Abstract
Objective: Peritonitis is a commonly seen disease with high morbidity and mortality. It is prevalently considered that the impaired intestinal barrier during peritonitis is the access point of gut microbes into the blood system, and acts as the engine of the following systemic infection. In our previous study, we found that Sodium Butyrate (NaB) was protective on intestinal barrier function. In this study, we aim to evaluate the effects of NaB on overwhelming infection animal models of peritonitis. Methods: Mouse cecal ligation and puncture (CLP) model was used to study the effects of NaB on the intestinal barrier. Experimental animals were fed of NaB by gavage. Post-CLP mortality, gut permeability and intestinal histological alterations were studied. Results: Gastrointestinal NaB pharmacodynamics profiles after medication were studied. Measurements of NaB concentration in chyme showed significantly higher intestinal concentration of NaB in the NaB treated group than that of the control group. CLP-induced mortality was significantly decreased by oral NaB treatments. Gut permeability was largely increased after CLP, which was partially prevented by NaB feeding. Histological study showed that intestinal, especially ileal injury following peritonitis was substantially alleviated by NaB treatments. Moreover, tissue regeneration was also prompted by NaB. Conclusion: NaB has a potential protective effect on intestinal barrier function in peritonitis.
Keywords: Sodium butyrate, intestinal barrier, peritonitis mice
Introduction
Peritonitis is commonly seen among postoperative patients as a complication of various surgeries including perforative peptic ulcer, suppurative appendicitis, cholecystitis, trauma, and postoperative anastomotic leakage [1,2]. Without proper treatments, peritonitis can cause sepsis, systemic inflammatory response syndrome (SIRS), and ultimately multiple organ failure (MOF). In patients with peritonitis, the microbe-filling peritoneal cavity becomes the engine of systemic infection. The impaired intestinal barrier also facilitates gut microbe transmission into the blood system. It was reported that the small bowel is the origin of bacteremia in acute diffuse peritonitis [3]. Protection of the intestinal barrier function is now a key focus in preventing peritonitis from deterioration.
The intestinal epithelium is a single layer of cells that function as both selective filters and foreign antigen barriers [4]. Its permeability allows nutrients to pass through the gut while the barrier function keeps noxious substances and foreign antigens from entering into the circulation system and other tissues. The barrier is further composed of two parts: the intrinsic barrier of continuous epithelial cell lining and the extrinsic mucus barrier combined of goblet cells secretions and the epithelium [5]. It is clear that the intestinal barrier plays a key role in the innate and adaptive immune system and its function under infections like peritonitis is more crucial [6]. The damage of intestinal barrier leading to the leakage of large amount of antigens into the blood system symbolized the deterioration of peritonitis during its pathological progression. In this view, protection of the intestinal barrier is a key step in treating peritonitis. Recently, several compounds have been reported protective on intestinal epithelial through various underlying mechanisms [7-9]. In our previous study, we focused on the protective effects of sodium butyrate (NaB) on intestinal barrier function under severe stress. To make further evaluations in vivo, we studied the protective effects of NaB on mice cecal ligation and puncture (CLP) model aiming to provide a potential pharmaceutical for treating peritonitis and improve the prognosis.
Butyrate is one of the major metabolites in the intestines of human and animals from bacterial fermentation. It has also been shown that butyrate is a critical mediator of the intestinal inflammatory response [10]. Through regulating IFN-γ/STAT1 signaling pathways, inhibiting histone deacetylase (HDAC) and interact with T cells, butyrate is involved in both intestinal homeostasis and under inflammation situation [11]. NaB (C3H7COO) and its effects have been intensively studied on cultured cell lines and in vivo experiments. Several groups have already reported the protective effects of NaB on intestinal barrier in recent years [12,13]. However, in vivo evaluation of NaB on barrier function is still unclear.
These accumulating findings motivated us to study whether NaB has an alleviative effects on the mortality rate in animal models of peritonitis. In this study, we used mice CLP models in order to simulate the peritonitis condition. From our results, we found that NaB could reduce peritonitis-related mortality, decrease intestinal permeability in peritonitic animals, and alleviate intestinal mucosal damage by infection.
Materials and method
This work was supported by the ethics committee of Jingxi Campus of Beijing Chaoyang Hospital Affiliated to Capital Medical University.
Animals & CLP model
Male C57BL/6N mice, 20~25 g, were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd and housed in the animal research facility of the Peking University First Hospital. Mice were allowed to acclimatize for 3 d with free access to standard chow diet (Beijing Ke Ao Xie Li Feeds Co., Ltd) and water under controlled conditions of temperature and humidity with a 12:12 hour light: dark cycle. CLP procedure was modified from the method reported by Habbard et al [14]. Briefly, mice were anesthetized by pentobarbital sodium (50 mg/kg i.p.) and the abdominal skin was prepared by hair removal and Iodophor disinfection. All operations were performed following aseptic procedures. After laparotomy, the distal portion of the cecum (1 cm) was ligated with 5-0 silk suture. The ligated cecum (1 cm) was then punctured with a 21-gauge syringe needle and gently compressed until a small amount of feces appeared. In the sham-operated animals, the cecum was manipulated but without ligation and puncture, and placed back into the peritoneum. The incision was closed with a 2-layer procedure: 5-0 polyglactin suture on the muscle layer and silk suture on the skin respectively. Mice received a subcutaneous injection of 1mL of saline for fluid resuscitation at the time of closure, and were placed in an incubator (37°C) for 15 min.
NaB Administration
NaB solution (1% w/v) was administered by gavage (0.2 g/kg, every 12 h) 24 h prior to surgery and continued 24 h after the operation. The control group was gavaged with an equal volume of normal saline.
Gastrointestinal (GI) Butyrate Concentration Measurements
GI digestive matter (chyme) was collected by laparotomy in euthanized animals. Butyrate content was measured using gas chromatography (GC), modified from the method previously described by He et al [15]. Briefly, samples were prepared as follows, 20 ul 2 mg/ml isobutyrate in water (internal standard), 1880 ul of sample or butyrate standards were mixed with 2 ml ethyl acetate. 100ul phosphoric acid was added for acidification. The analysis is based on headspace GC. GC was performed using an Agilent 6890 Series N Gas Chromatograph (Agilent, US) with FID flame ionization detector, equipped with a 30 cm × 530 μm × 10 μm Agilent 125-7032 capillary column.
Mortality study
Animals after either CLP or sham surgery were placed back to clean environments with free access to chow diet and drinking water. All the animals were inspected twice daily, and dead individuals were removed and recorded. The mortality observations ended at the tenth day after surgery. We did not collect either blood or tissue samples from these mice. The lethality was shown in a survival curve.
Intestinal permeability assay
Fluorescein isothiocyanate-dextran, molecular weight 40 kD, (FD40), (Sigma-Aldrich, USA), was used as a tracer of intestinal permeability. Animals were gavaged with 60 mg/kg FD40 4 h prior to blood sampling from the orbital sinus. Plasma was separated (Heperin anticoagulation, 4°C, 2000 rpm, 10 min) for FD40 measurement. Plasma FD40 was measured by high performance liquid chromatography (HPLC) using fluorescence detection model SIL-20A/SIL-20AC prominence liquid chromatograph (Waters-Millipore) (SHIMADZU Company, Japan). The chromatography system for FITC analysis included the following parameters: Autosampler (SIL-20A); RF-10 AXL fluorescence detector set at an excitation and emission wavelength of 494 and 518 nm respectively; Polysep-GFC-P linear size exclusion column (300 × 7.8 mm I.D., Phenomenex, Torrance, CA, USA) was used as the analytical column. The buffer component of the mobile phase (0.05 M phosphate buffer) was prepared with deionized water and the pH was adjusted to 7.0. The mobile phase was filtered through a 0.45-μm nylon filter and degassed under ultra-sound and vacuum for 15 min. The mobile phase was delivered at a flow rate of 1 ml/min. The mobile phase consisted of acetonitrile: 0.05 M phosphate buffer (12:88, v/v). The assay was linear (R ≥ 0.99) and inter-day precision ranged from 0.5-5.5%.
Morphological analysis
The ileal tissue of experimental animals was collected for morphological study by Hematoxylin and Eosin (H&E) staining. Villus length was determined on HE stained ileal sections by measuring the distance in micrometers from the crypt neck to the villus tip. A minimum of 12 well-oriented villi from each section were measured. The Chiu scale [16] of mucosal injury was used to evaluate the degree of histological alteration on ten 1 mm-thick sections of tissue, for a total measurement of 1 cm sample per animal. The scale consists of values from 0 to 5, where 0 reflected normal mucosa: 1, development of sub-epithelial (Gruenhagen’s) spaces; 2, extension of the sub-epithelial space with moderate epithelial lifting from the lamina propria; 3, extensive epithelial lifting with occasional denuded villi tips; 4, denuded villi with exposed lamina propria and dilated capillaries; and 5, disintegration of the lamina propria, hemorrhage, and ulceration.
Statistics
The values of NaB concentration and plasma FD40 concentration are shown as means ± S.E.M. from three independent samples. The significance of the difference between means was determined by analysis of variance. The level of significance was determined using Duncan’s multiple range test. Survival rates are compared for significance by Log-Rank test of Kaplan-Meier curve using statistical software package SPSS 13.0.
Results
GI butyrate pharmacokinetics following Oral Administration
Butyrate acid is one of the essential SCFAs produced by bacterial fermentation mainly in the colon of mammals. The bioactive form of butyrate acid is its negative ion. NaB as a stable sodium salt of butyrate, was used for introducing an exogenous supply of butyrate acid radicals. Little is known about butyrate anion’s effect on the upper alimentary tract and small intestine. Thus it is worthy to investigate the kinetics of butyrate in the GI tract after oral intake. Animals fed with regular chow diet with or without the addition of NaB solution were subjected to butyrate concentration measurements. The fresh GI chyme was sampled by laparotomy. Butyrate content from fecal matters of the stomach, jejunum, ileum, and cecum were examined. The butyrate concentration was constant in the cecum despite additional NaB. As shown in Table 1, undetectable amounts of butyrate (i.e. less than the sensitivity threshold of 0.01 mM) were observed in the stomach and small intestine of animals fed with normal chow, whereas a heightened level of butyrate was seen in the content of the proximal GI tracts of mice compulsively fed with NaB solution. The ingestion of NaB solution (0.2 g/kg, twice daily) resulted in a NaB concentration of 2 mmol/kg in stomach chyme. After a certain amount of absorption by the stomach, butyrate concentration in the food mass remained constant throughout subsequent digestive processes of the small intestine.
Table 1.
GI butyrate anion concentration (mmol/kg)
| Stomach | Jejunum | Ileum | cecum | |
|---|---|---|---|---|
| Sham | ND | ND | ND | 5.12 ± 0.43 |
| CLP | ND | ND | ND | 6.13 ± 0.59 |
| CLP + NaB | 2.08 ± 0.19* | 1.23 ± 0.24* | 1.64 ± 0.11* | 5.09 ± 0.44 |
difference is statistically significant compared with CLP.
NaB reduces mortality in peritonitic mice
As shown in Figure 1, CLP-induced peritonitis led to significant early stage death in experimental mice. By the fifth day after CLP, more than 50% of the individuals have died. The remaining mice were able to survive the CLP injury as long as they made it through the first five days. The overall survival rate of the CLP group was 46.2% (6/13). Without cecal ligation and puncture, the sham surgery group showed no mortality. Mice fed with NaB demonstrated better outcomes with only three out of thirteen mice dying, leading to a 76.9% (10/13) survival rate after CLP. The difference between CLP and CLP + NaB group was statistically significant (Log-Rank Χ 2 = 5.113 P = 0.024). Enteral administration of NaB proved to bear protective potential against peritonitis.
Figure 1.
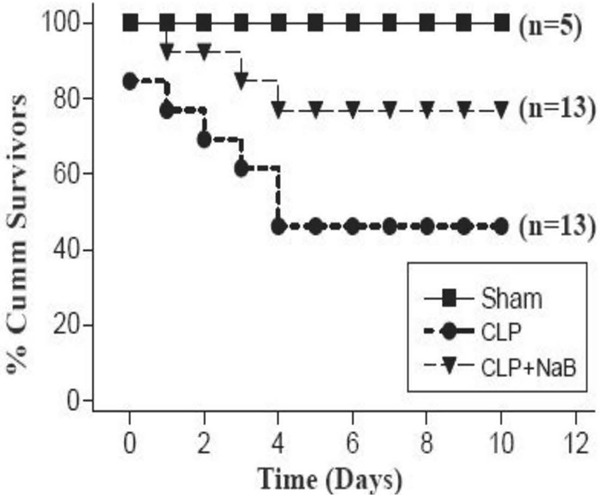
Lethalities after CLP. The curves represent the survival rate of sham, CLP, and CLP + NaB group.
NaB attenuated the intestinal barrier permeability in peritonitic mice
To further address the mechanisms through which NaB protects experimental animals against peritonitis and sepsis, we investigated whether the intestinal barrier integrity was altered by CLP as well as NaB (Figure 2). Fluorescence labeled macro-molecule FD40 was used as a tracer of intestinal permeability. After enteral administration of FD40, the intestinal permeability was represented by the plasma FD40. In healthy animals, only a trace amount of FD40 was transmitted across the gut wall into the blood stream. In post-CLP animals, the plasma FD40 concentration increased to nearly 0.3 ug/ml, 6 folds higher than the sham group. However, plasma FD40 in CLP mice treated with NaB was reduced to 0.13 ug/ml, suggesting that NaB significantly preserved gut barrier function in times of severe illness.
Figure 2.
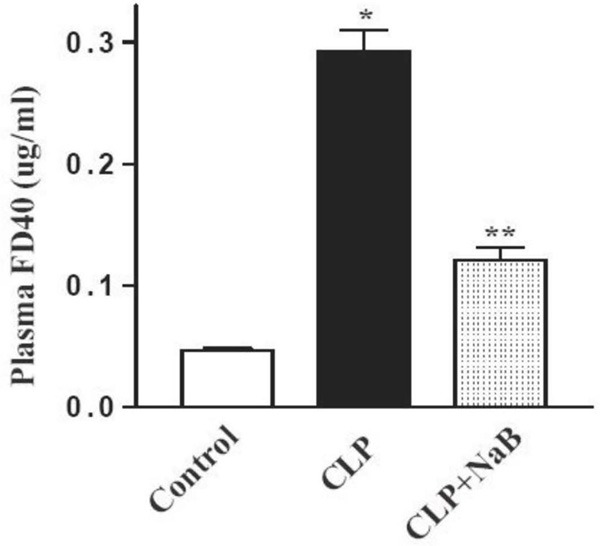
Intestinal permeability alteration. Stomach gavaged FD40 was used as the indicator for the permeability of gut mucosa. 4 h after stomach gavage, FD40 concentrations of blood plasma were measured using HPLC method. *P < 0.05 compared with sham. **P < 0.05 compared with CLP.
NaB alleviated the intestinal mucosal tissue damage caused by CLP and prompted tissue recovery
Intestinal histological changes were studied by H&E staining. The most dramatic microscopic injury was observed in the ileum. Figure 3 showed ileal mucosa damage from CLP-peritonitis. The left column (Figure 3A, 3C, 3E, 3G, 3I, 3K) showed the time course of microscopic tissue injury and recovery, with the most severe tissue harm seen 1 d after the onset of peritonitis. Significant villi destruction was seen, along with remarkable ulcerations and necrosis occurring at the upper tip portion (Figure 3E, inset). The recovery of villous structures in the ileal mucosa startedon the second day. The denuded villous tips were re-epithelialized and complete restitution of the mucosal structure was accomplished 4 d after the onset of peritonitis. The villi regained its original height and ulcerations at the tips were healed.
Figure 3.
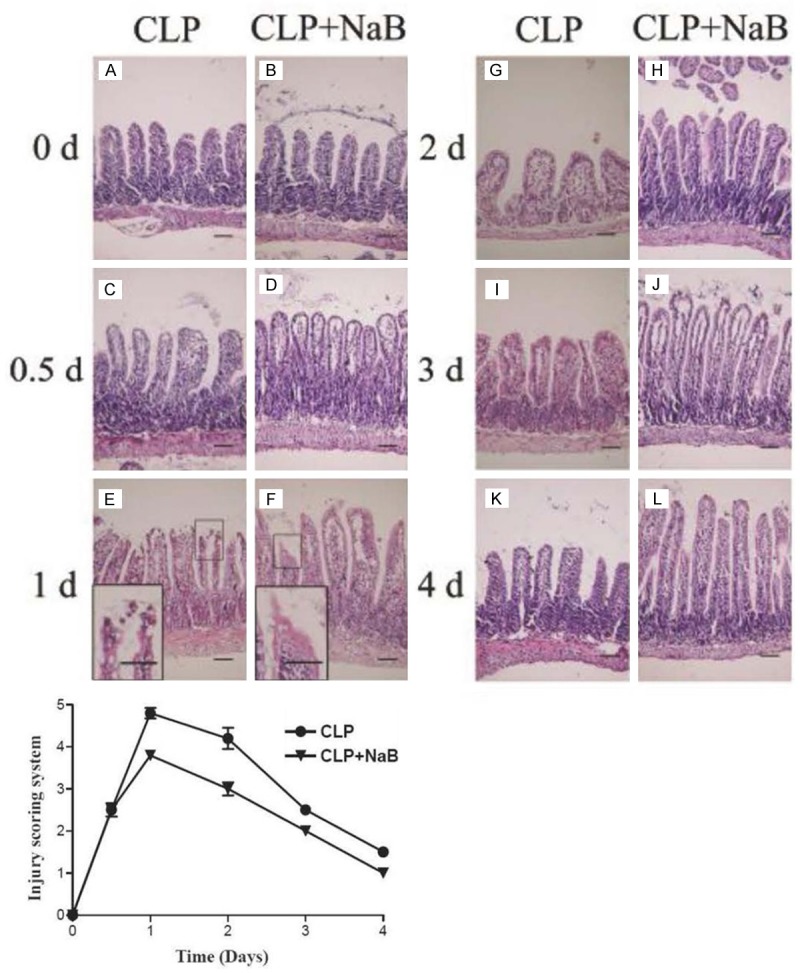
Morphological change of ileum following CLP was alleviated by NaB. Mice ileal tissue was harvest at scheduled time point. Paraffin sections were H&E stained. Magnification: ×200. Scale bars: 100 μm, 50 μm (insets of E and F). Lower chart: the injury scoring of the microscopic damages aforementioned. The data were shown in the mean ± SE. The samples were collected from three independent animals for each time point. Chiu scorings were done for 10 different fields of vision.
In a side by side comparison, a smaller degree of mucosal damage followed by a quicker histological recovery was witnessed in the ileal tissue sample of NaB fed mice (Figure 3B, 3D, 3F, 3H, 3J, 3L). There was fewer ulceration and necrosis by the first day after CLP (Figure 3F). An injury scoring evaluation system showed similar results (Figure 3, bottom chart). In the re-construction of villous structure from the second day through the fourth day, the NaB fed group showed a quicker re-gaining of villous height (Figure 3H, 3J, 3L).
Discussion
The human gut is not only a digestive tract, but also a gigantic warehouse of microbes. In healthy individuals, the flora is restrained in the lumen by a single barrier comprised of the continuous intestinal epithelium and intercellular junctions. In the event of severe sickness or stress, the intestinal barrier function can be impaired, thus allowing the microbes to translocate beyond the gut lumen and give rise to systemic infection. In critically ill patients, the pathogens of bacteremia are often Gram negative gut bacteria verifying the origin of the term “Gut-derived infection” [17]. Strengthening the intestinal barrier would be a promising way to reduce bacterial translocation. Numerous studies have been done in recent years seeking for intestinal barrier protective factors. Nutrients like glutamine and certain types of growth factors have been shown to safeguard gut barrier function [18-20]. CLP is a well-recognized model to mimic peritonitis and sepsis [14]. In this study, we proved that CLP animals showed increased gut permeability. We further used CLP mice to screen potential intestinal barrier protecting factors. NaB was found to enhance the integrity of mice GI mucosa under serious stress leading to better prognosis.
Butyrate acid is one of the SCFAs produced mainly within the colon lumen by bacterial fermentation of undigested dietary carbohydrates. Normally, SCFAs function mainly as the energy fuel of the colon epithelium. So far, there has been no report about butyrate quantity in the small intestine, but a rationale postulation would be that the concentration is low because of two reasons. Firstly, saccharolytic bacterial fermentation occurs predominantly in the colon. Secondly, over 95% of SCFAs are rapidly absorbed and metabolized by the colonic epithelium [21]. In this study, butyrate concentration in the proximal part of the GI tract was examined. In mice fed with regular diet, no detectable levels (or less than 0.01 mM) of butyrate was found in the chyme of the stomach and small intestine. This finding proved that the upper GI tract has a blank background, which is ideally suited for observing the effect of enteral butyrate on the small intestine. Because SCFAs are weak acids (pKa ≈ 4.8) and more than 90% exist in the anionic status dissociated form in the colonic lumen [22]. Therefore, sodium salt of butyrate was chosen for medication. The chyme sample from a different region of the GI tract of NaB fed mice was found to contain 1-2 mM butyrate anion. The NaB was absorbed only in the stomach, as implied by our data that there was a decrease in NaB after food mass passed through the stomach whereas NaB density remained constant in the small intestine.
NaB was observed to improve the survival of CLP mice in this study. Several possible underlying mechanisms might include the facilitating ability of NaB to dehydrate toxins, boost host immunity and repress the inflammatory reaction. Two groups recently reported the protective effects of butyrate was intimately related to tight junctions. Ma X et al. reported that butyrate had a positive effect on the expression of tight junction proteins occludin and zonula occluden protein-1 in the IPEC-J2 cell model [12]. Wang HB et al. reported the up-regulatory effect of butyrate on tight junction protein Claudin-1 transcription [13]. Besides the well-studied inhibitory role of butyrate on HDAC function, it is reported that butyrate is suppressive on hypoxia-inducible factor-1 (HIF-1) activity [23] in intestinal epithelial cells under hypoxic condition revealing another underlying mechanism of its protective effects. In earlier studies, butyrate was shown to ameliorate dextran sulfate colitis by inhibiting heat shock protein 70 (HSP70) expression and NF-kappaB activation [24]. Although most studies are consistent on the conclusion that butyrate has a protective role on intestinal barrier, it was also reported that butyrate could also induce colonic injuries and down-regulate intestinal trefoil factor gene expression both in vivo and in vitro [25].
Our observations from the intestinal mucosa integrity and permeability assay provided evidence that NaB increases CLP animal survival through, or at least partially through, strengthening of the epithelial barrier. Moreover, morphological observations yielded consistent results. NaB treatment alleviated CLP associated epithelial tissue damage and sped up tissue restitution. The most prominent morphological effect of NaB was observed in the ileum. These findings suggested that enteral NaB has a profoundly positive effect from the proximal GI tract to the cecum. The elevated permeability of the small bowel in peritonitis is harmful, however, it can be partially reversed by NaB treatments.
Conclusion
In summary, the present study demonstrated that treatment with NaB alleviates peritonitis-associated mortality through strengthened intestinal barrier function in mice CLP models. Taking considerations that NaB protects intestinal barrier function through a various of underlying mechanisms, in support of the previous in vitro studies, we evaluated NaB administration in vivo hoping to provide new insights in the exploration of treating intestinal barrier damage related diseases.
Disclsoure of conflict of interest
None.
References
- 1.Ruggiero R, Sparavigna L, Docimo G, Gubitosi A, Agresti M, Procaccini E, Docimo L. Post-operative peritonitis due to anastomotic dehiscence after colonic resection. Multicentric experience, retrospective analysis of risk factors and review of the literature. Ann Ital Chir. 2011;82:369–375. [PubMed] [Google Scholar]
- 2.Weber DG, Bendinelli C, Balogh ZJ. Damage control surgery for abdominal emergencies. Br J Surg. 2014;101:e109–118. doi: 10.1002/bjs.9360. [DOI] [PubMed] [Google Scholar]
- 3.Peregudov SI, Khanevich MD. The small intestine as the origin of bacteremia in acute diffuse peritonitis. Nutr Hosp. 1996;11:317–320. [PubMed] [Google Scholar]
- 4.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 21-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc. 2004;1:54–61. doi: 10.1513/pats.2306016. [DOI] [PubMed] [Google Scholar]
- 6.Hasnain SZ, Thornton DJ, Grencis RK. Changes in the mucosal barrier during acute and chronic Trichuris muris infection. Parasite Immunol. 2011;33:45–55. doi: 10.1111/j.1365-3024.2010.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penrose HM, Marchelletta RR, Krishnan M, McCole DF. Spermidine stimulates T cell protein-tyrosine phosphatase-mediated protection of intestinal epithelial barrier function. J Biol Chem. 2013;288:32651–32662. doi: 10.1074/jbc.M113.475962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cindric M, Cipak A, Zapletal E, Jaganjac M, Milkovic L, Waeg G, Stolc S, Zarkovic N, Suzana Borovic S. Stobadine attenuates impairment of an intestinal barrier model caused by 4-hydroxynonenal. Toxicol In Vitro. 2013;27:426–432. doi: 10.1016/j.tiv.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Azuma T, Shigeshiro M, Kodama M, Tanabe S, Suzuki T. Supplemental naringenin prevents intestinal barrier defects and inflammation in colitic mice. J Nutr. 2013;143:827–834. doi: 10.3945/jn.113.174508. [DOI] [PubMed] [Google Scholar]
- 10.Sunkara LT, Achanta M, Schreiber NB, Bommineni YR, Dai G, Jiang W, Lamont S, Lillehoj HS, Beker A, Teeter RG, Zhang G. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One. 2011;6:e27225. doi: 10.1371/journal.pone.0027225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 12.Ma X, Fan PX, Li LS, Qiao SY, Zhang GL, Li DF. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J Anim Sci. 2012;90(Suppl 4):266–268. doi: 10.2527/jas.50965. [DOI] [PubMed] [Google Scholar]
- 13.Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57:3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 14.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 15.He T, Priebe MG, Harmsen HJ, Stellaard F, Sun X, Welling GW, Vonk RJ. Colonic fermentation may play a role in lactose intolerance in humans. J Nutr. 2006;136:58–63. doi: 10.1093/jn/136.1.58. [DOI] [PubMed] [Google Scholar]
- 16.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 17.Gianotti L, Pyles T, Alexander JW, Fukushima R, Babcock GF. Identification of the blood component responsible for increased susceptibility to gut-derived infection. Transfusion. 1993;33:458–465. doi: 10.1046/j.1537-2995.1993.33693296806.x. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Chen Y, Zhang J, Zhu JF, Liu ZJ, Liang SY, Sun K, Liao WY, Gong JP. Protective effect of glutamine-enriched early enteral nutrition on intestinal mucosal barrier injury after liver transplantation in rats. Am J Surg. 2010;199:35–42. doi: 10.1016/j.amjsurg.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzo-Zuniga V, Rodríguez-Ortigosa CM, Bartolí R, Martínez-Chantar ML, Martínez-Peralta L, Pardo A, Ojanguren I, Quiroga J, Planas R, Prieto J. Insulin-like growth factor I improves intestinal barrier function in cirrhotic rats. Gut. 2006;55:1306–1312. doi: 10.1136/gut.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banan A, Fields JZ, Zhang LJ, Shaikh M, Farhadi A, Keshavarzian A. Zeta isoform of protein kinase C prevents oxidant-induced nuclear factor-kappaB activation and I-kappa-Balpha degradation: a fundamental mechanism for epidermal growth factor protection of the microtubule cytoskeleton and intestinal barrier integrity. J Pharmacol Exp Ther. 2003;307:53–66. doi: 10.1124/jpet.103.053835. [DOI] [PubMed] [Google Scholar]
- 21.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 22.Velazquez OC, Lederer HM, Rombeau JL. Butyrate and the colonocyte. Production, absorption, metabolism, and therapeutic implications. Adv Exp Med Biol. 1997;427:123–134. [PubMed] [Google Scholar]
- 23.Miki K, Unno N, Nagata T, Uchijima M, Konno H, Koide Y, Nakamura S. Butyrate suppresses hypoxia-inducible factor-1 activity in intestinal epithelial cells under hypoxic conditions. Shock. 2004;22:446–452. doi: 10.1097/01.shk.0000140664.80530.bd. [DOI] [PubMed] [Google Scholar]
- 24.Venkatraman A, Ramakrishna BS, Shaji RV, Kumar NS, Pulimood A, Patra S. Amelioration of dextran sulfate colitis by butyrate: role of heat shock protein 70 and NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2003;285:G177–184. doi: 10.1152/ajpgi.00307.2002. [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Peng L, Itzkowitz S, Holzman IR, Babyatsky MW. Short-chain fatty acid induces intestinal mucosal injury in newborn rats and down-regulates intestinal trefoil factor gene expression in vivo and in vitro. J Pediatr Gastroenterol Nutr. 2005;41:607–611. doi: 10.1097/01.mpg.0000179659.09210.ff. [DOI] [PubMed] [Google Scholar]


