Abstract
Background: The association between the peroxisome proliferator-activated receptor-γ (PPARγ) Pro12Ala polymorphism and colorectal cancer (CRC) risk was inconclusive. We conducted a meta-analysis to evaluate the association between PPARγ Pro12Ala polymorphism and CRC risk. Material and Method: We searched Pubmed, EMBASE, and China National Knowledge Infrastructure databases. Data were extracted and pooled odds ratios (OR) with 95% confidence intervals (CI) were calculated. Results: A total of 17 case-control studies with 12635 and 15803 controls were included in this meta-analysis. Overall, PPARγ Pro12Ala polymorphism was associated with CRC risk (OR = 0.84, 95% CI 0.75-0.94, P = 0.003, I2 = 35%). In the subgroup analysis by ethnicity, a significant association was found among Caucasians (OR = 0.85, 95% CI 0.75-0.96, P = 0.007, I2 = 38%) but not among Asians (OR = 0.76, 95% CI 0.51-1.12, P = 0.17, I2 = 28%). In the subgroup analysis by CRC site, a significant association was found among colon cancer (OR = 0.81, 95% CI 0.66-0.98, P = 0.03, I2 = 16%) but not among rectal cancer (OR = 0.83, 95% CI 0.57-1.21, P = 0.34, I2 = 63%). The sensitivity analysis did not influence the result by omitting low-quality studies (OR = 0.76, 95% CI 0.63-0.93, P = 0.006, I2 = 51%). Conclusions: In conclusion, this meta-analysis suggested that PPARγ Pro12Ala polymorphism was significant associated with CRC risk.
Keywords: Colorectal cancer, peroxisome proliferator-activated receptor, meta-analysis, polymorphism
Introduction
Colorectal cancer (CRC) was diagnosed in 1.2 million persons worldwide in 2008, and it accounted for close to 10% of all cancers. Risk factors for CRC include advanced age, medical history of benign adenomatous polyps and inflammatory bowel diseases, family history of CRC, low intake of vegetables and fruits and high intake of dietary fat (particularly animal fat) and processed meat [1,2]. Several lines of evidence indicate that inherited genetic factors influence the development and progression of CRC [3].
Peroxisome proliferator-activated receptor-γ (PPARγ) is part of a family of transcription factors. The PPARγ gene is expressed in many tissues, including high levels of expression in normal colonic mucosa, colorectal adenocarcinomas, and colon cancer cell lines [4,5]. PPARγ activation also may provide a molecular link between a high-fat diet and increased risk of CRC, since studies in mice have shown that mice treated with a PPARγ ligand had greater number of polyps in the colon [6]. A relatively common variant of the PPARγ gene (substitution of Ala for Pro at codon 12) has been found, which was associated with improved insulin sensitivity, smaller body size, and reduced risk of type-2 diabetes [7]. Several studies investigated the association between PPARγ Pro12Ala polymorphism and CRC risk. However, the results remained inconclusive [8-24]. Meta-analysis is an useful method for investigating associations between genetic factors and diseases, because a quantitative approach is used to combine the results from different studies on the same topic, thereby providing more reliable conclusions. Thus, we performed a meta-analysis to assess the association of PPARγ Pro12Ala polymorphism with CRC risk.
Methods
Publication search
In this meta-analysis, we searched the articles using the search terms “Colorectal cancer”, “Peroxisome proliferator-activated receptor-γ” and “polymorphism” in the PubMed, EMBASE and Chinese National Knowledge Infrastructure (CNKI) databases, and the last search updated on October 2014. Additional studies were identified by a hand search of references of original studies or review articles on the association between PPARγ Pro12Ala polymorphism with CRC risk. No publication date or language restriction were imposed.
Inclusion and exclusion criteria
The following inclusion criteria were used: (1) evaluation of the PPARγ Pro12Ala polymorphism with CRC risk, (2) using a case-control or cohort design, and (3) genotype distributions in both cases and controls should be available for estimating an odds ratio (OR) with 95% confidence interval (CI).
Studies were excluded if one of the following existed: (1) not relevant to CRC or PPARγ polymorphism, (2) not designed as case-control or cohort design studies, (3) genotype frequencies or number not offered, (4) animal studies, and (5) editorials, reviews and abstracts. If more than one study used the same cases, the one with the most comprehensive population were included.
Data extraction and quality assessment
The following data were collected from each study: first author’s surname, year of publication, ethnicity, CRC location, sample size, and source of controls. To assess the quality of the included studies, the Newcastle-Ottawa Scale was adopted. The studies were judged by 8 items of 3 aspects. The highest quality studies were awarded a maximum of one star of each item, except that the item of comparability allowed a maximum of two stars. Studies that controlled for age received one star, whereas studies that controlled for other important factors received an additional star. The Newcastle-Ottawa Scale score ranged from zero up to nine stars. And the high-quality study was defined ≥ 7 stars.
Statistical analysis
The strength of the associations between the PPARγ Pro12Ala polymorphism and CRC risk was measured by ORs and 95% CIs. The random-effects model was used. The statistical significance of summary OR was determined with Z test. The Q statistic and the I2 statistic were used to assess the degree of heterogeneity among the studies included in the meta-analysis. The source of heterogeneity was detected by using Galbraith plot. Subgroup analyses were carried out by ethnicity and CRC location. Sensitivity analysis was performed by excluding low-quality studies. The potential publication bias was examined visually in a funnel plot of log [OR] against its standard error (SE), and the degree of asymmetry was tested using Egger’s test [25]. All statistical tests were performed using STATA 11.0 software (Stata Corporation, College Station, TX, USA) and Reviewer Manager 5.1. A P value < 0.05 was considered statistically significant.
Results
Study characteristics
A total of 17 case-control studies with 12635 and 15803 controls on the association between PPARγ Pro12Ala polymorphism and CRC risk were included for this meta-analysis. There were 3 studies of Asians and 14 studies of Caucasians. The characteristics of each case-control study are listed in Table 1. Quality scores of each study were summarized in Table 2. The study scores ranged from 5 to 9 stars.
Table 1.
Main characteristics of selected studies
| Author | Year | Ethnicity | CRC location | Cases | Controls | Source of controls |
|---|---|---|---|---|---|---|
| Landi | 2003 | Caucasian | Rectal, colon | 377 | 326 | Population-based |
| Gong | 2005 | Caucasian | Rectal, colon | 163 | 212 | Hospital-based |
| Jiang | 2005 | Asian | Rectal, colon | 301 | 291 | Hospital-based |
| McGreavey | 2005 | Caucasian | NR | 484 | 738 | Hospital-based |
| Murtaugh | 2005 | Caucasian | Rectal, colon | 2371 | 2972 | Population-based |
| Siezen | 2005 | Caucasian | NR | 384 | 403 | Population-based |
| Gunter | 2006 | Caucasian | NR | 244 | 231 | Hospital-based |
| Koh | 2006 | Asian | Rectal, colon | 362 | 1164 | Population-based |
| Kuriki | 2006 | Asian | NR | 128 | 238 | Hospital-based |
| Theodoropoulos | 2006 | Caucasian | NR | 222 | 200 | Hospital-based |
| Vogel | 2007 | Caucasian | NR | 355 | 753 | Population-based |
| Küry | 2008 | Caucasian | NR | 1023 | 1121 | Population-based |
| Slattery | 2009 | Caucasian | NR | 1839 | 2014 | Population-based |
| Hawken | 2010 | Caucasian | NR | 1257 | 1336 | Population-based |
| Abulí | 2011 | Caucasian | NR | 515 | 515 | Hospital-based |
| Crous-Bou | 2012 | Caucasian | NR | 812 | 1479 | Hospital-based |
| Sainz | 2012 | Caucasian | NR | 1798 | 1810 | Population-based |
Table 2.
Methodological quality of the included studies
| Selection | Comparability | Exposure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Author | Year | Adequate definition of cases | Representativeness of cases | Selection of controls | Definition of controls | Control for important factors | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non- response rate | Total stars |
| Landi | 2003 | ☆ | — | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 |
| Gong | 2005 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Jiang | 2005 | — | — | ☆ | ☆ | ☆☆ | ☆ | ☆ | — | 5 |
| McGreavey | 2005 | ☆ | ☆ | — | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 |
| Murtaugh | 2005 | ☆ | — | ☆ | ☆ | — | — | ☆ | ☆ | 5 |
| Siezen | 2005 | ☆ | ☆ | ☆ | ☆☆ | ☆ | — | ☆ | 7 | |
| Gunter | 2006 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Koh | 2006 | — | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 |
| Kuriki | 2006 | ☆ | ☆ | — | ☆ | ☆☆ | ☆ | — | ☆ | 6 |
| Theodoropoulos | 2006 | ☆ | ☆ | ☆ | ☆ | — | ☆ | ☆ | ☆ | 7 |
| Vogel | 2007 | ☆ | — | ☆ | ☆ | ☆☆ | ☆ | ☆ | — | 7 |
| Küry | 2008 | ☆ | ☆ | — | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 |
| Slattery | 2009 | ☆ | ☆ | ☆ | — | ☆ | — | ☆ | 5 | |
| Hawken | 2010 | ☆ | ☆ | — | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 |
| Abulí | 2011 | — | — | ☆ | ☆ | ☆☆ | — | ☆ | — | 5 |
| Crous-Bou | 2012 | ☆ | ☆ | — | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 |
| Sainz | 2012 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
Overall and subgroup meta-analysis results
The result suggested that PPARγ Pro12Ala polymorphism was associated with CRC risk (OR = 0.84, 95% CI 0.75-0.94, P = 0.003, I2 = 35%, Figure 1). In the subgroup analysis by ethnicity, a significant association was found among Caucasians (OR = 0.85, 95% CI 0.75-0.96, P = 0.007, I2 = 38%) but not among Asians (OR = 0.76, 95% CI 0.51-1.12, P = 0.17, I2 = 28%). In the subgroup analysis by CRC site, a significant association was found among colon cancer (OR = 0.81, 95% CI 0.66-0.98, P = 0.03, I2 = 16%) but not among rectal cancer (OR = 0.83, 95% CI 0.57-1.21, P = 0.34, I2 = 63%). The sensitivity analysis did not influence the result by omitting low-quality studies (OR = 0.76, 95% CI 0.63-0.93, P = 0.006, I2 = 51%). The Galbraith plot was used to find the source of the heterogeneity. As shown in Figure 2, two studies were the outliers. After excluding these studies, the between-study heterogeneity effectively decreased and there was no obvious heterogeneity among the remaining studies (I2 = 0%, P = 0.65). Besides, the result was still statistically significant (OR = 0.92, 95% CI 0.86-0.99, P = 0.04).
Figure 1.
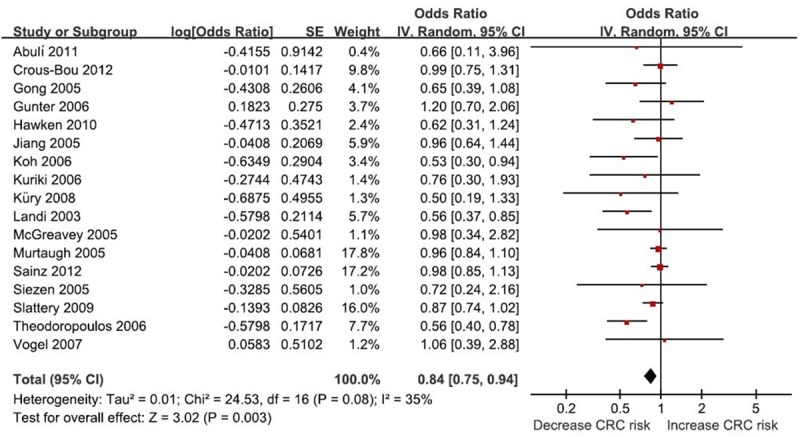
Meta-analysis of the association between between PPARγ Pro12Ala polymorphism and CRC risk.
Figure 2.
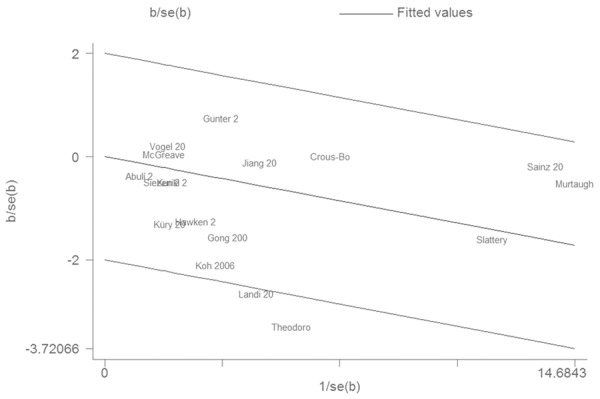
Galbraith plot of associations between PPARγ Pro12Ala polymorphism and CRC risk.
Funnel plot and Egger’s test were both performed to access the publication bias of this meta-analysis. The shape of the funnel plot seemed symmetrical (Figure 3). Egger’s test showed no evidence of publication bias (P = 0.110).
Figure 3.
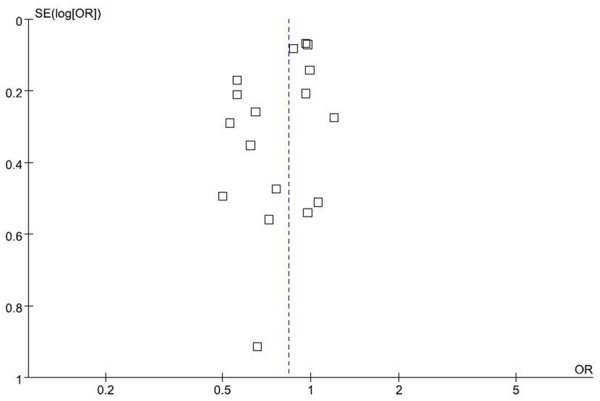
Funnel plot for associations between PPARγ Pro12Ala polymorphism and CRC risk.
Discussion
The main finding of this meta-analysis was that PPARγ Pro12Ala polymorphism was a potential protective factor for developing CRC In the subgroup analysis of ethnicity, no significant association was found in Asians, while a significant association was found in Caucasians. It was possible that different lifestyles, diets, and environments may account for this apparent discrepancy. These issues should be investigated in the future studies. Only three studies with Asians were included in this meta-analysis. Thus, more studies with Asians should be conducted to determine the association between PPARγ Pro12Ala polymorphism and risk of CRC. In the subgroup analysis by CRC site, we found that there was a significant association between PPARγ Pro12Ala polymorphism and risk of colon cancer, suggesting that PPARγ Pro12Ala polymorphism might influence the etiology of colon cancer.
Laboratory studies have indicated a complex role of PPARγ at the cellular level through regulation of cell growth, differentiation, and apoptosis [26]. A persistent state of inflammation might lead to colon cancer, as exemplified by the high risk of colon cancer associated with ulcerative colitis [27]. Chemically induced colonic inflammation and aberrant crypt foci have been diminished in animal models by administration of PPARγ ligands [28]. The Pro12Ala polymorphism of PPARγ gene has been associated with altered lipid profiles, lower fasting insulin concentrations, improved insulin sensitivity and a reduced risk of type II diabetes and the metabolic syndrome [29]. This amino acid was located in the PPARγ domain that enhanced ligand-independent activation [30]. The Pro to Ala change may cause a conformational change in the protein, thus affecting its activity and CRC risk.
Some limitations should be acknowledged. First, only published studies that were included in the selected electronic databases were identified. It was possible that some relevant published or unpublished studies may have been missed. Second, the effects of gene-gene and gene-environment interactions were not addressed in this meta-analysis, because of limited available data. Third, there was moderate heterogeneity in this meta-analysis. However, when the main source of heterogeneity was excluded, the result was still statistically significant.
In conclusion, this meta-analysis found significant associations between PPARγ Pro12Ala polymorphism and CRC risk.
Acknowledgements
This work was supported by Binzhou Medical University college project (No. BY2013KJ05) and Planning project of science and technology in Colleges and universities in Shandong Province (No. J12LE58).
References
- 1.van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, Jenab M, Boshuizen HC, Ros MM, Casagrande C, Tjønneland A, Olsen A, Overvad K, Thorlacius-Ussing O, Clavel-Chapelon F, Boutron-Ruault MC, Morois S, Kaaks R, Linseisen J, Boeing H, Nöthlings U, Trichopoulou A, Trichopoulos D, Misirli G, Palli D, Sieri S, Panico S, Tumino R, Vineis P, Peeters PH, van Gils CH, Ocké MC, Lund E, Engeset D, Skeie G, Suárez LR, González CA, Sánchez MJ, Dorronsoro M, Navarro C, Barricarte A, Berglund G, Manjer J, Hallmans G, Palmqvist R, Bingham SA, Khaw KT, Key TJ, Allen NE, Boffetta P, Slimani N, Rinaldi S, Gallo V, Norat T, Riboli E. Fruit, vegetables, and colorectal cancer risk: The European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2009;89:1441–52. doi: 10.3945/ajcn.2008.27120. [DOI] [PubMed] [Google Scholar]
- 2.Gerber M. Background review paper on total fat, fatty acid intake and cancers. Ann Nutr Metab. 2009;55:140–61. doi: 10.1159/000229000. [DOI] [PubMed] [Google Scholar]
- 3.Valle L. Genetic predisposition to colorectal cancer: where we stand and future perspectives. World J Gastroenterol. 2014;20:9828–49. doi: 10.3748/wjg.v20.i29.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fajas L, Auboeuf D, Raspé E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272:18779–89. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 5.Lefebvre M, Paulweber B, Fajas L, Woods J, McCrary C, Colombel JF, Najib J, Fruchart JC, Datz C, Vidal H, Desreumaux P, Auwerx J. Peroxisome proliferator-activated receptor gamma is induced during differentiation of colon epithelium cells. J Endocrinol. 1999;162:331–40. doi: 10.1677/joe.0.1620331. [DOI] [PubMed] [Google Scholar]
- 6.Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM, Thomazy VA, Evans RM. Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat Med. 1998;4:1058–61. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 7.Deeb SS, Fajas L, Nemoto M, Pihlajamäki J, Mykkänen L, Kuusisto J, Laakso M, Fujimoto W, Auwerx J. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20:284–7. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- 8.Landi S, Moreno V, Gioia-Patricola L, Guino E, Navarro M, de Oca J, Capella G, Canzian F Bellvitge Colorectal Cancer Study Group. Association of common polymorphisms in inflammatory genes interleukin (IL) 6, IL8, tumor necrosis factor alpha, NFKB1, and peroxisome proliferator-activated receptor gamma with colorectal cancer. Cancer Res. 2003;63:3560–6. [PubMed] [Google Scholar]
- 9.Gong Z, Xie D, Deng Z, Bostick RM, Muga SJ, Hurley TG, Hebert JR. The PPAR{gamma} Pro12Ala polymorphism and risk for incident sporadic colorectal adenomas. Carcinogenesis. 2005;26:579–85. doi: 10.1093/carcin/bgh343. [DOI] [PubMed] [Google Scholar]
- 10.Jiang J, Gajalakshmi V, Wang J, Kuriki K, Suzuki S, Nakamura S, Akasaka S, Ishikawa H, Tokudome S. Influence of the C161T but not Pro12Ala polymorphism in the peroxisome proliferator-activated receptor-gamma on colorectal cancer in an Indian population. Cancer Sci. 2005;96:507–12. doi: 10.1111/j.1349-7006.2005.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGreavey LE, Turner F, Smith G, Boylan K, Timothy Bishop D, Forman D, Roland Wolf C, Barrett JH Colorectal Cancer Study Group. No evidence that polymorphisms in CYP2C8, CYP2C9, UGT1A6, PPARdelta and PPARgamma act as modifiers of the protective effect of regular NSAID use on the risk of colorectal carcinoma. Pharmacogenet Genomics. 2005;15:713–21. doi: 10.1097/01.fpc.0000174786.85238.63. [DOI] [PubMed] [Google Scholar]
- 12.Murtaugh MA, Ma KN, Caan BJ, Sweeney C, Wolff R, Samowitz WS, Potter JD, Slattery ML. Interactions of peroxisome proliferator-activated receptor {gamma} and diet in etiology of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1224–9. doi: 10.1158/1055-9965.EPI-04-0681. [DOI] [PubMed] [Google Scholar]
- 13.Siezen CL, van Leeuwen AI, Kram NR, Luken ME, van Kranen HJ, Kampman E. Colorectal adenoma risk is modified by the interplay between polymorphisms in arachidonic acid pathway genes and fish consumption. Carcinogenesis. 2005;26:449–57. doi: 10.1093/carcin/bgh336. [DOI] [PubMed] [Google Scholar]
- 14.Gunter MJ, Canzian F, Landi S, Chanock SJ, Sinha R, Rothman N. Inflammation-related gene polymorphisms and colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1126–31. doi: 10.1158/1055-9965.EPI-06-0042. [DOI] [PubMed] [Google Scholar]
- 15.Koh WP, Yuan JM, Van Den Berg D, Ingles SA, Yu MC. Peroxisome proliferator-activated receptor (PPAR) gamma gene polymorphisms and colorectal cancer risk among Chinese in Singapore. Carcinogenesis. 2006;27:1797–802. doi: 10.1093/carcin/bgl001. [DOI] [PubMed] [Google Scholar]
- 16.Kuriki K, Hirose K, Matsuo K, Wakai K, Ito H, Kanemitsu Y, Hirai T, Kato T, Hamajima N, Takezaki T, Suzuki T, Saito T, Tanaka R, Tajima K. Meat, milk, saturated fatty acids, the Pro12Ala and C161T polymorphisms of the PPAR gamma gene and colorectal cancer risk in Japanese. Cancer Sci. 2006;97:1226–35. doi: 10.1111/j.1349-7006.2006.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theodoropoulos G, Papaconstantinou I, Felekouras E, Nikiteas N, Karakitsos P, Panoussopoulos D, Lazaris ACh, Patsouris E, Bramis J, Gazouli M. Relation between common polymorphisms in genes related to inflammatory response and colorectal cancer. World J Gastroenterol. 2006;12:5037–43. doi: 10.3748/wjg.v12.i31.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel U, Christensen J, Dybdahl M, Friis S, Hansen RD, Wallin H, Nexø BA, Raaschou-Nielsen O, Andersen PS, Overvad K, Tjønneland A. Prospective study of interaction between alcohol, NSAID use and polymorphisms in genes involved in the inflammatory response in relation to risk of colorectal cancer. Mutat Res. 2007;624:88–100. doi: 10.1016/j.mrfmmm.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Küry S, Buecher B, Robiou-du-Pont S, Scoul C, Colman H, Le Neel T, Le Houérou C, Faroux R, Ollivry J, Lafraise B, Chupin LD, Sébille V, Bézieau S. Low-penetrance alleles predisposing to sporadic colorectal cancers: a French case-controlled genetic association study. BMC Cancer. 2008;8:326. doi: 10.1186/1471-2407-8-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slattery ML, Wolff RK, Curtin K, Fitzpatrick F, Herrick J, Potter JD, Caan BJ, Samowitz WS. Colon tumor mutations and epigenetic changes associated with genetic polymorphism: insight into disease pathways. Mutat Res. 2009;660:12–21. doi: 10.1016/j.mrfmmm.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawken SJ, Greenwood CM, Hudson TJ, Kustra R, McLaughlin J, Yang Q, Zanke BW, Little J. The utility and predictive value of combinations of low penetrance genes for screening and risk prediction of colorectal cancer. Hum Genet. 2010;128:89–101. doi: 10.1007/s00439-010-0828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abulí A, Fernández-Rozadilla C, Alonso-Espinaco V, Muñoz J, Gonzalo V, Bessa X, González D, Clofent J, Cubiella J, Morillas JD, Rigau J, Latorre M, Fernández-Bañares F, Peña E, Riestra S, Payá A, Jover R, Xicola RM, Llor X, Carvajal-Carmona L, Villanueva CM, Moreno V, Piqué JM, Carracedo A, Castells A, Andreu M, Ruiz-Ponte C, Castellví-Bel S Gastrointestinal Oncology Group of the Spanish Gastroenterological Association. Case-control study for colorectal cancer genetic susceptibility in EPICOLON: previously identified variants and mucins. BMC Cancer. 2011;11:339. doi: 10.1186/1471-2407-11-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crous-Bou M, Rennert G, Salazar R, Rodriguez-Moranta F, Rennert HS, Lejbkowicz F, Kopelovich L, Lipkin SM, Gruber SB, Moreno V. Genetic polymorphisms in fatty acid metabolism genes and colorectal cancer. Mutagenesis. 2012;27:169–76. doi: 10.1093/mutage/ger066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sainz J, Rudolph A, Hoffmeister M, Frank B, Brenner H, Chang-Claude J, Hemminki K, Försti A. Effect of type 2 diabetes predisposing genetic variants on colorectal cancer risk. J Clin Endocrinol Metab. 2012;97:e845–51. doi: 10.1210/jc.2011-2565. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–35. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes JM, Campbell BJ. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med. 2002;8:10–6. doi: 10.1016/s1471-4914(01)02194-3. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T, Kohno H, Yoshitani S, Takashima S, Okumura A, Murakami A, Hosokawa M. Ligands for peroxisome proliferator-activated receptors alpha and gamma inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Res. 2001;61:2424–8. [PubMed] [Google Scholar]
- 29.Swarbrick MM, Chapman CM, McQuillan BM, Hung J, Thompson PL, Beilby JP. A Pro12Ala polymorphism in the human peroxisome proliferator-activated receptor-gamma 2 is associated with combined hyperlipidaemia in obesity. Eur J Endocrinol. 2001;144:277–82. doi: 10.1530/eje.0.1440277. [DOI] [PubMed] [Google Scholar]
- 30.Stumvoll M, Häring H. The peroxisome proliferator-activated receptor-gamma2 Pro12Ala polymor phism. Diabetes. 2002;51:2341–7. doi: 10.2337/diabetes.51.8.2341. [DOI] [PubMed] [Google Scholar]


