Abstract
Background: Cardiomyocyte hypoxia causes cardiac hypertrophy and other major myocardial injuries. We investigated the molecular mechanism of microRNA-26b (miR-26b) in regulating hypoxia-induced apoptosis in rat neonatal cardiomyocytes. Methods: Neonatal rat cardiomyocytes was prepared in vitro and hypoxia was induced. Apoptotic cardiomyocytes were examined by TUNEL staining and the expression of miR-26b were monitored by qRT-PCR. The effect of mir-26b downregulation on hypoxia-induced apoptosis, or expression of PTEN in cardiomyocytes was monitored. PTEN was knocked down in cardiomyocytes by siRNA to further investigate its association with miR-26b. Results: Hypoxia induced severe apoptosis and upregulated miR-26b in neonatal rat cardiomyocytes in vitro. Down-regulation of miR-26b markedly ameliorated hypoxia-induced apoptosis and up-regulated PTEN. Luciferase reporter assay confirmed PTEN was directly targeted by miR-26b, and knocking down PTEN reduced cytotoxicity induced by miR-26b upregulation. Conclusion: Downregulation of miR-26b protected cardiomyocytes from hypoxia-induced apoptosis, and the protective effect was very likely to be associated with PTEN regulation.
Keywords: Cardiomyocytes, hypoxia, miR-26b, PTEN
Introduction
Human cardiovascular disease (CVD) is one of the major causes of death among both children and adults in the world. In the United States alone, around 80 million adults (1 in 3) have one or more types of CVD and about 8 millions are estimated to have an acute myocardial infarction [1,2]. The current clinical treatment of acute myocardial infarction is trying to restore coronary blood flow as soon as possible. Unfortunately, myocardial reperfusion often leads to further cardiac damage of myocardial ischemia or reperfusion (I/R) injury [3,4], and the apoptosis of cardiomyocytes was the major mechanism in the development of cardiac injury induced by myocardial ischemia [5-7]. Therefore, inhibition of myocardial apoptosis may reduce I/R damage and finding new therapeutic target for the treatment of I/R injury could certainly benefit large population of CVD patients.
MicroRNAs (miRNAs) are groups of short sequence noncoding RNAs that silencing transcription through suppressing or degrading messenger RNAs [8]. They are important regulators of gene expression, participating in both normal heart development and cardiovascular diseases [9-11]. Recent studies demonstrated that the family of microRNA 26, including miR-26a and miR-26b was actively involved in the development of cardiac hypertrophy [12], and Atrial fibrillation [13].
In our current study, we cultured neonatal rat cardiomyocytes in vitro and introduce hypoxia to re-produce the in vivo I/R injury. Based on this model, we investigated the effect of hypoxia on cardiomyocyte apoptosis and the expression of miR-26b. In order to elucidate the underlying mechanisms of miR-26b during hypoxia, we applied antisense oligonucleotide of miR-26b to down-regulate miR-26b in cardiomyocytes and examined its effect on cardiomyocyte apoptosis and explored its molecular association with PTEN pathway. The results of our experiments would help seeking novel treatment strategy to prevent myocardial ischemia in patients suffered from CVD.
Materials and methods
Rat cardiomyocyte culture and model of hypoxia
In vitro culture of neonatal rat cardiomyocytes and induction of hypoxia were performed according to the method described in previous study [14]. Briefly, postnatal 2 to 4 days old Sprague-Dawley rats were anesthetized and decapitated. The ventricles were quickly extracted and minced into pieces in Hanks’ balanced salt solution and digested with 1% collagenase (Sigma, USA) in an incubator with 5% CO2 at 37°C for 15 minutes. The floating cells were collected and pre-plated in DMEM supplemented with 5% fetal bovine serum to purify and enrich cardiomyocytes. Cardiomyocytes were further cultured in DMEM with 10% fetal bovine serum and 1 microM Ara C for another 24 to 72 hours before hypoxia treatment. Then, to induce severe hypoxia, the cultured rat cardiomyocytes was treated with 95% N2 and 5% CO2 gas induction for 48 hours and subsequent 6 hours of re-oxygenation followed by further procedures.
TUNEL assay
The apoptotic cardiomyocytes in vitro was detected by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay according to manufacturer’s protocol (Molecular Probes, USA). Briefly, cultured cardiomyocytes were washed with PBS and fixed in 4% paraformaldehyde for 15 min and further fixed in ice cold ethanol mix of acetic acid (2:1) for additional 5 min at -20°C. After antoher wash with PBS, the cells were incubated with the reaction mixture of TUNEL for 1.0 h at 37°C in a humidified box. The percentage of cardiomyocytes was determined by counting cells nuclei (DAPI stained) in 500-um2 spaces in triplicate plates.
Quantitative real-time reverse transcription-PCR (qRT-PCR)
Total RNA or miRNA fractions were extracted from rat cardiomyocytes with Trizol reagent according to manufacturer’s protocol (Invitrogen, USA). Total RNA concentrations were measured with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, USA) at 260 and 280 nm (A260/280), and examined with an Agilent 2100 Bioanalyzer (Agilent Technologies, USA). Quantitative real-time reverse transcription-PCR (qRT-PCR) assays were done using the TaqMan miRNA Assay according to manufacturer’s protocol (Applied Biosystems, USA). The amplification conditions were 38 cycles of 15 s at 94°C and 1 min at 62°C. The expression levels of miR-26b and PTEN were normalized by the expression level of house keeping gene U6 and GAPDH, respectively. Experiments were performed in triplicate.
miRNA transfection
Cardiomyocytes were re-plated in six-well plates and transfected with 100 nM miR-26b antisense inhibitor (miR-26b-as), miR-26b mimics (miR-26b-mimics) or scrambled non-specific control miRNA (miR-NC) (Ambion, Inc, USA), using a Lipofectamine™ RNAiMAX Transfection Reagent according to manufacturer’s instruction (Invitrogen, USA). Subsequent experiments or analyses, including hypoxia induction, RNA extraction amd qRT-PCR were performed 24 hours after miRNA transfection.
miRNA target prediction
Three commonly used bioinformatics databases were used to predict the putative miRNA-gene targets, including TargetScan version 5.2 (http://www.targetscan.org/index.html), miRBase (http://microrna.sanger.ac.uk) and miRanda (http://www.microrna.org).
Luciferase reporter assays
Regular PCR was performed on the cardiomyocytes to amplify PTEN wild-type (WT) 3’-UTR and mutant (MUT) 3’-UTR according to predicted rat miR-26b binding site (rno-miR-26-b, Figure 3A). The WT 3’-UTR and MUT 3’-UTR sequences of PTEN were then constructed into a luciferase reporter vector (pmiR-REPORT, Ambion, USA) to generate constructs of Luc-PTEN and Luc-PTEN-mu (verified by DNA sequencing). The pmiR-REPORT control vector (Luc-control), along with and Luc-FOXC1, Luc-FOXC1-mu were co-transfected with β-galactosidase and rat miR-26b into HEK293 cells in 12-well plates with a Lipofectamine 2000 transfection kit according to the manufacturer’s protocol (Invitrogen, USA). The luciferase activity was examined in 24 hours. The signals were normalized to the β-galactosidase activity of Luc-control.
Figure 3.
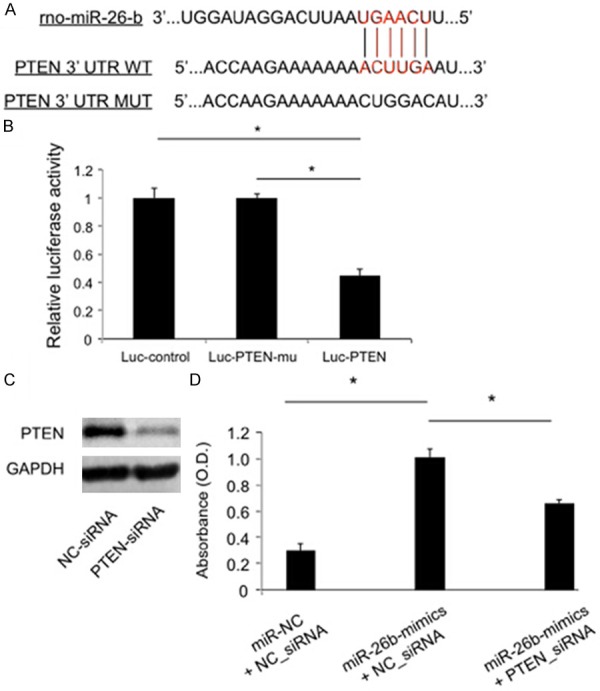
miR-26b targeted PTEN to rescue hypoxia-induced apoptosis in rat cardiomyocytes. A. The predicted binding site of rat miR-26b (rno-miR-26-b) on wild type 3’-UTR of PTEN (PTEN 3’UTR WT), along with mutated 3’-UTR of FOXC1 (PTEN 3’UTR MUT) were shown. B. HEK 293T cells were transfected with the pmiR-REPORT control construct (Luc-control), mutant 3’-UTR PTEN (Luc-PTEN-mu) or wild-type 3’-UTR PTEN (Luc-PTEN), along with β-galactosidase and miR-26b. After 24 hours, cells were examined by a luciferase assay and the signals were normalized to β-galactosidase activity of control vector. (*: P < 0.05). C. Rat cardiomyocytes were transfected with siRNA targeting PTEN (PTEN-siRNA, 50 nM), or its non-specific siRNA (NC-siRNA, 50 nM), followed by western blotting analysis of PTEN. D. Rat cardiomyocytes were treated with miR-NC plus NC-siRNA, miR-26b-mimics plus NC-siRNA or miR-26b-mimics plus PTEN-siRNA. LDH essay was applied to measure the cytotoxicity level among cardiomyocytes (*: P < 0.05).
PTEN siRNA transfection
The specific PTEN siRNA (PTEN-siRNA, 50 nM) and its non-specific scramble siRNA (NC_siRNA, 50 nM) were purchased from Stanta Cruz (Santa Cruz Biotechnology, USA), and the transfection of siRNAs was performed for 48 hours with a Lipofectamine 2000 transfection kit according to manufacturer’s recommended protocol. The efficiency of siRNA on knocking down PTEN was also examined by western blotting analysis.
Western blotting analysis
The cardiomyocytes were suspended and the lysis was collected. The protein concentration was determined using a BCA kit. Total amount of 20 μg protein was used to run on a 9% SDS-PAGE gel after electrophoresis at 70 V for 4 h, and then transferred to a film and incubated with primary antibody against rat PTEN (1:250, Santa Cruz Technology, Santa Cruz, CA). Horseradish peroxidase-ECL method was using for X-ray film exposure and the measurement of PTEN protein expression levels. GAPDH was the internal control.
Lactate dehydrogenase (LDH) assay
A cytotoxicity detection kit was used to measure the level of lactate dehydrogenase (LDH) released by cultured rat cardiomyocytes according to manufacturer’s protocol (Roche Applied Sciences, USA). The absorbance at 490 nm was examined by a microplate reader and presented as arbitrary unit of optical density (O.D.).
Statistical analysis
All data were presented as the mean ± SD and evaluated with a Student’s t test in SPSS software (version 11.0). Statistically significance was determined if P < 0.05. All experiments were repeated at least three times.
Results
Hypoxia induced apoptosis and up-regulated miR-26b in cultured rat cardiomyocytes
We cultured neonatal cardiomyocytes from postnatal 2 days old (P2) rats and treated those cells with 95% N2/5% CO2 to induce hypoxia and apoptosis [14]. After 48 hours of culture, TUNEL positive cardiomyocytes were evident under hypoxia condition whereas little or no TUNEL signals were detected under normal condition with hypoxia induction (Figure 1A). Quantitative measurement demonstrated that the percentage of TUNEL positive cardiomyocytes with hypoxia treatment was significantly higher than the percentage without hypoxia treatment (Figure 1B, P < 0.05). Through qRT-PCR, we found that the expression level of miR-26b was subsequently upregulated in hypoxia-induced cardiomyocytes (Figure 1C, P < 0.05).
Figure 1.
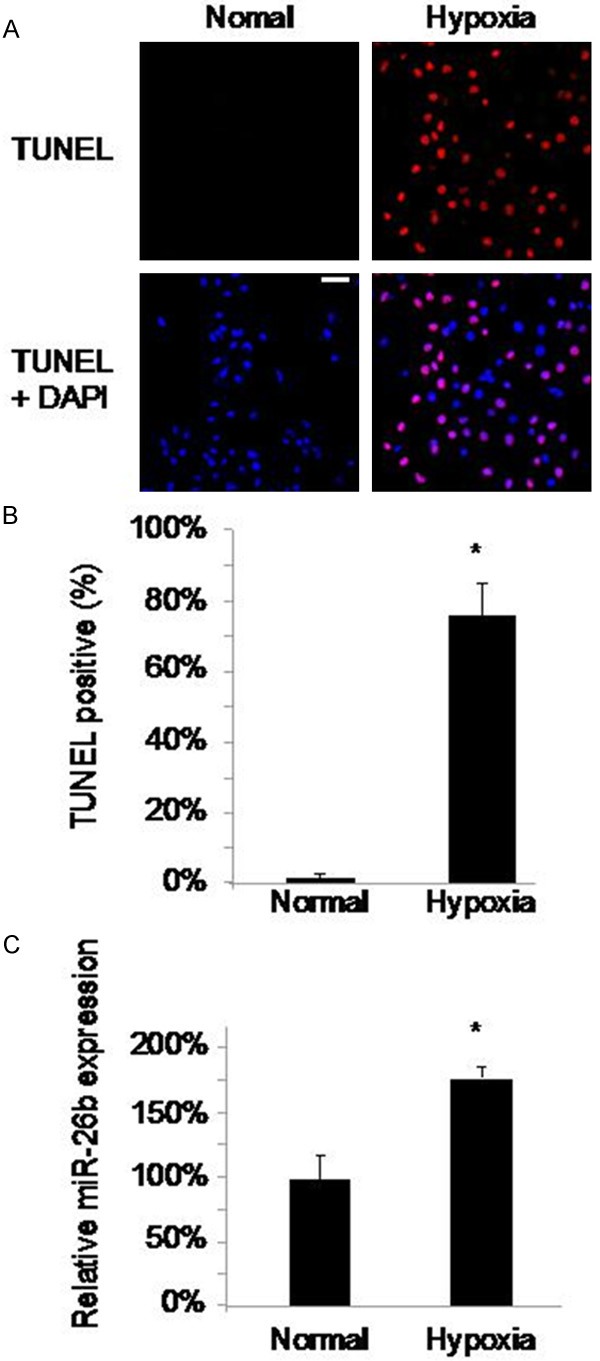
Apoptosis and aberrant expression of miR-26b in rat cardiomyocytes by hypoxia. The cultured cardiomyocytes extracted from P2 rats were either treated with N2 to induce hypoxia or with O2 (as normal condition) for 48 hours. Apoptosis among cardiomyocytes were examined with TUNEL staining (A) (scale bar: 20 m). The percentages of TUNEL positive cells were compared between hypoxia and normal conditions (B) (*, P < 0.05). The expression levels of miR-26b were measured and compared between hypoxia and normal conditions (C) (*: P < 0.05).
Down-regulation of miR-26b rescued hypoxia-induced apoptosis in cardiomyocytes and upregulated PTEN
Since miR-26b was upregulated by hypoxia in cardiomyocytes, we then sought to investigate whether down-regulating miR-26b would protect the apoptotic effect of hypoxia. First, we examined the efficiency of miRNA knocking down by transfecting the cultured rat cardiomyocytes with miR-26b antisense inhibitor (miR-26b-as) and measuring the corresponding change of miR-26b mRNA levels. Parallel transfection of non-specific miRNA (miR-NC) was also conducted as control. The qRT-PCR result showed that 100 nM miR-26b-as was sufficient to significantly reduce the endogenous expression level of miR-26b in cardiomyocytes (Figure 2A). Then, the protective effect of miR-26b on hypoxia-induced apoptosis was examined by transfecting cultured rat cardiomyocytes with miR-26b-as for 24 hours, followed by 48 hours of hypoxia treatment. After that, TUNEL staining demonstrated that the number of apoptotic cardiomyocytes was markedly reduced in cardiomyocytes transfected with miR-26b-as than the number in cardiomyocytes transfected with miR-NC (Figure 2B).
Figure 2.
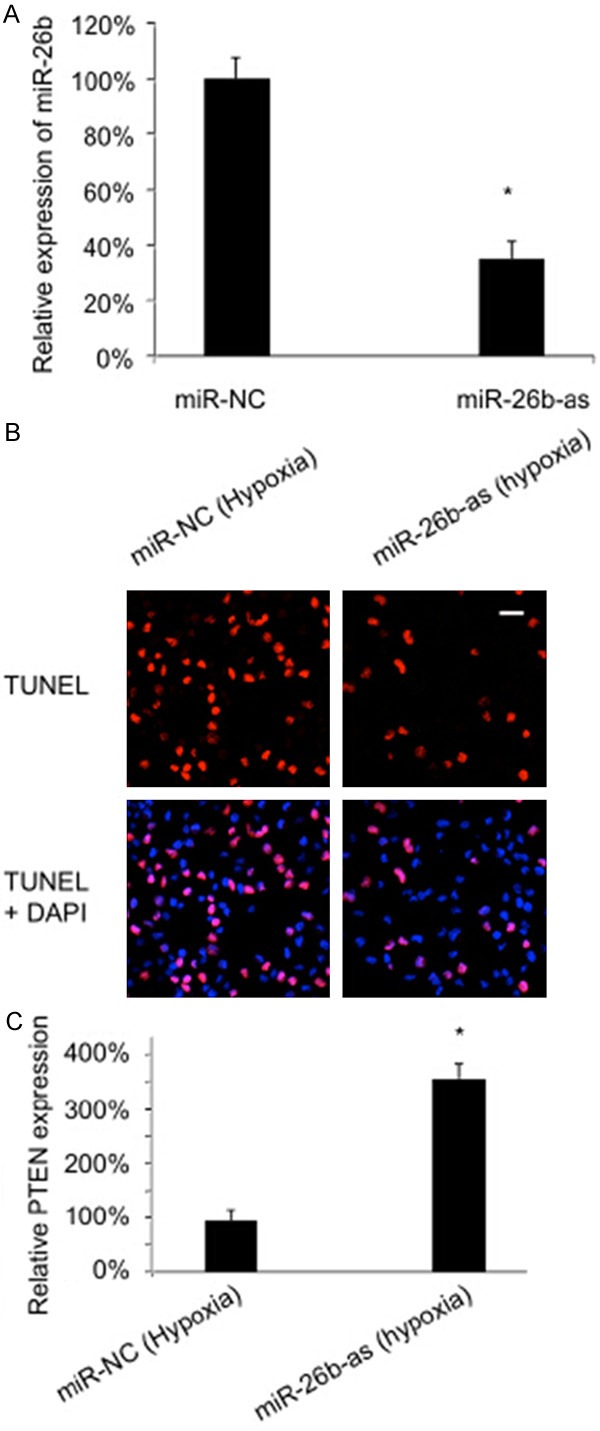
Downregulation of miR-26b rescued hypoxia-induced apoptosis and up-regulated PTEN in rat cardiomyocytes. A. Cardiomyocytes were transfected with 100 nM miR-26b antisense inhibitor (miR-26b-as) and its non-specific miRNA (miR-NC) for 24 hours, followed by qRT-PCR to measure the efficiency of miRNA transfection (*: P < 0.05). B. Twenty-four hours after miRNA transfection, hypoxia was induced in cultured rat cardiomyocytes for 48 hours. TUNEL staining was conducted to compare apoptotic cardiomyocytes between miR-26-as and miR-NC treatments. C. After miRNA transfection and hypoxia induction, qRT-PCR was conducted to compare the the expression levels of PTEN between miR-26-as and miR-NC treatments.
We also examined the effect of miR-26b on apoptosis associated pathway and found that PTEN was specifically up-regulated by miR-26b during hypoxia induction (Figure 2C).
miR-26b modulated hypoxia-induced apoptosis cardiomyocytes through PTEN
We then sought to investigate whether the regulation of miR-26b on hypoxia-induced apoptosis in rat cardiomyocytes were through PTEN. Through bioinformatics prediction softwares including TargetScan, miRBase and miRanda, we discovered that PTEN was very likely to be the direct target of rat miR-26b (rno-miR-26-b) (Figure 3A). We then use a luciferase reporter essay to confirm that miR-26b was directly acting on PTEN in rat cardiomyocytes (Figure 3B).
Based on these findings, we suspected that PTEN was directly involved in the modulation of miR-26b in cardiomyocytes and decided to use siRNA to genetically knock down PTEN in rat cardiomyocytes in vitro. The efficiency of siRNA transfection was evaluated by a western blotting assay and the result demonstrated that PTEN siRNA (PTEN-siRNA) was efficient in down-regulating PTEN protein in cardiomyocytes (Figure 3C). Then, we pre-treated rat cardiomyocytes with either miR-NC or miR-26b mimics (miR-26-mimics), followed by transfection of either NC_siRNA or PTEN_siRNA. The result demonstrated that the cytotoxicity level was significantly elevated in cardiomyocytes by miR-26b-mimics, but rescued by PTEN_siRNA, suggesting that knocking down PTEN exerted similar protective mechanisms on rat cardiomyocytes as down-regulating miR-26b (Figure 3C).
Discussions
In recent decades, microRNAs have emerged as new genetic regulators or even therapeutic candidates for various cardiovascular diseases, including ischemic heart disease [15-17]. Although family of miR-26 had been suggested to play role in cardiac hypertrophy [12] or atrial fibrillation [13], there has been no evidence showing miR-26b was directly modulated or actively participating in the process of cardiac apoptosis induced by I/R injury.
For the first time ever, in the present study, we demonstrated that miR-26b was upregulated during the process of hypoxia, in close association with apoptosis in cultured rat neonatal cardiomyocytes. More importantly, through the experiment of genetic manipulation, we showed that down-regulation of miR-26b could ameliorate hypoxia-induced apoptosis in cardiomyocytes. Thus, the above experiments strongly indicated an active role of miR-26b in regulating I/R injury and supported the notion that miR-26b might be a novel molecular target for cardiovascular diseases.
In the current study, we also explored the possible pathways that might be targeted by miR-26b in regulating hypoxia-induced apoptosis in cardiomyocytes. We found that PTEN, a tumor suppressor gene involved in various aspects of cardiovascular diseases [18-20], was a direct target of miR-26b. Down-regulating miR-26b under the condition of hypoxia subsequently up-regulated PTEN in neonatal cardiomyocytes. In addition, we used siRNA to genetically silence endogenous PTEN in cardiomyocytes. The results showed that knocking down PTEN exerted similar protective effect as down-regulating miR-26b by reducing the cytotoxicity in cardiomyocytes induced by over-expressing miR-26b. The meaning of these findings is two folds. First, it presented direct evidence demonstrating that in cardiomyocytes, miR-26b negatively regulates PTEN expression during I/R injury. Second, as PTEN was the direct target of miR-26b during the regulation of I/R injury, it demonstrated that miR-26b could be activating similar down-stream targets and closely associated with other apoptotic pathways, such as AKT/PI3K pathways during the process of I/R injury.
In summary, our findings demonstrated that miR-26b played an important role in PTEN-associated protective effect against hypoxia-induced apoptosis in cardiomyocytes. Although the study was performed in animal models and the experimental results may not be directly related to human study, the findings certainly brought attention for further investigation to evaluate whether miR-26b regulation could be extrapolated in future clinical practice.
Disclosure of conflict of interest
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpert JS, Thygesen KA, White HD, Jaffe AS. Diagnostic and therapeutic implications of type 2 myocardial infarction: review and commentary. Am J Med. 2014;127:105–8. doi: 10.1016/j.amjmed.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 3.Frassdorf J, De Hert S, Schlack W. Anaesthesia and myocardial ischaemia/reperfusion injury. Br J Anaesth. 2009;103:89–98. doi: 10.1093/bja/aep141. [DOI] [PubMed] [Google Scholar]
- 4.Dong Y, Undyala VV, Gottlieb RA, Mentzer RM Jr, Przyklenk K. Autophagy: definition, molecular machinery, and potential role in myocardial ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther. 2010;15:220–230. doi: 10.1177/1074248410370327. [DOI] [PubMed] [Google Scholar]
- 5.Elsasser A, Suzuki K, Lorenz-Meyer S, Bode C, Schaper J. The role of apoptosis in myocardial ischemia: a critical appraisal. Basic Res Cardiol. 2001;96:219–226. doi: 10.1007/s003950170052. [DOI] [PubMed] [Google Scholar]
- 6.Freude B, Masters TN, Robicsek F, Fokin A, Kostin S. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol. 2000;32:197–208. doi: 10.1006/jmcc.1999.1066. [DOI] [PubMed] [Google Scholar]
- 7.Freude B, Masters TN, Kostin S, Robicsek F, Schaper J. Cardiomyocyte apoptosis in acute and chronic conditions. Basic Res Cardiol. 1998;93:85–89. doi: 10.1007/s003950050066. [DOI] [PubMed] [Google Scholar]
- 8.Choudhuri S. Small noncoding RNAs: biogenesis, function, and emerging significance in toxicology. J Biochem Mol Toxicol. 2010;24:195–216. doi: 10.1002/jbt.20325. [DOI] [PubMed] [Google Scholar]
- 9.Yu S, Li G. MicroRNA expression and function in cardiac ischemic injury. J Cardiovasc Transl Res. 2010;3:241–245. doi: 10.1007/s12265-010-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang N, Zhou Z, Liao X, Zhang T. Role of microRNAs in cardiac hypertrophy and heart failure. IUBMB Life. 2009;61:566–571. doi: 10.1002/iub.204. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Luo X, Lu Y, Yang B. miRNAs at the heart of the matter. J Mol Med (Berl) 2008;86:771–783. doi: 10.1007/s00109-008-0341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang ZH, Li J, Liu BR, Luo CF, Dong Q. MicroRNA-26 was decreased in rat cardiac hypertrophy model and may be a promising therapeutic target. J Cardiovasc Pharmacol. 2013;62:312–319. doi: 10.1097/FJC.0b013e31829b82e6. [DOI] [PubMed] [Google Scholar]
- 13.Luo X, Pan Z, Shan H, Xiao J, Sun X. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest. 2013;123:1939–1951. doi: 10.1172/JCI62185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka M, Ito H, Adachi S, Akimoto H, Nishikawa T. Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circulation research. 1994;75:426–433. doi: 10.1161/01.res.75.3.426. [DOI] [PubMed] [Google Scholar]
- 15.Thum T, Catalucci D, Bauersachs J. MicroRNAs: novel regulators in cardiac development and disease. Cardiovasc Res. 2008;79:562–570. doi: 10.1093/cvr/cvn137. [DOI] [PubMed] [Google Scholar]
- 16.Frost RJ, van Rooij E. miRNAs as therapeutic targets in ischemic heart disease. J Cardiovasc Transl Res. 2010;3:280–289. doi: 10.1007/s12265-010-9173-y. [DOI] [PubMed] [Google Scholar]
- 17.Fasanaro P, Greco S, Ivan M, Capogrossi MC, Martelli F. microRNA: emerging therapeutic targets in acute ischemic diseases. Pharmacol Ther. 2010;125:92–104. doi: 10.1016/j.pharmthera.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82:250–260. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 20.Schwartzbauer G, Robbins J. The tumor suppressor gene PTEN can regulate cardiac hypertrophy and survival. J Biol Chem. 2001;276:35786–35793. doi: 10.1074/jbc.M102479200. [DOI] [PubMed] [Google Scholar]


