Abstract
Background: Hedyotis diffusa is a well-known herb in traditional Chinese Medicine (TCM) which is used to treat various cancers including prostate cancer. Recently, lots of cyclotides possessing anti-cancer activities were found in Hedyotis family plants, suggesting that H.diffusa may also contain these bioactive ingredients. Cyclotides are heat-stable macrocyclic peptides from plants that display a wide range of biological activities. Currently, over 250 cyclotides have been discovered. Objective: This study tried to isolate novel cyclotides from H.diffusa and further investigate their anti-cancer activities for the prostate cancer cells in vitro and in vivo. Methods: The novel cyclotides from H.diffusa were isolated and purified by High-performance liquid chromatography (HPLC), amino acid sequences in their primary structure were confirmed using Edman degradation and gene cloning. Colorimetric cell viability assay (CCK8 assay), wound healing assay and human prostate cancer xenograft were used to analyze their anti-prostate cancer activity in vitro and in vivo. Results: Three novel cyclotides, termed as Diffusa cyclotide 1 to 3 (DC1-3) from the leaves and root of H.diffusa, were isolated firstly based on my knowledge. Using Edman degradation sequencing and gene cloning, we confirmed their amino acid sequence and obtained precursors of these peptides. By CCK8 assay, all present cyclotides showed potent cytotoxicity against all three prostate cancer cell lines, especially for DC3. In migration assay and wound healing assay, DC3 inhibited the cell migration and invasion Of LNCap cells. By model of prostate xenograft, DC3 could significantly inhibit development of the tumor in weight and size compared to the placebo. Conclusion: The novel cyclotides extracted from H.Diffusa have anti-cancer effects, and they are potential bioactive ingredients in H.diffusa.
Keywords: Cyclotides, H.diffusa, LNcap, prostate, migration and proliferation
Introduction
Cyclotides are plant-derived cyclic peptides containing 28 to 37 residues [1]. They can be considered as mini-proteins because of their well-defined 3D structures with an end-to-end circular peptide backbone cross-braced by three disulfide bonds in a cysteine-knot arrangement of cysteine I-IV, II-V, III-VI [1,2]. The absence of both termini and the presence of the knotted disulfide arrangement in a cyclic structure attributed to the extraordinary proteolytic, thermal or chemical stability of the cyclotides [3].
The discovery of the first cyclotide, kalata B1, from the Rubiaceae plant, Oldenlandia affinis, dated back in the early 1970s [4]. Kalata B1 produces an oxytocic activity and constitutes one of the active ingredients that cause the uterotonic decoction kalata-kalata to accelerate child delivery in the tribal women in Congo. Since then, over 250 cyclotide sequences [2]have been found in the Rubiaceae [5], Violaceae [6], Cucurbitaceae [7] and Fabaceae [8] families. Cyclotides likely played a role of host defense in plants and exhibited diverse biological activities such as anti-HIV [9], anti-cancer, antimicrobial [10], hemolysis [11], uterotensin [12], insecticidal [13] and nematicidal [14]. With respect to their anti-cancer effect, lots of studies have been done to investigate if cyclotides may produce cytotoxic and chemo-sensitization effects on various cancer cells [15-17].
Hedyotis diffusa is a Chinese herbal medicine widely used in combination with other herbal medicines such as Scutellaria barbata to treat various types of cancer [18]. Late-stage and recurrent cancer patients usually use H.diffusa during chemotherapy in expecting to achieve additive or synergistic therapeutic effects. Several classes of active ingredients, including anthraquinones, iridoid glucosides and stigmasterols have been isolated and characterized from H.diffusa [19,20]. Recently, some anti-cancer cyclotides have been isolated and confirmed from hedyotis biflora, which is quite homologous with H.diffusa [21]. Cyclotides have a well-known anti-cancer activity, which inspires us to investigate whether H.diffusa also have such active peptides.
Based on my knowledge, there is no related cyclotide report in H.diffusa so far. In the current study, we tried to find whether cyclotides existed in H.diffusa, and for further investigating their anti-cancer effects.
Materials and methods
Screening cyclotides in H.Diffusa
Previous procedure by Tam et al [22] and Qian [21] was conducted to screen and isolate the new cyclotides. Briefly, 500 mg plant materials of H.diffusa were macerated and extracted with 2 mL of 50% ethanol. The extracts were diluted five-fold and subjected to C18 solid phase extraction (SPE) columns. The SPE columns were washed with 20% acetonitrile (ACN) and eluted with 80% ACN. The eluted fractions were subjected to MALDI-TOF MS to scan for mass signals in the range between the 2-4 kDa. Plant extracts showing positive signal in the desired mass range were subjected to further S-reduction and S-alkylation to verify the number of disulfide.
Isolation and purification of cyclotides
Fresh aerial materials (5 kg) of H.diffusa were macerated with 5 L of 50% ethanol and partitioned with 2.5 L of dichloromethane for defatting. After removal of plant debris, the ethanol/water fraction was dried in vacuum and dissolved in 200 mL of 10% ethanol. The concentrated extract was subjected to flash column packed with 100 g of C18 media (Agilent, USA). The column was washed with 20% ACN and eluted with 80% ACN to obtain a cyclotide-enriched fraction containing main peptides. Isolation of individual peptides was then achieved by repetitive RP-HPLC using Agilent system.
S-reduction and Edman degradation
20 μg of each peptide was dissolved in 100 μL NH4HCO3 buffer (100 mM, pH 7.8) containing 10 mM dithiothreitol (DTT) and incubated for 1 hr at 37°C. Two-fold excess of iodoacetamide (IAA) over the total thiol was added and incubated for 1 hr at 37°C. S-alkylated peptides were purified by RP-HPLC. All the purified peptides were sent to Zhong Xiu Biotechnology Co. Ltd (Shanghai, China) for Edman degradation sequencing.
RNA cloning of cyclotides
Total RNA was extracted from fresh plant materials (leaves, flowers, and roots) using the PureLinkTM RNA purification system (Invitrogen). cDNA libraries were prepared from total RNA using the SmarterTM RACE cDNA amplification kit (Takara). We used DC1 as a sample to explain the cloning procedure. First, 3’ RACE PCR was conducted using degenerate primers designed against DC1 (5-GGIGYRTTYATHAARTGYGGIGA-3’ encoding GAFLKCGE). The PCR products were then cloned into pGEM-T Easy vector (Promega) and sent for sequencing. The 5’-end partial gene was obtained from the same procedure using the Universal Primer A Mix primer from the kit and a specific primer against the 3’-untranslated region of the newly found gene. The full transcript was then assembled from the two partial genes. This strategy was also applied to other diffusa cyclotides.
Cell culture
Human prostate cancer cell lines PC3, DU145 and LNCap were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). The cells were cultured in RPMI 1640 medium (Gibco Laboratories, Life Technologies Inc.; Grand Island, NY, USA) with 10% fetal bovine serum (FBS; Gibco, USA) at 37°C in a humidified 5% CO2 atmosphere.
Cytotoxicity assay
Various types of diffusa cyclotides were assessed for their cytotoxic activities observed in prostate cancer cells. Briefly, the cells were incubated in a 96 well flat-bottomed microtiter plate (2 × 103). After 12 h of culture, the cells were treated with cyclotides at a dose (μM) of 0.1, 0.5, 1, 4, 10 and 20, in 200 μl media for 72 h. Cell viability was evaluated with Cell Counting Kit-8 (Beyotime) according to the protocol of the manufacture. After treated with CCK8 at 37°C for 1 hour, three kinds of prostate cells were used to measure the absorbency at 450 nm using a microplate reader (MD, USA).
Wound healing motility assay
LNcap cells were grown in six-well tissue culture dishes until confluence. Cultures were incubated for 10 min with Moscona buffer. A scrape was made through the confluent monolayer with a plastic pipette tip of 1 mm diameter. Afterwards, the dishes were washed twice and incubated at 37°C in fresh RPMI containing 10% fetal bovine serum in the presence and absence of DC3 (0.01 and 0.05 μM). At the bottom side of each dish, two arbitrary places were marked where the width of the wound was measured with an inverted microscope (objective × 4) (Olympus 1X71, Japan). Motility was expressed as Mean ± SEM coming from the difference between time interval measurements (0, 12 h and 24 h).
Matrigel invasion assay
The cellular invasiveness of LNCap treated with DC3 (0.01 and 0.05 μM) was tested using BD Matrigel Invasion Chamber (8-μm pore size; BD Biosciences, Le Pont de Claix, France) according to the manufacturer’s protocol. Briefly, cells (1 × 105 cells in 0.5 mL media) and DC3 with indicated concentrations were incubated into the upper chambers of the system, the bottom wells in the system were filled with RPMI 1640 supplemented with 10% fetal bovine serum as a chemo-attractant and then incubated at 37°C for 24 h. Non-penetrating cells on the upper surface of the filter were removed using a cotton swab. The cells that had migrated through the Matrigel were fixed with 4% formaldehyde, stained with crystal violet and counted in 25 random fields under a microscope. The assay was carried out in duplicate and repeated three times for quantitative analysis.
Tumor xenografts
16 female Balb/c nude mice (5-6 weeks of age) weighing 17-21 g were used for the anti-cancer efficacy study. The mice were randomly divided into 2 groups: (a) Control group (n = 8) receiving xenograft tumors alone in 0.9% NaCl at 10 mL/kg; (b) DC3-treated group (n = 8) receiving xenograft tumors associated with intravenous administration of 1 mg/kg DC3. The mice were housed in a laminar air-flow cabinet under specific pathogen-free conditions and provided food and water ad libitum under a constant temperature (22 ± 2°C) and illuminated 7:00 a.m. to 7:00 p.m.
To establish LNCap xenograft tumor model in nude mice, the animals were intraperitoneally injected with 1 × 106 LNCap cells (in 0.2 mL medium) using a 23-gauge needle. Once the tumors became palpable (mean size of 0.1 mm3 at day 12) after the injection, the mice were treated with either 0.9% NaCl or 1 mg/kg DC3 per day for consecutive 14 days. Tumor size was measured every three days using a caliper and tumor volume was calculated using the formula: V = a × b2/2, where a and b were the largest and smallest perpendicular diameters, respectively. On day 14 after the treatment, the mice were sacrificed and the tumors were harvested for weight measurement. The inhibition rate of DC3 on the tumor growth was calculated by the following formula:
Inhibition Rate = [1 - Tumor Weight (treatment)/Tumor Weight (control)] × 100%.
Statistical analysis
All values were expressed as Mean ± SEM. The significance of the data was determined by one-way analysis of variance (ANOVA) followed by Student’s paired t test or Student-Newman-Keuls analysis. A p value was less than 0.05 was considered significant.
Results
Screening for novel cyclotides from H.diffusa
Fresh H.diffusa was collected from Tai Zhou city, Zhe Jiang province of China for the purpose to find novel cyclotides. Finally three peaks with molecular weights (Da) from 3000 to 3400 were found using mass spectrometry. They were confirmed as cyclotides by comparing the mass difference before and after S-alkylation with IAA. Each S-alkylated half-cystine residue caused a mass increase of 58 Da. All of them displayed a mass shift of 348 Da, indicating the presence of three cysteine bonds.
Isolation and sequence determination of DC1 to DC3
Three novel cyclotides with m/z of 3394, 3283, and 3157 Da were isolated from the H.diffusa by C18 reverse-phase HPLC. They were designated as Diffusa cyclotide 1 to 3 (abbreviated as DC1 to DC3). All of the cyclotides were sequenced by Edman degradation in support of Zhong Xiu biotech Ltd (Shang Hai, China).
The primary sequences of DC1 to DC3 were summarized in Table 1. Database information revealed that all peptides were novel and belonged to the cyclotide family.
Table 1.
Biological characteristics of three novel cyclotides from Hedyotis diffusa
| Hedyotide | Sequence | MW (Da) | Net Charge |
|---|---|---|---|
| DC1 | GAFLKCGESCVYLPCLTTVVGCSCQNSVCYRD | 3394 | 0 |
| DC2 | GAVPCGETCVYLPCITPDIGCSCQNKVCYRD | 3283 | -1 |
| DC3 | G-TSCGETCVLLPCLSSVLGCTCQNKRCYKD | 3157 | +1 |
Note: Molecular weight is expressed as the monoisotopic mass of the molecule. Cysteine residues are highlighted in yellow.
Encoding cDNAs of DC1 to DC3
To determine the encoding cDNAs of DC1 to DC3, degenerate primers based on the CGETC and CGESC sequences of loop 1 were used for 3’ RACE amplification. This resulted in the identification of three unique partial clones encoding for DC1 to DC3, respectively. Reverse specific primers based on the cDNA sequences of each clone were then used for 5’RACE amplification to obtain the remaining encoding sequences. Their full-length clones were designated as dcc1, dcc2 and ddc3 (diffusa cyclotide clone 1 to 3 corresponding to diffusa cyclotde 1 to 3, respectively). Overall, the three clone has a similar arrangement to the oak1 and hcf-1 clones from O. affinis [13] and H. centranthoides [5], respectively. Each contains an ER signal sequence, an N-terminal propeptide, a cyclotide domain and a hydrophobic tail (Figure 1).
Figure 1.
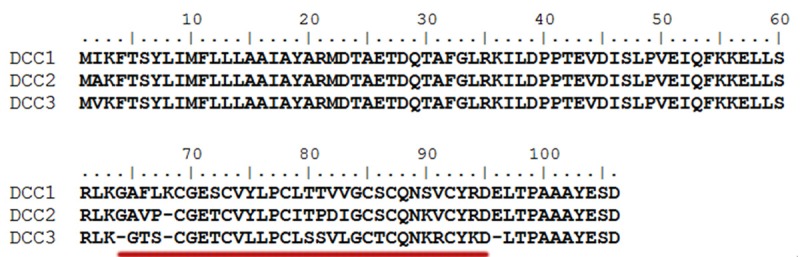
Peptide sequence of DC1 to DC3 precursors. Their primary sequences were deduced from cDNA clones and aligned using Bioedit software. The mature cyclotide domains were underlined with red color.
Cytotoxicity of hedyotide
All three cyclotides were tested for their cytotoxicity against the three prostate cancer cells. As shown in Table 2, all of them demonstrated potent cytotoxicity and DC3 had a lowest IC50 value.
Table 2.
Sensitivities of the human prostate cancer cells to diffusa cyclotides
| IC50 (μM) | |||
|---|---|---|---|
|
|
|||
| LNcap | PC3 | DU145 | |
| DC1 | 5.03 | 2.24 | 3.32 |
| DC2 | 4.31 | 2.65 | 3.11 |
| DC3 | 0.21 | 0.76 | 0.55 |
Effects of DC3 on invasive and migratory abilities of LNcap cells
To study the effect of diffusa cyclotide on cell invasion and motility which were the characteristics of cancer metastasis, we selected DC3, the most cytotoxic one, to test its effect on the LNcap cells. In an invasion assay, we calculated the number of cells migrated to the bottom side of the membrane on a chamber where the cells were seeded. At a concentration of 0.01 μM DC3, it did not show any significant effect on invasion ability. However, at a concentration of 0.05 μM, DC significantly suppressed the invasion ability of LNcap cells (average of 503 and 161 cells per viewed field in untreated and DC3 group, respectively. P < 0.05) (Figure 2A).
Figure 2.
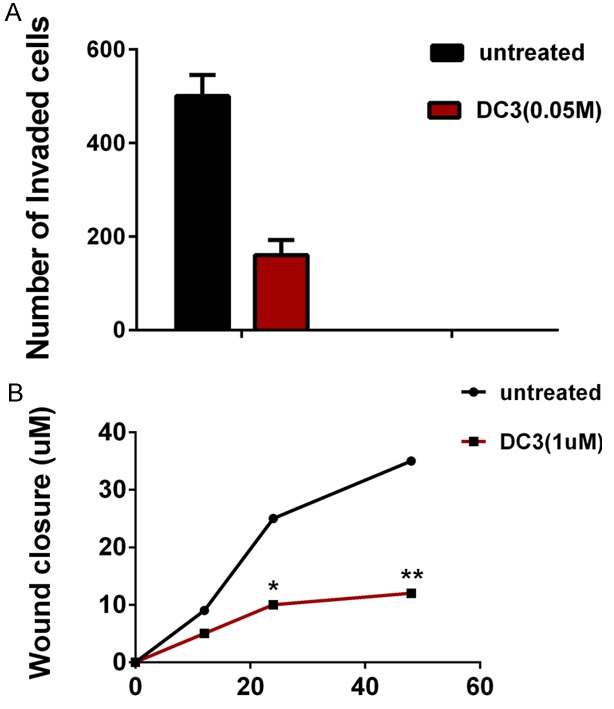
DC3 (0.05 μM) inhibits prostate cancer cell invasion and migration in vitro. A. Cells invaded into Matrigel were counted as described in Materials and Methods. B. The distance from the wound edge was measured in three independent wound sites per group; Cell motility tests were quantitated as described in “Materials and methods”. The graph was expressed as MEAN + SEM. *, P < 0.05, **, P < 0.01 vs control.
To examine whether DC3 had any effect on the migratory ability of the LNcap cells, we performed a wound-healing experiment. Similar to the invasion assay, the data showed that DC3 could significantly inhibit the migration of LNcap cells in both 12, 24 h and 48 h at the concentration of 0.05 but not 0.01 μM, the average of distance from the wound edge were 9.1, 24.9, 35.3 and 5.2, 9.9, 12.2 in DC3 and untreated group at 12, 24 h and 48 h respectively (Figure 2B).
Inhibition of LNcap proliferation by DC3 in xenograft
The effects of DC3 on the LNcap xenograft model are shown in Figure 3. Tumor weight of capan2 xenografts inoculated subcutaneously in nude mice and treated with DC3 (1 mg/kg) or control carrier solution were average of 0.25 and 0.36 g respectively. At a dose of 1 mg/kg, DC3 showed significant anti-cancer effect (P < 0.01), and inhibition of rate of tumor growth (weight) was 40.23% (Figure 3A).
Figure 3.
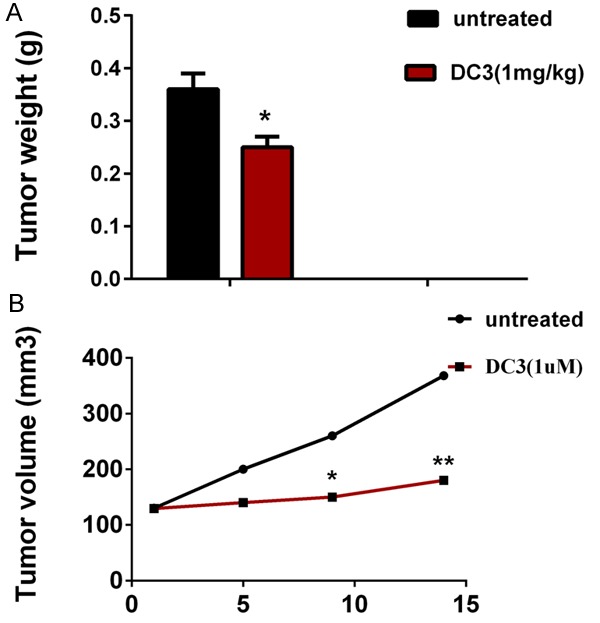
DC3 induces regression of established LNcap xenografts. A. Tumor weight of capan2 xenografts inoculated subcutaneously in nude mice and treated with DC3 (1 mg/kg) or control carrier solution, alone, for a total of 14 days. B. Tumor volume obtained from the same control and treated nude mice. Data points represent the mean ± SEM of 8 mice per group. *Significantly different at P < 0.05, and **represents P < 0.01.
By comparison with the control group, the effect of DC3 on tumor volume exhibited a significant difference at a dose of 1 mg/kg with a value of 1.39 of RTV (relative tumor volume) while control has a 2.82 RTV (Figure 3B) (Tumor volume obtained were 129.5, 141.1, 152.0, 179.9 and 130.3, 200.4, 261.1, 367.9 mm3 respectively at 1, 5, 9, 14 day respectively, P < 0.01).
Discussion
It has been reported that H.diffusa is one of the most popular traditional herbal medicines in China [23]. It is noteworthy that H.diffusa has various pharmacological activities such as anti-cancer [24], anti-inflammatory, hepatoprotective [25], and neuroprotective activities [26]. H.diffusa is used as a therapy to treat hepatoma, lung cancer, colon cancer, pancreatic cancer, and leukemia [27]. As a well-known herb, its effect has been confirmed in clinical practice. In the past few years, lots of research has been done to identify the bioactive ingredients such as 2-hydroxy-3-methylanthraquinoneand 1-methoxy-2-hydroxyanthraquinone, which have been proved to be of inhibitory activity for protein tyrosine kinases v-src and pp60src and arrested the growth of SPC-1-A, Bcap37 and HepG2 cancer cells [28]. Recently, a series of cyclotides have been found in the Hedyotis family plants, which encourage us to investigate whether the H.diffusa also contains these bioactive ingredients.
In this study, three novel cyclotides from H.diffusa were isolated. By searching database, we confirmed that their sequences were never published. The encoding genes of DC1 to DC3 were subsequently cloned, revealing biosynthesis and processing of cyclotides in plants.
A previous study has demonstrated that most cyclotides had potent cytotoxic activity [29]. In the present study, all of the three cyclotides from H.diffusa showed potent cytotoxic activity against three prostate cancer cell lines with their IC50 values shown at the level below 10 μM, especially for DC3 whose IC50 value was lower than 1 μM. We speculated that this was due to a change in its net charge status since the bioactivities displayed by cyclotides were correlated with their net charge status, whereas cyclotides containing positive charges in their net charges had the more potent cytotoxicity [30]. The underlying mechanism could be considered that the positively charged cyclotides exerted their cytotoxicity by binding to the outer membrane of cancer cells presenting 3%-9% anionic phosphatidylserine, consequently disrupted the integrity of cell membrane [31].
Few studies reported the effect of cyclotides on invasion and migration of the cancer cells, although they have been considered as anti-cancer agents. In the present study, DC3 was tested for its effects on invasion and migration of the LNcap cells. The results at non-toxic concentration (0.05 uM) of DC3 showed that it had significantly inhibitory effects on the abilities of both invasion and migration of the LNcap cells, raising such a possibility that its cancer-targeting ability should be further investigated.
Finally, we established a mouse xenograft model of human prostate cancer (LNcap), in which anti-cancer activity of DC3 was studied in vivo. Although previous studies did not report significant anti-cancer activity of the plant cyclotides in vivo [16], in this study DC3 (1 mg/kg) showed a 40% inhibition in the LNcap-based model, suggesting its potential in cancer therapy.
In conclusion, in our study, three novel cyclotides were obtained from H.diffusa and they exhibited potent anticancer bioactivity in the mouse xenograft model of human prostate cancer.
Disclosure of conflict of interest
None.
References
- 1.Craik DJ, Daly NL, Bond T, Waine C. Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J Mol Biol. 1999;294:1327–1336. doi: 10.1006/jmbi.1999.3383. [DOI] [PubMed] [Google Scholar]
- 2.Sze SK, Wang W, Meng W, Yuan R, Guo T, Zhu Y, Tam JP. Elucidating the structure of cyclotides by partial acid hydrolysis and LC-MS/MS analysis. Anal Chem. 2009;81:1079–1088. doi: 10.1021/ac802175r. [DOI] [PubMed] [Google Scholar]
- 3.Colgrave ML, Craik DJ. Thermal, chemical, and enzymatic stability of the cyclotide kalata B1: the importance of the cyclic cystine knot. Biochemistry. 2004;43:5965–5975. doi: 10.1021/bi049711q. [DOI] [PubMed] [Google Scholar]
- 4.Grain L. Isolation of oxytocic peptides from Oldenlandia affinis by solvent extraction of tetraphenylborate complexes and chromatography on sephadex LH-20. Lloydia. 1973;36:207–208. [PubMed] [Google Scholar]
- 5.Gruber CW, Elliott AG, Ireland DC, Delprete PG, Dessein S, Goransson U, Trabi M, Wang CK, Kinghorn AB, Robbrecht E, Craik DJ. Distribution and evolution of circular miniproteins in flowering plants. Plant Cell. 2008;20:2471–2483. doi: 10.1105/tpc.108.062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trabi M, Svangard E, Herrmann A, Goransson U, Claeson P, Craik DJ, Bohlin L. Variations in cyclotide expression in viola species. J Nat Prod. 2004;67:806–810. doi: 10.1021/np034068e. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez JF, Gagnon J, Chiche L, Nguyen TM, Andrieu JP, Heitz A, Trinh Hong T, Pham TT, Le Nguyen D. Squash trypsin inhibitors from Momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry. 2000;39:5722–5730. doi: 10.1021/bi9929756. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen GK, Zhang S, Nguyen NT, Nguyen PQ, Chiu MS, Hardjojo A, Tam JP. Discovery and characterization of novel cyclotides originated from chimeric precursors consisting of albumin-1 chain a and cyclotide domains in the Fabaceae family. J Biol Chem. 2011;286:24275–24287. doi: 10.1074/jbc.M111.229922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gustafson KR, McKee TC, Bokesch HR. Anti-HIV cyclotides. Curr Protein Pept Sci. 2004;5:331–340. doi: 10.2174/1389203043379468. [DOI] [PubMed] [Google Scholar]
- 10.Tam JP, Lu YA, Yang JL, Chiu KW. An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystine-knot disulfides. Proc Natl Acad Sci U S A. 1999;96:8913–8918. doi: 10.1073/pnas.96.16.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goransson U, Luijendijk T, Johansson S, Bohlin L, Claeson P. Seven novel macrocyclic polypeptides from Viola arvensis. J Nat Prod. 1999;62:283–286. doi: 10.1021/np9803878. [DOI] [PubMed] [Google Scholar]
- 12.Gran L, Sandberg F, Sletten K. Oldenlandia affinis (R&S) DC. A plant containing uteroactive peptides used in African traditional medicine. J Ethnopharmacol. 2000;70:197–203. doi: 10.1016/s0378-8741(99)00175-0. [DOI] [PubMed] [Google Scholar]
- 13.Jennings C, West J, Waine C, Craik D, Anderson M. Biosynthesis and insecticidal properties of plant cyclotides: the cyclic knotted proteins from Oldenlandia affinis. Proc Natl Acad Sci U S A. 2001;98:10614–10619. doi: 10.1073/pnas.191366898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colgrave ML, Kotze AC, Huang YH, O’Grady J, Simonsen SM, Craik DJ. Cyclotides: natural, circular plant peptides that possess significant activity against gastrointestinal nematode parasites of sheep. Biochemistry. 2008;47:5581–5589. doi: 10.1021/bi800223y. [DOI] [PubMed] [Google Scholar]
- 15.Gerlach SL, Rathinakumar R, Chakravarty G, Goransson U, Wimley WC, Darwin SP, Mondal D. Anticancer and chemosensitizing abilities of cycloviolacin 02 from Viola odorata and psyle cyclotides from Psychotria leptothyrsa. Biopolymers. 2010;94:617–625. doi: 10.1002/bip.21435. [DOI] [PubMed] [Google Scholar]
- 16.Burman R, Svedlund E, Felth J, Hassan S, Herrmann A, Clark RJ, Craik DJ, Bohlin L, Claeson P, Goransson U, Gullbo J. Evaluation of toxicity and antitumor activity of cycloviolacin O2 in mice. Biopolymers. 2010;94:626–634. doi: 10.1002/bip.21408. [DOI] [PubMed] [Google Scholar]
- 17.He W, Chan LY, Zeng G, Daly NL, Craik DJ, Tan N. Isolation and characterization of cytotoxic cyclotides from Viola philippica. Peptides. 2011;32:1719–1723. doi: 10.1016/j.peptides.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Khandekar JD, Beck HH 3rd. Delayed recurrences in primary fallopian tube carcinoma. South Med J. 1993;86:1314–1315. doi: 10.1097/00007611-199311000-00033. [DOI] [PubMed] [Google Scholar]
- 19.Ayhan A, Deren O, Yuce K, Tuncer Z, Mocan G. Primary carcinoma of the fallopian tube: a study of 8 cases. Eur J Gynaecol Oncol. 1994;15:147–151. [PubMed] [Google Scholar]
- 20.Rosen AC, Graf AH, Hacker GW, Klein M. Prognostic impact of DNA content and a classification system for ploidy (AUER classification) in primary fallopian tube carcinoma (FTC) Eur J Cancer. 1994;30A:1907–1908. doi: 10.1016/0959-8049(94)00203-h. [DOI] [PubMed] [Google Scholar]
- 21.Ding X, Bai D, Qian J. Novel cyclotides from Hedyotis biflora inhibit proliferation and migration of pancreatic cancer cell in vitro and in vivo. Medicinal Chemistry Research. 2014;23:1406–1413. [Google Scholar]
- 22.Nguyen GK, Zhang S, Wang W, Wong CT, Nguyen NT, Tam JP. Discovery of a linear cyclotide from the bracelet subfamily and its disulfide mapping by top-down mass spectrometry. J Biol Chem. 2011;286:44833–44844. doi: 10.1074/jbc.M111.290296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin CC, Kuo CL, Lee MH, Hsu SC, Huang AC, Tang NY, Lin JP, Yang JS, Lu CC, Chiang JH, Chueh FS, Chung JG. Extract of Hedyotis diffusa Willd influences murine leukemia WEHI-3 cells in vivo as well as promoting T- and B-cell proliferation in leukemic mice. In Vivo. 2011;25:633–640. [PubMed] [Google Scholar]
- 24.Chen XH, Gao RL, Qian XD, Wang X, Tan PL, Yin LM, Zhou YH. [Inhibition effect of hedyotis diffusa wild injection on HL-60 cells and its mechanism] . Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16:1035–1038. [PubMed] [Google Scholar]
- 25.Lin CC, Ng LT, Yang JJ, Hsu YF. Anti-inflammatory and hepatoprotective activity of peh-hue-juwa-chi-cao in male rats. Am J Chin Med. 2002;30:225–234. doi: 10.1142/S0192415X02000405. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Park EJ, Kim J, Kim Y, Kim SR, Kim YY. Neuroprotective constituents from Hedyotis diffusa. J Nat Prod. 2001;64:75–78. doi: 10.1021/np000327d. [DOI] [PubMed] [Google Scholar]
- 27.Kuo YJ, Yang JS, Lu CC, Chiang SY, Lin JG, Chung JG. Ethanol extract of Hedyotis diffusa willd upregulates G0/G1 phase arrest and induces apoptosis in human leukemia cells by modulating caspase cascade signaling and altering associated genes expression was assayed by cDNA microarray. Environ Toxicol. 2014 doi: 10.1002/tox.21989. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Shi Y, Wang CH, Gong XG. Apoptosis-inducing effects of two anthraquinones from Hedyotis diffusa WILLD. Biol Pharm Bull. 2008;31:1075–1078. doi: 10.1248/bpb.31.1075. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqi SA, Kamal AK. My patient’s brain MRI has cerebral microbleeds--what does this finding mean? J Pak Med Assoc. 2011;61:1029–1030. [PubMed] [Google Scholar]
- 30.Craik DJ. Host-defense activities of cyclotides. Toxins (Basel) 2012;4:139–156. doi: 10.3390/toxins4020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Yang H, Wan L, Cai HW, Li SF, Li YP, Cheng JQ, Lu XF. Enhancement of cytotoxicity of antimicrobial peptide magainin II in tumor cells by bombesin-targeted delivery. Acta Pharmacol Sin. 2011;32:79–88. doi: 10.1038/aps.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]


