Abstract
This study aimed to investigate the neuroprotective effect and its mechanism of lentivirus mediated VEGF on rat model with cerebral ischemic injury. 45 rats with cerebral ischemic injury constructed by the suture method were randomly divided into sham group, model group, vector group and VEGF group. The packaged vector lentivirus and lentivirus carrying VEGF gene were injected into the lateral ventricular of rats in vector group and VEGF group respectively. The equal volume of PBS buffer was injected in sham group and model group respectively. The expression of VEGF and protein in brain tissue were detected by real-time fluorescence quantitative PCR and Western blot. The change of brain tissue vascular density was analyzed by immunohistochemistry. The brain infarction area and the degree of nervous functional defect of the rats were analyzed. VEGF mRNA and protein levels were significantly higher in brain tissue of rats in VEGF group than those in model group and vector group (P < 0.05). The brain tissue vascular density increased significantly in VEGF group (P < 0.05). Compared with sham group, the infarction area of brain tissue and the degree of nervous functional defect increased significantly in model group, vector group and VEGF group, but the VEGF group was significantly lower than those in model group and vector group (P < 0.05). In conclusion, the overexpression of VEGF in cerebral ischemia injury contributed to the angiogenesis in brain tissues, reduced the brain injury caused by cerebral ischemia and protected brain neuronal function.
Keywords: Cerebral ischemia injury, vascular endothelial growth factor (VEGF), lentivirus
Introduction
Ischemic cerebral injury was one of the common types of cerebral vascular disease. Its incidence was secondary to malignant tumor and became one of the diseases which seriously affects human health [1,2]. The main treatment of cerebral ischemia injury was thrombolytic therapy, secondly all kinds of brain protective agents were used to prevent brain cells apoptosis [3]. However, the time window of thrombolysis therapy was relatively short, the time when the majority of patients visited the doctors exceeded thrombolysis time window. The brain protective agents usually only had protective effects on ischemic penumbra cells, and had minor role on the ischemic core area [4]. In recent years, with the continuous development of ischemic brain damage mechanism and gene therapy technology, the means of gene target treatment became one of the methods to treat ischemic brain injury. Vascular endothelial growth factor (VEGF) was a potent mitogen. It could act on vascular endothelial cells in the form of paracrine in normal physiological and pathological conditions, promote angiogenesis, and increase vascular especially microvascular permeability [5-7]. A large number of animal and clinical studies showed that hypoxia or ischemia could induce high expressions of VEGF, its receptor mRNA and protein level in brain tissue during short period. However VEGF synthesis obstacles appeared after ischemia for a long time [8-11]. This result suggested that the high expression of VEGF in the early stage of the disease could promote brain tissue angiogenesis, and compensatorily protect brain tissue from necrosis and apoptosis. Therefore, a certain dose of VEGF could protect brain tissue from necrosis and apoptosis at time of ischemic cerebral injury. Under the guidance of this theory, the effects of high expression of VEGF on brain tissue cells and neural function in rats with cerebral ischemic injury were investigated through the expression in ischemic brain damage model carrying exogenous VEGF gene taking lentivirus as a vector.
Materials and methods
Model construction
45 SD rats (female, 180-200 g) were purchased from Experimental Animal Center of Xinxiang Medical University. The rat model with ischemic brain injury was constructed according to LONGA method [12]. 0.4% pentobarbital sodium was injected into abdominal cavity. A midline incision 1.6-2.0 cm long was made on the neck of rat, the right common carotid artery was exposed, and external carotid artery was ligated. The vagus nerve accompanied with common carotid artery was separated and the common carotid artery was ligated. In the distal of carotid artery ligation, the thread was retained. The common carotid artery was closed down with tiny artery clamp and the incision was made near the spare thread. No. 5 thread was inserted into the incision. 5/0 nylon suture was inserted upwards into the initiation site of middle cerebral artery along the common carotid artery and internal carotid artery. If the insertion encountered resistance from the common carotid bifurcation, it should be stopped. That was the middle cerebral artery blood supply disorder rat model. The vessel was only isolated but without occlusion in sham group. The signs of successful modeling included: The rats were dispirited within 2 h after the establishment of modeling, Horner’s syndrome on the same side, contralateral forelimb ptosis, adduction and internal rotation, spontaneously whirling to the affected side, which illustrated that the modeling was successful. The model was unsuccessful without the above performances. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD, USA). Eighth Edition, 2010. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Xinxiang Medical University.
Experimental grouping and treatment
The blood vessels were only isolated but without occlusion on 10 rats in sham group. 100 μl PBS buffer was injected into lateral ventricles; The remaining 30 cerebral ischemia injury model rats were randomly divided into model group, vector group and VEGF group. 100 μl PBS buffer was injected into lateral ventricles in model group; 100 μl lentivirus solution packaged with vector plasmid was injected into lateral ventricles in vector group; 100 μl lentivirus solution packaged with the expression of VEGF was injected in VEGF group. The lentivirus solution and control solution were injected after modeling for 2 h.
Real time fluorescent quantitative PCR
Four groups of rats were sacrificed 72 h after modeling and treatment. 0.1 g brain tissue was used for extracting total RNA with RNA extraction kit (TaKaRa, Dalian, China). RNA was transcribed into cDNA with reverse transcription kit (TaKaRa, Dalian, China) for the following experiments. According to the gene sequence of VEGF, real-time fluorescent quantitative PCR primers were designed, and the amplified length was 200 bp, meanwhile, β-actin was designed as internal standard. Primer sequences were as follows: VEGF-F: 5’-GAGCAGAAGTCCCATGAAGTGA-3’; VEGF-R; 5’-CACAGGACGGCTTGAAGATGT-3’; Actin-F: 5’-AGAAGGCTGGGGCTCATTTG-3’; Actin-R: 5’-AGGGGCCATCCACAGTCTTC-3’. PCR reaction system was prepared according to the following system: 10 μl 2*SYBR Green general qPCR Master Mix (TaKaRa, Dalian, China), 0.6 μl upstream/downstream primer respectively (10 μmmol•L-1), 8.8 μl 1:100 diluted cDNA. The total reaction volume was 20 μl. Then the reaction mixture was swung to the bottom of test tube with 1500 rpm/min centrifugal. PCR was undertaken according to the following reaction conditions: pre degenerated for 30 s at 95°C; degeneration for 3 s at 95°C; annealing and extension for 30 s at 60°C; The dissolution curve was constructed. Finally, the data were directly read from the real-time fluorescence quantitative PCR instrument (Applied Biosystems, Foster City, CA, USA).
Western blot
Four groups of rats were sacrificed after modeling and treatment for 72 h. 0.1 g brain tissue was taken out and cut into pieces. 300 μl tissue lysate was added, homogenized in tissue homogenate instrument and centrifuged by 12000 rpm/min for 30 min. The supernatant was extracted, 5*Loading buffer was added and boiled in the boiling water for 10 min, then centrifuged by 12000 rpm/min for 5 min. The supernatant was extracted for SDS-PAGE. The protein was transferred onto NC membrane after SDS-PAGE, closed with 5% BSA PBST solution for 30 min, incubated overnight using the diluted VEGF (1:2000; Abcam, Cambridge, UK) at 4°C, washed with PBST for 3 times, 5 min each time and incubated with Goat anti rat HRP labeled secondary antibodies (1:2000; ZSGB-BIO, Beijing, China) at room temperature for 1 h. HRP substrate was added on film and colored. The expression level of VEGF was analyzed.
Immunohistochemical analysis
Four groups of rats were sacrificed after modeling and treatment for 72 h. The brain tissue was taken out, embedded with paraffin and cut into 5 μm thick sections. After the sections were dewaxed and hydrated, the endogenous catalase was removed with 3% hydrogen peroxide. Then the tissue was closed with 10% goat serum for 1 h. CD31 antibody (1:500; Abcam, Cambridge, UK) was dropped on the slides, incubated overnight at 4°C and washed with PBST for 3 times the next day, 5 min each time. The reaction system was incubated with Goat anti rat HRP labeled secondary antibodies (1:2000; ZSGB-BIO, Beijing, China) at room temperature for 1 h, washed with PBST for 3 times, 5 min each time, colored with TMB and stained with hematoxylin. The sections were successively immersed in 75%, 80%, 90% and 100% ethanol for 1 min, placed in xylene for 30 min and closed with neutral resin. The brain tissue vascular density was observed under the microscope.
TTC staining
The rats were sacrificed 48 h later. The brain tissue was taken out. The olfactory bulb, cerebellum and lower brainstem were resected. The coronal sections were undertaken for the remainder. The sections were placed in 2% of TTC phosphate buffer for staining for 20 min at 37°C. Then paraformaldehyde was used for fixation. The normal tissue was red, while the infarcted tissue was white. The infarcted tissue was separated with normal brain tissue under the microscope. The wet weights of normal tissue and infarction tissue were weighed with analytical balance. The infarction range was the percentage of infarction tissue weight accounting for the total mass of infarction tissue and normal brain tissues.
Effects of different treatments on nervous functional defect degree of rats
The nervous functional defects were analyzed in sham group, model group, vector group and VEGF group after modeling for 24 h, 48 h and 72 h with Bederson standard score [13]. 0: without nervous functional defect; 1: right forepaw was not fully extended; 2: whirling to the left; 3: dumping to the right; 4: could not walk spontaneously and loss of consciousness.
Statistical analysis
All data were analyzed using SPSS13.0 statistical software (SPSS Inc, Chicago, IL, USA). The measurement data were expressed by XS. The measurement data were compared using analysis of variance. t test was used for the two-two comparison between groups. P < 0.05 showed that the difference had statistical significance.
Results
Analysis of expression of lentivirus mediated VEGF in brain tissues
The level of VEGF mRNA in brain tissue in different group of rats was analyzed using real-time fluorescence quantitative PCR. As shown in Figure 1A, compared with sham group, model group and vector group, the level of VEGF mRNA increased significantly in brain tissues of rats in VEGF group after treatment for 72 h (P < 0.05). Compared with sham group, the levels of VEGF mRNA in brain tissue of rats in model group and vector group increased after treatment for 72 h (P > 0.05), but were still significantly lower than that in VEGF group (P < 0.05). Western blot results showed that compared with sham group, the expression level of VEGF protein in brain tissue of the rats increased significantly in model group, vector group and VEGF group (P < 0.05). However compared with model group and vector group, the expression level of VEGF protein in brain tissue of the rats increased significantly in VEGF group (P < 0.05) (Figure 1B).
Figure 1.
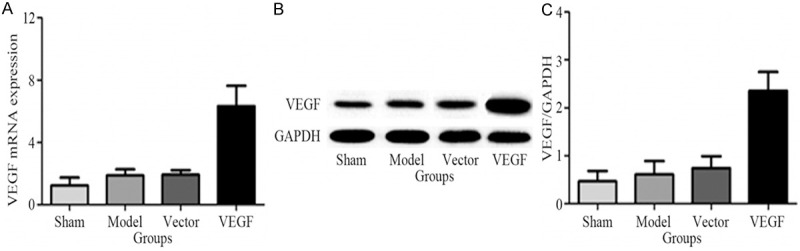
Comparison of VEGF mRNA and protein in brain tissues in different treatment groups. A. VEGF mRNA in brain tissues in different treatment groups were analyzed by real-time fluorescence quantitative PCR; B. VEGF protein in brain tissues in different treatment groups were analyzed by Western blot; C. Quantitative analysis results of Western blot.
Comparison of brain tissue neovascular density in different treatment groups
The neovascular density of brain tissue in rats was analyzed by immunohistochemistry in four groups. The number of vascular tubular structures with CD31 positive in brain tissues of the rats in sham group was fewer, the vascular density in brain tissue of the rats in model group and vector group increased to a certain degree, but the vascular density in brain tissue of the rats in VEGF group significantly increased (Figure 2A). The results of quantitative analysis were shown in Figure 2B, compared with sham group, the vascular density in brain tissue of the rats in model group and vector group increased, but the difference was no statistically significant (P < 0.05), while compared with sham group, model group and vector group, the vascular density in brain tissue of the rats in VEGF group increased significantly and the difference was statistically significant (P < 0.05).
Figure 2.
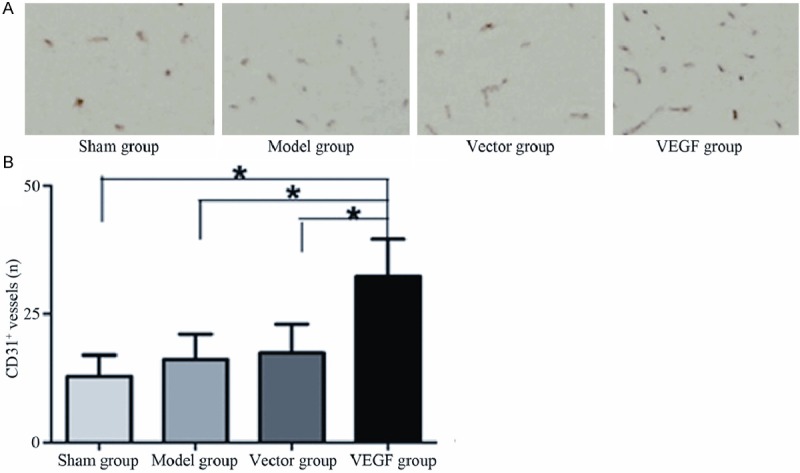
Comparison of microvessel density of rat brain tissue in different treatment groups. A. Microvessel density of brain tissue displayed by CD31 (Magnification ×400); B. Microvessel density of brain tissue in different treatment groups by quantitative analysis.
Comparison of brain infarction areas of rats in different treatment groups
Cerebral ischemia for long time could lead to brain tissue apoptosis and necrosis. This research analyzed the effect of different treatments on rat brain tissue infarction in quick succession. The infarct area was white after coloration by TTC. The normal brain tissue was red. The infarct tissue was separated and the infarction size was analyzed (Figure 3A). Compared with sham group, model group and vector group, the infarct size in brain tissue of rats in VEGF group decreased significantly (P < 0.05), while sham group and model group showed no statistical significance with vector group (P < 0.05) (Figure 3B).
Figure 3.
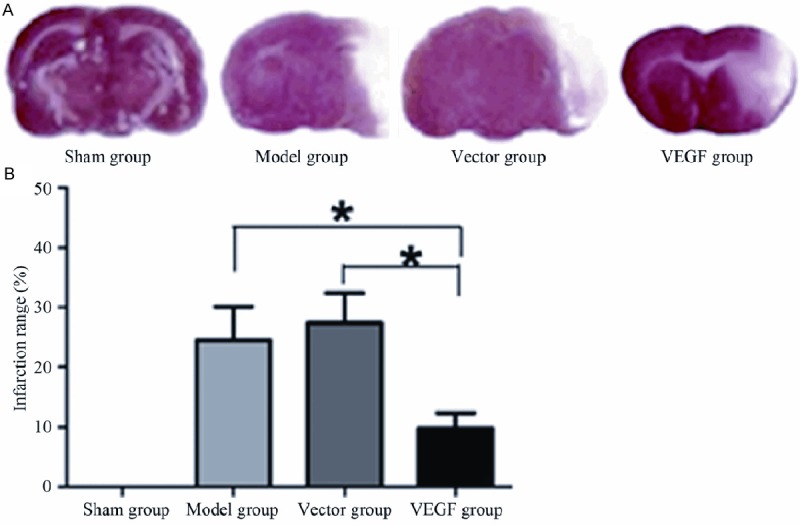
Comparison of brain infarction areas of rats in different treatment groups. A. TTC staining results (Magnification ×100); B. Quantitative analysis of brain infarction areas in sham group, model group, vector group and VEGF group.
Comparison of cerebral neural function defect in different treatment groups
The evaluation of cerebral neural function defect degree of rats after different treatments for 24 h, 48 h and 72 h showed that compared with sham group, cerebral neural function defect degree of rats increased significantly in model group, vector group and VEGF group. With the increase of time, the injury degree was heavier. However the degree of neural function damage of rats in VEGF group decreased significantly compared with model group and vector group and the difference was statistically significant (P < 0.05) (Table 1).
Table 1.
Comparison of cerebral neural function defect in different treatment groups
| Groups | n | Before treatment | After treatment | ||
|---|---|---|---|---|---|
|
| |||||
| 24 h | 48 h | 72 h | |||
| Sham group | 15 | 0.29±0.18 | 0.31±0.15 | 0.23±0.15 | 0.15±0.10 |
| Model group | 15 | 3.24±0.78* | 3.01±0.63* | 2.87±0.56* | 2.07±0.50* |
| Vector group | 15 | 3.36±0.81* | 2.96±0.52* | 2.79±0.60* | 2.14±0.47* |
| VEGF group | 15 | 3.54±0.79*,# | 2.01±0.53*,# | 1.51±0.38*,# | 0.65±0.32*,# |
Compared with sham group;
P < 0.05.
Compared with Model group and Vector group;
P < 0.05.
Discussion
Ischemic cerebral injury was one of the common clinical acute cerebral vascular diseases. The main means of the present treatment was to block the subsequent pathological changes of brain tissue penumbra cells by improving the hemodynamics parameters or other means, such as abnormal energy metabolism, electrolyte disturbances, large amount of free radicle release and a large number of apoptosis and necrosis of brain tissue, so as to protect penumbra tissue and cell injury and improve the disease [14-16]. In recent years, there were the expressions of specific genes accompanied by cerebral tissue injury in clinical studies. The expressions of these genes compensatorily protected brain tissue.
VEGF was one of the numerous genes. VEGF was called as vascular endothelial cell growth factor, which could promote angiogenesis and played an important role in the process of physiological and pathological neovascularization [17,18]. Research showed that in acute cerebral infarction model, VEGF expression level in both sides of brain tissue was improved after the blood vessel was blocked for 1 day, among them the highest level was in the edge of infarction [19]. Another study also showed that VEGF expressed in cerebral ischemia center and brain tissue to a certain degree after ischemia for 2 d [20]. Our study also showed the level of VEGF in brain tissue of rats increased compared with sham group after modeling, but which was not statistically significant. This phenomenon suggested that the up-regulation of VEGF in brain tissue might be a compensatory phenomenon and was a self protective mechanism of body, so as to promote neovascularization, increase the blood supply of ischemic tissues and decrease brain tissue apoptosis and necrosis. However this protection mechanism was limited. Therefore exogenous VEGF could achieve the purposes of promoting brain tissue angiogenesis, improving cerebral blood supply, oxygen supply and protect brain function.
The exogenous VEGF mediated by lentivirus was expressed in brain tissue. The results showed that lentivirus could successfully mediate the expression of VEGF plasmid in brain tissue. The expression level of VEGF mRNA and protein in brain tissue of rats increased obviously in VEGF group. Meanwhile the neovascularization density of brain tissue of rats in VEGF group increased significantly by immunohistochemistry, which was significantly higher than those in sham group, model group and vector group, indicating that VEGF plasmid mediated by lentivirus expressed the activated VEGF in brain tissue and promoted neovascularization in brain ischemic tissue. This had the vital significance to improve ischemic brain tissue. The experiment further analyzed the infarct area in brain tissue of rats after different treatments. The results showed that the infarction area of brain tissue of rats in VEGF group significantly decreased than those in model group and vector group, suggesting that neovascularization promoted by VEGF could alleviate cerebral ischemia in a certain extent, thereby to reduce the degree of brain tissue apoptosis and necrosis and reduce the infarct area. Neural function was one of the indicators reflecting brain tissue injury degree. Research results showed that cerebral neural function defect degree of rat in VEGF group was significantly lower than those in model group and vector group, but still higher than that in sham group, suggesting that lentivirus mediated VEGF had a certain protective function on cerebral ischemic injury.
In conclusion, the expression of VEGF mediated by lentivirus could promote neovascularization in brain tissue, improve cerebral ischemia condition in a great extent, thereby to reduce cerebral ischemic injury.
Acknowledgements
This work was supported by the Scientific Research Fund of Xinxiang Medical University (No. 2014QN126) and the Funding Program for Young Backbone Teachers in Colleges and Universities of Henan (No. 2012GGJS-134).
Disclosure of conflict of interest
None.
References
- 1.Fazekas F, Enzinger C, Schmidt R, Dichgans M, Gaertner B, Jungehulsing GJ, Hennerici MG, Heuschmann P, Holzhausen M, Kaps M, Kessler C, Martus P, Putaala J, Ropele S, Tanislav C, Tatlisumak T, Norrving B, Rolfs A sifap1 Investigators. MRI in acute cerebral ischemia of the young: the Stroke in Young Fabry Patients (sifap1) Study. Neurology. 2013;81:1914–1921. doi: 10.1212/01.wnl.0000436611.28210.ec. [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Clarke WR, Grubb RL Jr, Videen TO, Adams HP Jr, Derdeyn CP COSS Investigators. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA. 2011;306:1983–1992. doi: 10.1001/jama.2011.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nardi K, Engelter S, Strbian D, Sarikaya H, Arnold M, Casoni F, Ford GA, Cordonnier C, Lyrer P, Bordet R, Soinne L, Gensicke H, Duriez P, Baumgartner RW, Tatlisumak T, Leys D Lipid Profile in Thrombolysis Study Group. Lipid profiles and outcome in patients treated by intravenous thrombolysis for cerebral ischemia. Neurology. 2012;79:1101–1108. doi: 10.1212/WNL.0b013e3182608c82. [DOI] [PubMed] [Google Scholar]
- 4.Gaire BP, Kim H. Neuroprotective effects of FructusChebulae extracts on experimental models of cerebral ischemia. J Tradit Chin Med. 2014;34:69–75. doi: 10.1016/s0254-6272(14)60057-1. [DOI] [PubMed] [Google Scholar]
- 5.Hanawa K, Ito K, Aizawa K, Shindo T, Nishimiya K, Hasebe Y, Tuburaya R, Hasegawa H, Yasuda S, Kanai H, Shimokawa H. Low-intensity pulsed ultrasound induces angiogenesis and ameliorates left ventricular dysfunction in a porcine model of chronic myocardial ischemia. PLoS One. 2014;9:e104863. doi: 10.1371/journal.pone.0104863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang H, Oh MY, Kim YJ, Choi IY, Yang HS, Ryu WS, Lee SH, Yoon BW. Hydrogen sulfide treatment induces angiogenesis after cerebral ischemia. J Neurosci Res. 2014;92:1520–1528. doi: 10.1002/jnr.23427. [DOI] [PubMed] [Google Scholar]
- 7.Shen F, Fan Y, Su H, Zhu Y, Chen Y, Liu W, Young WL, Yang GY. Adeno-associated viral vector-mediated hypoxia-regulated VEGF gene transfer promotes angiogenesis following focal cerebral ischemia in mice. Gene Ther. 2008;15:30–39. doi: 10.1038/sj.gt.3303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Abe K, Suzuki H, Itoyama Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- 10.Beazley-Long N, Hua J, Jehle T, Hulse RP, Dersch R, Lehrling C, Bevan H, Qiu Y, Lagrèze WA, Wynick D, Churchill AJ, Kehoe P, Harper SJ, Bates DO, Donaldson LF. VEGF-A165b is an endogenous neuroprotective splice isoform of vascular endothelial growth factor A in vivo and in vitro. Am J Pathol. 2013;183:918–929. doi: 10.1016/j.ajpath.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonchar IA, Prudyvus IS, Stepanova IuI. Vascular endothelial growth factor expression in patients with acute ischemic stroke. Zh Nevrol Psikhiatr Im S S Korsakova. 2013;113:25–29. [PubMed] [Google Scholar]
- 12.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 13.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 14.Gong G, Xiang L, Yuan L, Hu L, Wu W, Cai L, Yin L, Dong H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS One. 2014;9:e89450. doi: 10.1371/journal.pone.0089450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G, Wang T, Wang T, Song J, Zhou Z. Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on cerebral ischemia rats. Biomed Rep. 2013;1:861–867. doi: 10.3892/br.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci. 2011;12:7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, Zhao YH. Therapeutic angiogenesis after ischemic stroke: Chinese medicines, bone marrow stromal cells (BMSCs) and their combinational treatment. Am J Chin Med. 2014;42:61–77. doi: 10.1142/S0192415X14500049. [DOI] [PubMed] [Google Scholar]
- 18.Reddy CL, Yosef N, Ubogu EE. VEGF-A165 potently induces human blood-nerve barrier endothelial cell proliferation, angiogenesis, and wound healing in vitro. Cell Mol Neurobiol. 2013;33:789–801. doi: 10.1007/s10571-013-9946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai T, Li M, Zheng L, Song Y, Xu X, Guo Y, Zhang Y, Zhang Z, Mei Y. Over-expression of VEGF in marrow stromal cells promotes angiogenesis in rats with cerebral infarction via the synergistic effects of VEGF and Ang-2. J Huazhong Univ Sci Technolog Med Sci. 2012;32:724–731. doi: 10.1007/s11596-012-1025-3. [DOI] [PubMed] [Google Scholar]
- 20.Plate KH, Beck H, Danner S, Allegrini PR, Wiessner C. Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J Neuropathol Exp Neurol. 1999;58:654–666. doi: 10.1097/00005072-199906000-00010. [DOI] [PubMed] [Google Scholar]


