Abstract
Objective: This study explored the potential of human cord blood mononuclear cell (HCMNC) transplantation as a treatment for premature ovarian failure (POF) in a nude mouse model. Methods: Female nude mice were randomly divided into three groups; a normal control group (n = 35), a POF group (POF plus vehicle, n = 35) and a POF plus cell transplantation group (HCMNCs were implanted into the ovaries, n = 35). HCMNCs were isolated by Ficoll density gradient centrifugation and labeled with BrdU. Four weeks after transplantation, the nude mice were sacrificed to determine serum levels of E2, FSH and LH as indicators of ovarian function, and the ovaries were examined both histologically and immunochemically. Results: The transplanted HCMNCs survived in the transplantation group and were detected by BrdU. In the transplantation group, serum levels of E2 significantly increased while serum levels of FSH and LH significantly decreased compared to the POF control group. Additionally, the transplantation group had a recovery in follicle number. Conclusion: HCMNCs can be successfully transplanted into the ovaries of nude mice and can improve ovarian function in POF.
Keywords: Fetal blood, ovarian function, gonadotropin, therapy
Introduction
Premature ovarian failure (POF) is a heterogeneous disorder defined as cessation of ovarian function with primary and secondary amenorrhea, low estrogen levels (E2 < 91750 pmol/ml) and high gonadotropin levels (FSH > 40 U/L, LH > 30 U/L), before or at 40 years of age [1]. POF used to be termed early menopause and it affects approximately one in 10,000 women by the age of 20 years, one in 1,000 women by the age of 30 years and one in 100 women by the age of 40 years [2], accounting for 10%-28% of primary amenorrhea and 4%-18% of secondary amenorrhea [3]. The etiology of POF is complex, involving genetic, immunologic, metabolic, infectious, environmental and iatrogenic factors; however, most POF disease is idiopathic [4,5]. The pathogenesis of POF is not clear, and there are no efficacious treatments.
Stem cell transplantation therapy utilizing cord blood stem cells is a new therapeutic approach that has been successful in the treatment of many diseases [6-8]. Human cord blood mononuclear cells (HCMNCs) can be used as a source of stem cells for transplantation as they contain a large number of mesenchymal stem cells, endothelial progenitor cells and immature stem/progenitor cells [9,10]. In this study, we examined HCMNC transplantation as a possible therapy for the treatment of POF in a nude mouse model.
Methods
Experimental animals
Female BALB/c nude mice (7 to 8 weeks old) were purchased from the Experimental Animal Center of Second Military Medical University. All mice were kept in the same conditions: body weight (20 ± 3 g); 20°C-22°C; light for 12 hours a day; and an unrestricted diet.
Isolation and labeling of human cord blood mononuclear cells
Ten cord blood samples (60-80 ml per cord) were collected from Department of Obstetrics and Gynecology, Changzheng Hospital, Second Military Medical University, Shanghai, China. All cord blood samples were from infants between 37 to 40 weeks gestational age. Cord blood was excluded if the mother had HIV, syphilis, hepatitis or other infectious diseases, or familial history of hereditary diseases. The blood was collected in triple disposable blood bags containing anticoagulants. The blood was separated within 6 hours after collection. The collected cord blood was diluted 1:1 with Hanks solution (balanced salt solution [g/L]: NaCl 8.00, NaHCO3 0.35, Na2HPO4 0.0477, KH2PO4 0.06, KCl 0.40, d-glucose 1.0, distilled water 1.0 L; pH 7.4), and the diluted solution was spread on 1.077 g/L ficoll-HYpaque separation medium (Sigma, USA) in a 2:1 ratio (v/v). Samples were centrifuged (30 min, 1500 rpm, 4°C), and the mononuclear cell layer was collected and washed twice with PBS (0.01 M, pH 7.4). Before the cells were transplanted, they were labeled with 10 μmol/L BrdU (Sigma) in L-DMEM medium (Sigma) with 10% fetal bovine serum at a 1 × 107/μL cell concentration, and incubated in a 5% CO2 humidified incubator for 24 hours.
Construction of animal models and HCMNC transplantation
BALB/c mice were randomly divided into three groups; Group A was the control group (n = 35), Group B was the POF control group (n = 35) and Group C was the POF + HCMNC transplantation group (n = 35). After 1 week of environmental adaptation, Groups B and C received 0.5 Gr cobalt 60 γ (11)-ray irradiation while Group A remained untreated. 30 days later, 15 mice in each group were sacrificed to determine successful POF development. After confirming the development of POF in Groups B and C, Group B received 10 μL-DMEM (1X high glucose; Gibco, Invitrogen, USA) medium injected into the ovaries while Group C received 10 μL BrdU-labeled HCMNC suspension. Group A received no injections.
Detection of ovarian function indicators FSH, LH and E2
30 days after transplantation, the three groups were sacrificed and serum was collected using standard techniques (3000 rpm centrifugation for 20 min, 4°C). Serum levels of FSH, LH and E2 were determined using ELISA kits (Biosource, USA). Both the intra- and inter-assay variability were < 10%.
Ovarian tissue morphology and follicle count
The ovaries of each nude mouse were measured, weighed, and formalin-fixed for 24 hours. Tissues were embedded in paraffin and cut into 6 μm thick serial sections. Sections were stained with hematoxylin & eosin (HE). Sections from the three groups were examined by light microscopy (400 × magnification), and the number of primordial follicles were counted. This number was multiplied by a constant [6] to calculate the final number of primordial follicles [12]. Additionally, the growth of ovarian follicles, the granulosa cells, and luteal and interstitial histological changes were observed.
Detection of BrdU-labeled cells
Ovarian sections from each group were dewaxed, incubated at room temperature for 10 min in 3% hydrogen peroxide, and washed three times with distilled water. Sections were incubated in 2 mol/L HCl solution at room temperature for 30 min, placed in 2% normal goat serum (Jackson Immunoresearch, West Grove, PA) at 37°C for 10 min, and washed 3 times with PBS. Anti-BrdU monoclonal antibody (Sigma) was added to the sections (1:200), which were incubated at 4°C overnight. The next day, sections were washed 3 times with PBS; subsequently cy3 labeled goat-rat (1:200) was applied according to the manufacturer’s instructions (Sigma). The surviving transplanted cells stained positive for BrdU.
Statistical analysis
SPSS18.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Analysis of variance and pairwise comparisons between groups were used to evaluate measurement data. If the variance was homogeneous, the LSD method was used; if heterogeneous, the Dunnett t-test was used. Fisher’s exact test was used to compare rates. P < 0.05 was considered statistically significant.
Results
HCMNC transplantation reduced estrous cycle disease in POF nude mice
The estrous cycle of normal nude mice averages 4-5 days. We observed 10 estrous cycles in this study. After Cobalt-60 γ-ray irradiation, the estrous cycle of Group A (normal untreated control) nude mice was characterized by the presence of epithelioid cells, keratinocytes, leukocytes as well as alternating cyclical changes. 10% of mice in Group A exhibited estrous cycle disorders, while 90% and 80% of the mice in Group B (POF) and C (POF), respectively, demonstrated estrous cycle disorders. The percentage of mice with estrous cycle disorders was significantly different between Group A and both Groups B (P = 0.001) and C (P = 0.003). Percentages between Groups B and C were not statistically different.
After HCMNC transplantation, Group C (POF, HCMNC-treated) had a small amount of vaginal smear keratinocytes, which gradually increased with time, and epithelial cells, keratinocytes, leukocytes and alternating cyclical changes emerged. The percent of estrous cycle disorders was reduced from 80% to 30%. The percent difference between Groups A (normal, untreated) and B (POF, untreated) was still statistically significant (P = 0.005) (Figure 1). However, Group C no longer demonstrated higher percentages of estrous cycle disorders as compared to normal controls.
Figure 1.
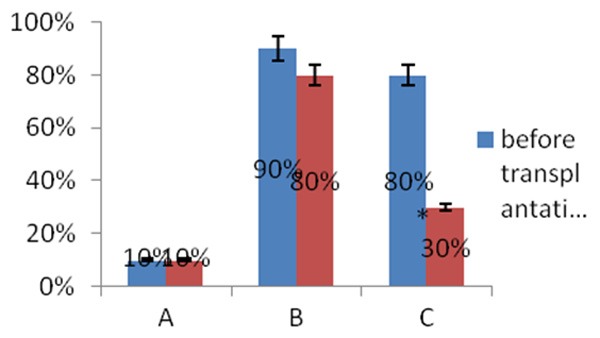
Percent of estrous cycle disorders before and after HCMNC transplantation in mice with POF. Group A, control group (n = 35); Group B, POF control group (n = 35); Group C, POF + HCMNC transplantation group (n = 35). The percent of estrous cycle disorders before and after transplantation were compared between the three groups. HCMNC: human cord blood mononuclear cell; POF: premature ovarian failure.
Ovarian function, as indicated by E2, FSH and LH serum levels, improved after HCMNC transplantation
E2, FSH, and LH are three indicators of ovarian function. Prior to HCMNC transplantation, serum levels of all three indicators in both POF groups (Groups B and C) were significantly different compared to the normal control group (P < 0.0001); serum levels in Group B and C were similar (Table 1).
Table 1.
Serum levels of E2, FSH and LH prior to HCMNC transplantation
| Group | n | E2 (pmol/l) | FSH (U/L) | LH (U/L) |
|---|---|---|---|---|
|
| ||||
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Group A | 35 | 42.504 ± 5.563 | 1.480 ± 0.450 | 5.236 ± 1.213 |
| Group B | 35 | 31.363 ± 4.833 | 2.593 ± 0.673 | 8.896 ± 1.426 |
| Group C | 35 | 32.790 ± 6.901 | 2.587 ± 0.490 | 8.469 ± 2.066 |
| F | 16.226** | 20.651** | 23.195** | |
HCMNC, human cord blood mononuclear cell; F, comparisons between three groups by statistical analysis;
P < 0.01.
Differences in E2, FSH, LH levels between the three groups were statistically significant (P < 0.01). Differences between Groups A and B and Groups A and C were statistically significant (P < 0.0001). Differences between Groups B and C were not statistically significant (P = 0.506, 0.974 and 0.471, all P > 0.05).
After HCMNC transplantation, E2 serum levels were increased and FSH serum levels were decreased in Group C compared to before transplantation (E2: 46.080 ± 5.211 vs. 32.790 ± 6.901; FSH: 1.953 ± 0.276 vs. 2.587 ± 0.490) and Group B E2: 46.080 ± 5.211 vs. 24.704 ± 3.090; FSH: 1.953 ± 0.276 vs. 2.902 ± 0.378). However, LH serum levels were increased in Group C compared to before transplantation (10.798 ± 2.082 vs. 8.469 ± 2.066) and decreased compared to group B (10.798 ± 2.082 vs. 13.052 ± 2.225) (Table 2).
Table 2.
Serum levels of E2, FSH and LH after HCMNC transplantation
| Group | n | E2 (pmol/L) | FSH (U/L) | LH (U/L) |
|---|---|---|---|---|
|
| ||||
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Group A | 35 | 48.773 ± 5.873 | 1.954 ± 0.296 | 10.503 ± 1.130 |
| Group B | 35 | 24.704 ± 3.090 | 2.902 ± 0.378 | 13.052 ± 2.225 |
| Group C | 35 | 46.080 ± 5.211 | 1.953 ± 0.276 | 10.798 ± 2.082 |
| F | 146.553** | 58.853** | 11.041** | |
HCMNC, human cord blood mononuclear cell; F, comparisons between three groups by statistical analysis;
P < 0.01.
Differences in E2, FSH, LH levels between the three groups were statistically significant (P < 0.01). Differences between Groups A and B and Groups B and C were statistically significant (P < 0.0001). Differences between Groups A and C were not statistically significant (P = 0.918, 0.996 and 0.621, all P > 0.05).
Ovarian morphology and number of ovarian follicles changed after HCMNC transplantation
Prior to transplantation, the number of ovarian follicles in Groups B and C were significantly decreased compared to Group A. Group A had well-developed ovarian follicles in large quantities and even demonstrated a slight increase in the number of atretic follicles. In Groups B and C, early ovarian primordial follicle growth significantly reduced the number of follicles (all P < 0.001). The number of follicles in Groups A, B and C were 816.000 ± 96.561, 303.333 ± 29.019 and 321.200 ± 45.631, respectively. There were no significant differences in the number of follicles between Groups B and C.
After HCMNC transplantation, the ovaries of Groups B (untreated) and C (treated) were characterized by follicles at different developmental stages. Group B had diminished partial normal ovarian tissue and an increased number of atretic follicles, antral follicles and mature follicles, while the number of corpus lutea were significantly reduced compared to before transplantation. Conversely, Group C had an increased number of atretic follicles, showing a small number of different developmental stages including mature follicles and corpus lutea (Figure 2).
Figure 2.
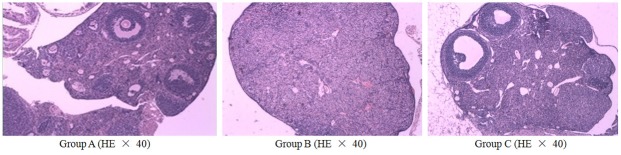
Ovarian follicle development in POF mice after HCMNC transplantation. Ovaries from untreated normal controls (Group A), untreated POF (Group B) and treated POF mice were HE stained to demonstrate follicle development. POF: premature ovarian failure; HCMNC: human cord blood mononuclear cell; HE: hematoxylin and eosin staining; POF: premature ovarian failure. Scale bar = 200 μm.
Additionally, the number of follicles was increased in Group C (750.300 ± 67.081) compared to Group B (325.800 ± 34.114) (P < 0.001). After transplantation, the number of follicles in Group C was no longer different from that of the normal untreated group (Group A). The number of follicles in Group A remained unchanged (785.200 ± 71.782).
BrdU-positive cells were detectable in the ovaries of HCMNC transplanted POF nude mice
BrdU-labeled HCMNCs were detected in the ovaries of transplanted mice using immunohistochemical staining (Figure 3). Thus, HCMNCs survived ovarian transplantation.
Figure 3.
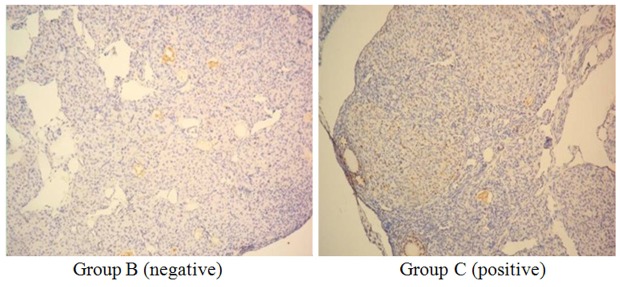
Detection of BrdU-labeled HCMNCs in the ovaries of HCMNC-untreated (Group B) and -treated (Group C) POF mice. Immunohistochemical examination showed that cells labeled with BrdU were not retained in group B, but were present in group C. BrdU was located in the nucleus as a brown granular or diffuse distribution. HCMNC: human cord blood mononuclear cell; POF: premature ovarian failure. Scale bar = 200 μm (× 100).
Discussion
Currently, the incidence of POF is increasing and POF is developing at a younger age [13]. The etiology of POF is complex and its pathogenesis is not clear; there is no effective treatment for this condition. Although hormone replacement therapy is commonly used to relieve symptoms, ovarian function in women affected by POF is not recovered and women often have adverse reactions to long-term hormone therapy [14,15]. In recent years, transplantation of live ovarian tissue has been successful in animal experiments. However, there are many technical difficulties that need to be resolved before human trials can be initiated.
Stem cell transplantation is a new method of treatment, accepted by the majority of researchers. HCMNCs are readily available, are a rich source of a multitude of progenitor and stem cells and have multi-potent differentiation capacity. Thus, with low immunogenicity, HCMNCs could allow stem cell transplantation to achieve a broader application [2,3]. Indeed, Leor et al. demonstrated that HCMNC transplantation improved myocardial infarction in a nude mouse model [16]. Large animal model experiments confirm that HCMNC transplantation can significantly improve the progression of nerve injury, such as stroke, muscle atrophy, Parkinson’s disease, Alzheimer’s disease and spinal cord injury [17]. Therefore, HCMNCs can be used for stem cell transplantation and potentially be utilized in the treatment of POF.
In this study, HCMNCs were injected directly into the ovaries of POF nude mice. HCMNC transplantation in POF mice led to increased ovarian function, as indicated by increased serum E2 levels and decreased serum FSH and LH levels, compared to untreated POF mice. The number of atretic follicles was similar to that of untreated normal controls. Taken together, these data demonstrate that ovarian function can be restored in POF mice with HCMNC transplantation, suggesting that mononuclear cells may be involved in radiation-induced ovarian injury and ovarian vascular repair.
Additionally, we found BrdU-labeled HCMNCs in the ovaries of HCMNC transplanted POF mice, confirming that some HCMNCs were alive in nude mice ovaries and can survive the transplantation process. Cord blood contains a large number of T suppressor cells, which can inhibit the secretion of soluble factors and non-specifically suppress host immune responses. Furthermore, with a strong differentiation potential, cord blood stem cells may induce tolerance in mouse recipients and allow for functional transplantation into nude mice.
HCMNCs have unmatched advantages that other stem cell sources do not. Cord blood mononuclear cells are readily available, easy to amplify and possess a robust differentiation potential [18,19]. Our results show that HCMNC transplantation can repair the ovarian function of POF mice, potentially by replacing damaged ovarian cells. Therefore, HCMNC transplantation may be a novel therapy for the treatment of POF. Understanding the mechanisms by which HCMNCs promote ovarian repair in POF warrants further investigation.
Disclosure of conflict of interest
None.
References
- 1.Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410. doi: 10.1093/humupd/dmi012. [DOI] [PubMed] [Google Scholar]
- 2.Van Kasteren YM. Premature ovarian failure. Ned Tijdschr Geneeskd. 2000;144:2142–2146. [PubMed] [Google Scholar]
- 3.Laml T, Schul Z, Lobmeyr I. Obrucaetal premature ovarian failure etiology and prospects. Gynecol Endocrinol. 2010;14:292. doi: 10.3109/09513590009167696. [DOI] [PubMed] [Google Scholar]
- 4.Beck-Peccoz P, Persani L. Premature ovarian failure. Orphanet J Rare Dis. 2006;1:9. doi: 10.1186/1750-1172-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rebar RW. Premature ovarian “failure” in the adolescent. Ann N Y Acad Sci. 2008;1135:138–145. doi: 10.1196/annals.1429.000. [DOI] [PubMed] [Google Scholar]
- 6.Kurtzberg J, Laughlin M, Graham M, Smith C, Olson JF, Halperin EC, Ciocci G, Carrier C, Stevens CE, Rubinstein P. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 7.Prasad VK, Kurtzberg J. Emerging trends in transplantation of inherited metabolic diseases. Bone Marrow Transplant. 2008;41:99–108. doi: 10.1038/sj.bmt.1705970. [DOI] [PubMed] [Google Scholar]
- 8.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, Verfaillie CM, Wagner JE. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 9.Hirata Y, Sata M, Motomura N, Takanashi M, Suematsu Y, Ono M, Takamoto S. Human umbilical cord blood cells improve cardiac function after myocardial infarction. Biochem Biophys Res Commun. 2005;327:609–614. doi: 10.1016/j.bbrc.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 10.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 11.Gosden RG, Wade JC, Fraser HM, Sandow J, Faddy MJ. Impact of congenital or experimental hypogonadotrophism on the radiation sensitivity of the mouse ovary. Hum Reprod. 1997;12:2483–2488. doi: 10.1093/humrep/12.11.2483. [DOI] [PubMed] [Google Scholar]
- 12.White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–21. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilly JL. Ovarian follicle counts not as simple as 1, 2, 3. Reprod Biol Endocrinol. 2003;1:11. doi: 10.1186/1477-7827-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blumenfeld Z. Fertility treatment in women with premature ovarian failure. Expert Rev Obstet Gynecol. 2011;6:321–30. [Google Scholar]
- 15.Tartagni M, Cicinelli E, De Pergola G, De Salvia MA, Lavopa C, Loverro G. Effects of pretreatment with estrogens on ovarian stimulation with gonadotropins in women with premature ovarian failure: a randomized, placebo-controlled trial. Fertili Steril. 2007;87:858–861. doi: 10.1016/j.fertnstert.2006.08.086. [DOI] [PubMed] [Google Scholar]
- 16.Leor J, Guetta E, Feinberg MS, Galski H, Bar I, Holbova R, Miller L, Zarin P, Castel D, Barbash IM, Nagler A. Human umbilical cord blood2derived CD133 + cells enhance function and repair of the infarcted myocardium. Stem Cells. 2006;24:772–780. doi: 10.1634/stemcells.2005-0212. [DOI] [PubMed] [Google Scholar]
- 17.Said RS, Nada AS, El-Demerdash E. Sodium selenite improves folliculogenesis in radiation-induced ovarian failure: a mechanistic approach. PLoS One. 2012;7:e50928. doi: 10.1371/journal.pone.0050928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kogler G, Trapp T, Critser P, Yoder M. Future of cord blood for non-oncology uses. Bone Marrow Transplant. 2009;44:683–97. doi: 10.1038/bmt.2009.287. [DOI] [PubMed] [Google Scholar]
- 19.Jurga M, Markiewicz I, Sarnowska A, Habich A, Kozlowska H, Lukomska B, Buzanska L, Domanska-Janik K. Neurogenic potential of human umbilical cord blood: neural-like stem cells depend on previous long-term culture conditions. J Neurosci Res. 2006;83:627. doi: 10.1002/jnr.20766. [DOI] [PubMed] [Google Scholar]


