48]. In RTOG 9402; after stratifying the enrolled patients in terms of age < 50 vs. ≥ 50, KPS of 60-70 vs. ≥ 80, and the degree of anaplasia, they were randomized to either experimental (intensive PCV ×4 q6wks cycles, then RT) or the standard RT arms [47]. Only 54% of patients could complete the 4 cycles. The reasons for early stopping were hematological toxicity and tumor progression. PCV toxicity was seen in 64% patient with grade III, IV or V. Salvage chemotherapy turned to be more common with RT only arm. Initial data revealed that despite an improved PFS, early PCV could not positively influence the overall survival (OS). Moreover, PCV-related improved PFS was a benefit associated with acute toxicity and was only seen in 1p/19q co-deleted subset [47].
Patients with co-deleted tumors lived much longer than other patients. This beneficial effect was independent of initial treatment. The OS results indicated a median survival of 4.6 years for PCV+RT vs. 4.7 years for RT alone, showing no OS benefit with PCV. Similarly in 1p/19q non-co-deleted patients, the median survival of 2.6 and 2.7 years for PCV+RT vs. RT alone arms; respectively, indicated no significant difference. On the other hand, 1p/19q co-deleted patients showed an extended median survival of 14.7 compared to 7.3 years in PCV+RT vs. RT alone arms, respectively [47].
Based on this pivotal trial, the upfront combination of RT and PCV demonstrated an improved survival in AOD patients who were shown to be 1p/19q co-deleted. Such association was not seen in non-co-deleted patients, and the statistical endpoint has been achieved only after sufficient time and error to increase tumor tissue acquisition [47].
Given the above, the clinical implications for patient care may include: 1-RT alone is no longer adequate for patients with AOD patients who show 1p/19q co-deletion; 2- the data support chemotherapy + RT as the first line treatment strategy in AOD; and 3- the optimal treatment regimen (PCV vs. TMZ) as well as the preferred paradigm (i.e. chemotherapy then RT vs. RT then chemotherapy) need to be further established.
Pediatric Neuro-Oncology, our local experience
HGGs are observed in about 20-30% of all cancer diagnoses mostly in children younger than 10 years [49,50]. It is reported that 5-year OS rate in children for GBM and anaplastic astrocytoma is about 5-15% and 20-40%, respectively [51]. The chromosome aberration is shown to be influential in tumor prognosis such as 5q, 6q, 9q and 12q for anaplastic astrocytoma and 1, 3 and 16 for GBM. On the other hand, total resection of the tumor is so effective in disease prognosis [52,53]. Moreover, chemotherapy in pediatric gliomacan improve PFS rate vs. RT alone [51,54]. This intervention however is not shown to yield a significant effect on the improvement of OS [54]. Based on the evidence, HGG in children remains with unfortunately poor prognosis and chemotherapy with TMZ has only provided trivial advantages [55]. Given the common cerebrospinal fluid (CSF) dissemination and quite high mortality rate in pediatric HGG, intense supportive care becomes warranted [55].
In pediatric low-grade gliomas, conformal radiotherapy under the stereotactic guide results to a notably less radiation-associated delayed toxicity. Meanwhile in some patients with HGGs, adjuvant or neo-adjuvant chemotherapy is used and resulted in improved survival. In patients with ependymomas, the extent of resection and the radiotherapy are considered as the most determinant prognostic factors [56]. With regard to the prognosis of primitive neuroectodermal tumors, some biological markers have been identified and are being applied to clinical practice. The emergence of a new standard treatment with reduced-dose craniospinal radiotherapy and platinum-based chemotherapy has introduced a new trend in treating localized medulloblastomas [56]. On the other hand, the future treatments of supratentorial primitive neuroectodermal tumors will largely aim at optimizing local control [56,57].
Considering the rarity of primary central nervous system malignancies in children, further progress can be reached through prospective clinical trials. Integrating the biological findings into clinical applications in an interdisciplinary research context within NOSC has been strongly encouraged.
Neuro-oncology research and practice in Iran and the NOSC’s umbrella; concluding remarks
NOSC has endeavored to fill the gaps in interdisciplinary practice of neuro-oncology across Iran. The working team concept within NOSC is being further consolidated as we proceed. The brain tumor collaborative registry (BTCR) software is a validated tool provided to foster integrated research works while allowing a more organized patient care [9]. NOSC scientific meetings, update sessions and round table discussions will continue to serve as a platform for shared initiatives towards improving care to brain tumor.
NOSC members have concurred on three major plans for the future NOSC-related activities. The prospective plans and action points include: (I) constitution of tumor board meetings in Tehran, (II) designing a multi-center trial to investigate the efficacy and safety of 12 cycles adjuvant therapy with TMZ as compared to 6 cycles in GBM patients, and (III) taking due steps towards drafting local guidelines for the management of HGGs in Iran.
Acknowledgements
Authors would like to thank Drs. Dindoust P, Salarian A, Hejazi Farahmand S.A.R., Nabil Y, and Mukhomorova L, for supporting the NOSC. Appreciation is extended to Ranjbar E, Rohani Najafabadi H and Afarid M for their invaluable assistance. This meeting received scientific and administrative support from Behestan Darou PJS and Behphar scientific Committee, Tehran, Iran.
Disclosure of conflict of interest
None.
References
- 1.Tabatabai G, Hattingen E, Schlegel J, Stummer W, Schlegel U. [Interdisciplinary neuro-oncology: part 1: diagnostics and operative therapy of primary brain tumors] . Nervenarzt. 2014;85:965–975. doi: 10.1007/s00115-014-4041-7. [DOI] [PubMed] [Google Scholar]
- 2.Boeker M, Muller C, Klar R, Lutterbach J. OncoCase: interdisciplinary case based teaching in Neuro-Oncology based on the campus platform. AMIA Annu Symp Proc. 2005:898. [PMC free article] [PubMed] [Google Scholar]
- 3.Anvari K, Bahadorkhan GH, Silanian-Toussi M, Rahighi S, Ghavamnasiri MR, Saeedi M, Mojarrad M, Tabatabaee yazdy SA, Nekooee S, Nowfersti GH, Salek R, Mashhadi Nezhad H, Taghzadeh kermani A, Homaei-Shandiz F, Aledavood SA, Ehsaei MR, Faraji-Rad M, Rafati AR, M A, Bahar Vahdat H, Safaie Yazdi A, Bidoyei F, Khoshroo F, Varshoee Tabrizi F, Fani Pakdel A, Shahid Sales S, Mirsadraee M, Yousefi AH, Hosseini S, Noori fard M, Dehestani M, Mirshahi J, Mukhomorova L, Afarid M, Hejazi-Farahmand SAR, Torabi-Nami M. From fundamental brain tumor science to interdisciplinary bedside care; the outcome report from the neuro-oncology scientific club second meet-up (nosc-2), 19th April 2012, Mashhad, Iran. International Journal of Medical and Clinical Research. 2012;3:168–175. [Google Scholar]
- 4.Stupp R, Weller M. Questions regarding the optimal use of bevacizumab in glioblastoma: a moving target. Neuro Oncol. 2014;16:765–767. doi: 10.1093/neuonc/nou092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt F, Fischer J, Herrlinger U, Dietz K, Dichgans J, Weller M. PCV chemotherapy for recurrent glioblastoma. Neurology. 2006;66:587–589. doi: 10.1212/01.wnl.0000197792.73656.c2. [DOI] [PubMed] [Google Scholar]
- 6.Levin VA, Uhm JH, Jaeckle KA, Choucair A, Flynn PJ, Yung WKA, Prados MD, Bruner JM, Chang SM, Kyritsis AP, Gleason MJ, Hess KR. Phase III randomized study of postradiotherapy chemotherapy with alpha-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N’-cyclohexyl-N-nitrosurea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiforme. Clin Cancer Res. 2000;6:3878–3884. [PubMed] [Google Scholar]
- 7.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 8.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 9.Torabi-Nami M, Hejazi Farahmand S, Mohammadzadeh F. Neuro-oncology scientific club and the national Iranian brain tumor registry. Neuro Oncol. 2012;14:iii1–iii94. [Google Scholar]
- 10.Amouheidari A, Hemati S, Sabouri M, Emami J, Mehrzad V, Hekmatnia H, Alian B, Rouhani Najafabadi H, Torabi-Nami M. The Nexus between Interdisciplinary Approach and Extended Survival in CNS Tumors, Neuro- Oncology Scientific Club (NOSC) Meeting Report, 27 December 2012, Isfahan, Iran. Research In Cancer and Tumor. 2013;2:1–9. [Google Scholar]
- 11.Anvari K, Bahadorkhan G, Nekooi S, Taghizadeh A, Kheradmand H, Nowferesti G, Seilanian Tousi M, Mashhadinejad H, Zahed Anaraki S, Makhdoomi Y, Homaee F, Ehsaee M, Fani Pakdel A, Varshoee Tabrizi F, Shahidsales S, Motlagh F, Mirsadraie M, Khoshroo F, Partoie R, Abili M, Rahighi S, Bidoyie F, Mirshahi J, Dehestani M, Noorifard M, Afarid M, Hejazi Farahmand S, Torabi-Nami M. Towards the Real Interdisciplinary Approach in Treating Brain Tumors: Report from the Neuro-Oncology Scientific Club opening meeting -NOSC 2011-13 October- Mashhad, IR Iran. WMC Oncology. 2011;10:WMC002381. [Google Scholar]
- 12.Haddad P, Zali A, Tabatabaeefar M, Nikoofar A, Hadizadeh Kharazi H, Ghadyani M, Fadavi P, Vossough P, Mousavizadeh M, Mehdizadeh M, Motlagh A, Amoozegar Hashemi F, Ameri A, Yaghobi Joibari A, Pazooki B, Rakhsha A, Arbabi F, Mehrvar A, Aghili M, Babaei M, Dehghan Menshadi H, Kamian S, Moeini B, Andalib B, Karimkhani S, Dadgar F, Khajei H, Behrouzi H, Abed Moghadam M, Mottahedi H, Tashvighi M, Faranoush F, Mojahed MM, Naderi A, Naseri S, Ibrahim S, Hejazi Farahmand SAR, Mohammadzadeh F, Mukhomorova L, Torabi-Nami M. Turning Interdisciplinary Brain Tumor Science into Survival; Report from the Neuro-Oncology Scientific Club Opening Session, NOSC 2012 -19 January- Tehran. IR Iran Research and Opinion. 2012;4:42–53. [Google Scholar]
- 13.Faranoush M, Torabi-Nami M, Mehrvar A, HedayatiAsl AA, Tashvighi M, Parsa RR, Fazeli MA, Sobuti B, Mehrvar N, Jafarpour A, Zangooei R, Alebouyeh M, Abolghasemi M, Vahabie AH, Vossough P. Classifying Pediatric Central Nervous System Tumors through near Optimal Feature Selection and Mutual Information: A Single Center Cohort. Middle East Journal of Cancer. 2013;4:153–162. [Google Scholar]
- 14.Anvari K, Bahadorkhan G, Etemad-Rezaie H, Silanian-Toussi M, Nekooei S, Samini F, Ehsaie M, Motlagh Pirooz F, Safaie Yazdi A, Taghizadeh Kermani A, Salek R, Nowferesti G, Zahed An- araki S, Varshoee Tabrizi F, Mirsadraee M, Rafati A, Alipour Tabrizi H, Baharvahdat H, Gholamin M, Dayani M, Bidouei F, Fazl Ersi M, Sadeghi-Ivari M, Nafarieh L, Afarid M, Torabi-Nami M. Prognostic Factors and Treatment Outcome in Glial Brain Tumors; Data from the Third Neuro-oncology Scientific Club’s Input Forum, 2013, Mashhad. Iran British Journal of Medicine and Medical Research. 2014;4:3538–3553. [Google Scholar]
- 15.Figarella-Branger D, Mokhtari K, Dehais C, Jouvet A, Uro-Coste E, Colin C, Carpentier C, Forest F, Maurage CA, Vignaud JM, Polivka M, Lechapt-Zalcman E, Eimer S, Viennet G, Quintin-Roue I, Aubriot-Lorton MH, Diebold MD, Loussouarn D, Lacroix C, Rigau V, Laquerriere A, Vandenbos F, Michalak S, Sevestre H, Peoch M, Labrousse F, Christov C, Kemeny JL, Chenard MP, Chiforeanu D, Ducray F, Idbaih A, Network P. Mitotic index, microvascular proliferation, and necrosis define 3 groups of 1p/19q codeleted anaplastic oligodendrogliomas associated with different genomic alterations. Neuro Oncol. 2014;16:1244–1254. doi: 10.1093/neuonc/nou047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malkki H. Neuro-oncology: Bevacizumab prolongs progression-free survival but not overall survival in newly diagnosed glioblastoma. Nat Rev Neurol. 2014;10:179. doi: 10.1038/nrneurol.2014.47. [DOI] [PubMed] [Google Scholar]
- 17.Diez Valle R, Tejada S. Resection in glioblastoma: maximal or safe. Acta Neurochir (Wien) 2014;156:325–326. doi: 10.1007/s00701-013-1967-7. [DOI] [PubMed] [Google Scholar]
- 18.Gulati S, Jakola AS, Nerland US, Weber C, Solheim O. The risk of getting worse: surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 2011;76:572–579. doi: 10.1016/j.wneu.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Chaichana KL, Halthore AN, Parker SL, Olivi A, Weingart JD, Brem H, Quinones-Hinojosa A. Factors involved in maintaining prolonged functional independence following supratentorial glioblastoma resection. Clinical article. J Neurosurg. 2011;114:604–612. doi: 10.3171/2010.4.JNS091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshikawa K, Kajiwara K, Morioka J, Fujii M, Tanaka N, Fujisawa H, Kato S, Nomura And S, Suzuki M. Improvement of functional outcome after radical surgery in glioblastoma patients: the efficacy of a navigation-guided fence-post procedure and neurophysiological monitoring. J Neurooncol. 2006;78:91–97. doi: 10.1007/s11060-005-9064-2. [DOI] [PubMed] [Google Scholar]
- 21.Dziedzic T, Bernstein M. Awake craniotomy for brain tumor: indications, technique and benefits. Expert Rev Neurother. 2014;14:1405–1415. doi: 10.1586/14737175.2014.979793. [DOI] [PubMed] [Google Scholar]
- 22.Lakicevic G, Splavski B, Brekalo Z. The value of stereotactic biopsy in improving survival and quality of life for malignant brain glioma patients. Coll Antropol. 2010;34(Suppl 1):93–97. [PubMed] [Google Scholar]
- 23.Muragaki Y, Chernov M, Maruyama T, Ochiai T, Taira T, Kubo O, Nakamura R, Iseki H, Hori T, Takakura K. Low-grade glioma on stereotactic biopsy: how often is the diagnosis accurate? Minim Invasive Neurosurg. 2008;51:275–279. doi: 10.1055/s-0028-1082322. [DOI] [PubMed] [Google Scholar]
- 24.Kieran MW. Time to rethink the unthinkable: Upfront biopsy of children with newly diagnosed diffuse intrinsic pontine glioma (DIPG) Pediatr Blood Cancer. 2015;62:3–4. doi: 10.1002/pbc.25266. [DOI] [PubMed] [Google Scholar]
- 25.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 26.Chang JH, Kim CY, Choi BS, Kim YJ, Kim JS, Kim IA. Pseudoprogression and pseudoresponse in the management of high-grade glioma : optimal decision timing according to the response assessment of the neuro-oncology working group. J Korean Neurosurg Soc. 2014;55:5–11. doi: 10.3340/jkns.2014.55.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kingwell K. Brain imaging: measures of functional brain connectivity can be used to predict outcome after glioma surgery. Nat Rev Neurol. 2012;8:532. doi: 10.1038/nrneurol.2012.187. [DOI] [PubMed] [Google Scholar]
- 28.Hygino da Cruz LC Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32:1978–1985. doi: 10.3174/ajnr.A2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amin A, Moustafa H, Ahmed E, El-Toukhy M. Glioma residual or recurrence versus radiation necrosis: accuracy of pentavalent technetium-99m-dimercaptosuccinic acid [Tc-99m (V) DMSA] brain SPECT compared to proton magnetic resonance spectroscopy (1H-MRS): initial results. J Neurooncol. 2012;106:579–587. doi: 10.1007/s11060-011-0694-2. [DOI] [PubMed] [Google Scholar]
- 30.Chen SD, Hou PF, Lou L, Jin X, Wang TH, Xu JL. The correlation between MR diffusion-weighted imaging and pathological grades on glioma. Eur Rev Med Pharmacol Sci. 2014;18:1904–1909. [PubMed] [Google Scholar]
- 31.Yamasaki F, Kurisu K, Aoki T, Yamanaka M, Kajiwara Y, Watanabe Y, Takayasu T, Akiyama Y, Sugiyama K. Advantages of high b-value diffusion-weighted imaging to diagnose pseudoresponses in patients with recurrent glioma after bevacizumab treatment. Eur J Radiol. 2012;81:2805–2810. doi: 10.1016/j.ejrad.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Hu X, Xie P, Li W, Li X, Ma L. Comparison of magnetic resonance spectroscopy and positron emission tomography in detection of tumor recurrence in posttreatment of glioma: A diagnostic meta-analysis. Asia Pac J Clin Oncol. 2014 doi: 10.1111/ajco.12202. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Gorlia T, Stupp R, Brandes AA, Rampling RR, Fumoleau P, Dittrich C, Campone MM, Twelves CC, Raymond E, Hegi ME, Lacombe D, van den Bent MJ. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48:1176–1184. doi: 10.1016/j.ejca.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Scott JG, Bauchet L, Fraum TJ, Nayak L, Cooper AR, Chao ST, Suh JH, Vogelbaum MA, Peereboom DM, Zouaoui S, Mathieu-Daude H, Fabbro-Peray P, Rigau V, Taillandier L, Abrey LE, DeAngelis LM, Shih JH, Iwamoto FM. Recursive partitioning analysis of prognostic factors for glioblastoma patients aged 70 years or older. Cancer. 2012;118:5595–5600. doi: 10.1002/cncr.27570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paravati AJ, Heron DE, Landsittel D, Flickinger JC, Mintz A, Chen YF, Huq MS. Radiotherapy and temozolomide for newly diagnosed glioblastoma and anaplastic astrocytoma: validation of Radiation Therapy Oncology Group-Recursive Partitioning Analysis in the IMRT and temozolomide era. J Neurooncol. 2011;104:339–349. doi: 10.1007/s11060-010-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Wang M, Won M, Shaw EG, Coughlin C, Curran WJ Jr, Mehta MP. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81:623–630. doi: 10.1016/j.ijrobp.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clara CA, Marie SK, de Almeida JR, Wakamatsu A, Oba-Shinjo SM, Uno M, Neville M, Rosemberg S. Angiogenesis and expression of PDGF-C, VEGF, CD105 and HIF-1alpha in human glioblastoma. Neuropathology. 2014;34:343–352. doi: 10.1111/neup.12111. [DOI] [PubMed] [Google Scholar]
- 38.Emara M, Allalunis-Turner J. Effect of hypoxia on angiogenesis related factors in glioblastoma cells. Oncol Rep. 2014;31:1947–1953. doi: 10.3892/or.2014.3037. [DOI] [PubMed] [Google Scholar]
- 39.Poulsen HS, Urup T, Michaelsen SR, Staberg M, Villingshoj M, Lassen U. The impact of bevacizumab treatment on survival and quality of life in newly diagnosed glioblastoma patients. Cancer Manag Res. 2014;6:373–387. doi: 10.2147/CMAR.S39306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke JL. Bevacizumab and other targeted agents in the upfront treatment of glioblastoma. Semin Radiat Oncol. 2014;24:273–278. doi: 10.1016/j.semradonc.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Fathpour P, Obad N, Espedal H, Stieber D, Keunen O, Sakariassen PO, Niclou SP, Bjerkvig R. Bevacizumab treatment for human glioblastoma. Can it induce cognitive impairment? Neuro Oncol. 2014;16:754–756. doi: 10.1093/neuonc/nou013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chamberlain MC, Glantz MJ. CPT-11 for recurrent temozolomide-refractory 1p19q co-deleted anaplastic oligodendroglioma. J Neurooncol. 2008;89:231–238. doi: 10.1007/s11060-008-9613-6. [DOI] [PubMed] [Google Scholar]
- 43.Gwak HS, Yee GT, Park CK, Kim JW, Hong YK, Kang SG, Kim JH, Seol HJ, Jung TY, Chang JH, Yoo H, Hwang JH, Kim SH, Park BJ, Hwang SC, Kim MS, Kim SH, Kim EY, Kim E, Kim HY, Ko YC, Yun HJ, Youn JH, Kim J, Lee B, Lee SH. Temozolomide salvage chemotherapy for recurrent anaplastic oligodendroglioma and oligoastrocytoma. J Korean Neurosurg Soc. 2013;54:489–495. doi: 10.3340/jkns.2013.54.6.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen SA, Stechishin OD, Luchman HA, Lun XQ, Senger DL, Robbins SM, Cairncross JG, Weiss S. Novel MSH6 mutations in treatment-naive glioblastoma and anaplastic oligodendroglioma contribute to temozolomide resistance independently of MGMT promoter methylation. Clin Cancer Res. 2014;20:4894–4903. doi: 10.1158/1078-0432.CCR-13-1856. [DOI] [PubMed] [Google Scholar]
- 45.Gorlia T, Delattre JY, Brandes AA, Kros JM, Taphoorn MJ, Kouwenhoven MC, Bernsen HJ, Frenay M, Tijssen CC, Lacombe D, van den Bent MJ. New clinical, pathological and molecular prognostic models and calculators in patients with locally diagnosed anaplastic oligodendroglioma or oligoastrocytoma. A prognostic factor analysis of European Organisation for Research and Treatment of Cancer Brain Tumour Group Study 26951. Eur J Cancer. 2013;49:3477–3485. doi: 10.1016/j.ejca.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 46.Ino Y, Betensky RA, Zlatescu MC, Sasaki H, Macdonald DR, Stemmer-Rachamimov AO, Ramsay DA, Cairncross JG, Louis DN. Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res. 2001;7:839–845. [PubMed] [Google Scholar]
- 47.Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W, Mehta M. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J. Clin. Oncol. 2013;31:337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, Bernsen HJ, Frenay M, Tijssen CC, Grisold W, Sipos L, Enting RH, French PJ, Dinjens WN, Vecht CJ, Allgeier A, Lacombe D, Gorlia T, Hoang-Xuan K. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013;31:344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 49.Fangusaro J, Warren KE. Unclear standard of care for pediatric high grade glioma patients. J Neurooncol. 2013;113:341–342. doi: 10.1007/s11060-013-1104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung TY, Lee JY, Kim DS, Park HJ, Kim CY, Ra YS, Lee MJ, Kim SH, Baek HJ, Kim IH, Park KD, Kim SK. Pediatric supratentorial high-grade glioma: multicenter retrospective observational study of the Korean Society for Pediatric Neuro-Oncology. J Neurooncol. 2015;121:413–9. doi: 10.1007/s11060-014-1653-5. [DOI] [PubMed] [Google Scholar]
- 51.Wolff JE, Driever PH, Erdlenbruch B, Kortmann RD, Rutkowski S, Pietsch T, Parker C, Metz MW, Gnekow A, Kramm CM. Intensive chemotherapy improves survival in pediatric high-grade glioma after gross total resection: results of the HIT-GBM-C protocol. Cancer. 2010;116:705–712. doi: 10.1002/cncr.24730. [DOI] [PubMed] [Google Scholar]
- 52.Diaz AK, Baker SJ. The genetic signatures of pediatric high-grade glioma: no longer a one-act play. Semin Radiat Oncol. 2014;24:240–247. doi: 10.1016/j.semradonc.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawkins CE, Bartels U, Bouffet E. Molecular genetic approaches and potential new therapeutic strategies for pediatric diffuse intrinsic pontine glioma. J. Clin. Oncol. 2011;29:3956–3957. doi: 10.1200/JCO.2011.37.8661. [DOI] [PubMed] [Google Scholar]
- 54.Chastagner P, Kalifa C, Doz F, Bouffet E, Gentet JC, Ruchoux MM, Bracard S, Desandes E, Frappaz D French Society of Pediatric Oncology (SFOP) Pilot Study. Outcome of children treated with preradiation chemotherapy for a high-grade glioma: results of a French Society of Pediatric Oncology (SFOP) Pilot Study. Pediatr Blood Cancer. 2007;49:803–807. doi: 10.1002/pbc.21051. [DOI] [PubMed] [Google Scholar]
- 55.Khaw SL, Coleman LT, Downie PA, Heath JA, Ashley DM. Temozolomide in pediatric low-grade glioma. Pediatr Blood Cancer. 2007;49:808–811. doi: 10.1002/pbc.21270. [DOI] [PubMed] [Google Scholar]
- 56.Saran F. Recent advances in paediatric neuro-oncology. Curr Opin Neurol. 2002;15:671–677. doi: 10.1097/01.wco.0000044762.39452.e3. [DOI] [PubMed] [Google Scholar]
- 57.Friedrich C, Muller K, von Hoff K, Kwiecien R, Pietsch T, Warmuth-Metz M, Gerber NU, Hau P, Kuehl J, Kortmann RD, von Bueren AO, Rutkowski S. Adults with CNS primitive neuroectodermal tumors/pineoblastomas: results of multimodal treatment according to the pediatric HIT 2000 protocol. J Neurooncol. 2014;116:567–575. doi: 10.1007/s11060-013-1327-8. [DOI] [PubMed] [Google Scholar]


