Abstract
Several studies have shown that CNS provides the regulation of gastric functions. Recent evidence indicated that the activation of melanocortin 4 receptors (MC4R) in brain nuclei played an important role in modulating gastric activity. This study was designed to assess whether MC4R signaling existed in autonomic circuitry modulated the activity of stomach by a virally mediated transsynaptic tracing study. Pseudorabies virus (PRV)-614 was injected into the ventral stomach wall in adult male MC4R-green fluorescent protein (GFP) transgenic mice (n = 5). After a survival time of 5 days, the mice were assigned to humanely sacrifice, and spinal cords and caudal brainstem were removed and sectioned, and processed for PRV-614 visualization. Neurons involved in the efferent control of the stomach were identified following visualization of PRV-614 retrograde tracing. The neurochemical phenotype of MC4R-GFP-positive neurons was identified using fluorescence immunocytochemical labeling. PRV-614/MC4R-GFP dual labeled neurons were detected in spinal IML and the dorsal motor nucleus of the vagus nerve (DMV). Our findings support the hypothesis that MC4R signaling in autonomic circuitry may participate in the modulation of gastric activity by the melanocortinergic-sympathetic pathway or melanocortinergic-parasympathetic pathway.
Keywords: Stomach, Melanocortin-4 receptor, spinal cord, autonomic nervous system, pseudorabies virus, transsynaptic tracing
Introduction
Knowledge on the neural bases of gastric function may help to explain many mechanisms associated with the gastrointestinal phenomena, e.g., gastric stress responses, gastric acid secretion and motility [1,2]. The past two decades have witnessed an explosion in the recognition of autonomic circuitry involved in gastric function [3-5]. Several studies have shown that the CNS cell groups that project to the gastric sympathetic preganglionic neurons were identified by the viral retrograde transneuronal labeling method [6-9]. Card et al used synapse-dependent retrograde transneuronal transport of pseudorabies virus (PRV) to trace autonomic emotional motor circuit development from the stomach wall to CNS [10]. Gao et al used PRV-152 expressed EGFP to inject into the stomach wall, and found that neurons expressed EGFP 60-72 h subsequent to PRV-152 inoculation of vagal terminals in the stomach wall were targeted in transverse brainstem slices [8].
Recent evidence indicated that the activation of melanocortin 4 receptors (MC4R) in brain nuclei played an important role in modulating gastric activity [11]. It is known that vagal afferents regulate energy balance by providing a link between the CNS and stomach [12]. In present study, we designed to assess whether MC4R signaling existed in autonomic circuitry modulated the activity of stomach by a virally mediated transsynaptic tracing study [13-16]. We seek to map the polysynaptic pathways between stomach and MC4R-expressing regions in the brainstem and spinal cord, using pseudorabies virus (PRV)-614 in MC4R-green fluorescent protein (GFP) transgenic mice.
Materials and methods
Animals
Male transgenic MC4R-GFP mice weighing between 25 g and 30 g (n = 5), which were first obtained from Dr. Joel Elmquist (UT Southwestern Medical Center, USA), were used for these experiments. Mice were genotyped as described by Rossi and colleagues [17]. Mice were housed under controlled conditions (12 h alternating light-dark cycle, food and water ad libitum). Experimental procedures and protocols were approved in advance by the local Animal Care and Use Committee.
Microinjection of virus into the ventral stomach wall
PRV-614 was generated by the Enquist laboratory at Princeton University and was made available through the Center for Neuroanatomy with Neurotropic Viruses (NIH P40 OD010996). PRV-614 was microinjected into the ventral stomach wall on male transgenic MC4R-GFP mice using a previously described approach [6,18]. After mice were anesthetized with isoflurane (1.5%-2%), a small transverse incision was made to expose the ventral stomach wall for injection under direct vision, and 2 μl injections of PRV-614 were inserted into the ventral stomach wall (0.5 μl per injection at 4 injection sites per one mouse). The incision was closed using tissue glue. To minimize discomfort during and after PRV-614 infection, the animals were treated with ketoprofen (3 mg/kg).
Fluorescence immunohistochemistry and tissue analysis
At 5 d after PRV-614 injection into the ventral stomach wall, he animals were humanely sacrificed under deep anesthesia with ketamine hydrochloride and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde-borate fixative (pH 9.5). Spinal cords and caudal brainstem were removed and sectioned into 30 μm coronal sections.
To determine which PRV-614-immunoreactive (IR) neurons coexpress MC4R-GFP, distinct fluorophores were used for PRV-614 and GFP. PRV-614 infected neurons express the red fluorescent protein for direct visualization under fluorescence microscope. Immunofluorescence studies were carried out to determine expression of MC4R-GFP in IML and DMV slices. according to published protocols [19,20]. The sections were pre-incubated for 1 h in 2% normal donkey serum followed by incubation for 24 h with a chicken polyclonal anti-GFP (1:1000) primary antibodies in 0.01 M PBS containing 0.5% Triton-X 100 at 4°C. Slices were then washed with PBS 3 times for 10 minutes and incubated for 2 h in Alexafluor 488-conjugated anti-chicken IgG (1:1000); then they were washed several times at room temperature. Sections were washed, mounted onto slides and cover slipped with mounting media.
To identify immunohistochemical co-localization of MC4R-GFP and stomach-related neurons, an Olympus IX81 photomicroscope was used. Double labeled neurons were merged by using Adobe Photoshop. These data were obtained from at least 4-5 sections.
Results
MC4R expression in the IML of spinal cord and in the DMV of brainstem
A typical example of the distribution of MC4R-GFP and the summary of MC4R-GFP immunostaining within the IML of spinal cord and in the DMV of brainstem is shown in Figures 1A and 2C. Prominent MC4R-GFP labeling was present in the DMV and the nucleus of the solitary tract (NTS). MC4R-GFP immunofluorescent labeling was more abundant in the DMV compared to the NTS. To test the specificity of the MC4R-GFP staining, we performed staining without a primary or secondary antibody. In both cases, staining was not observed.
Figure 1.
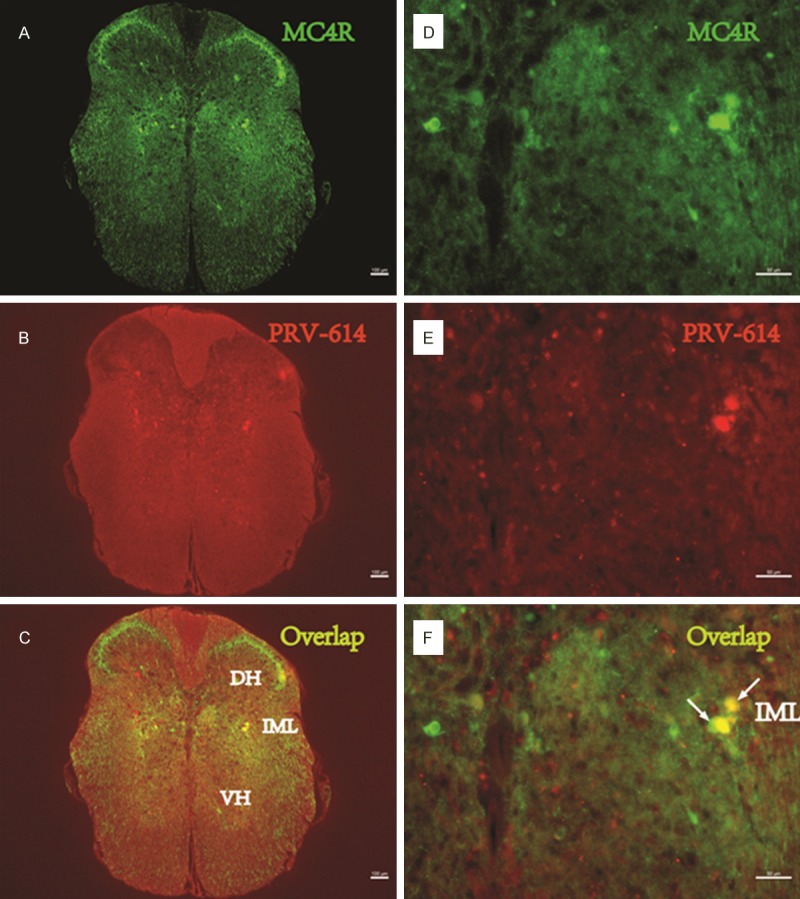
PRV-614/MC4R-GFP double-labeled neurons in the spinal cord. Images (A-C) were taken from an animal after injections of PRV-614. (A) Showed MC4R-GFP positive neurons in the spinal cord; (B) Showed neurons infected with PRV-614, which send transsynaptic projections to the stomach; (C) Showed overlaid images of (A) plus (B). Images (D-F) amplified views of (A-C), respectively. Arrows (white) indicate double-labeled neurons. IML, the intermediolateral cell column; DH, Dorsal horn; VH, ventral horn. Scale bars, 50 μm.
Figure 2.
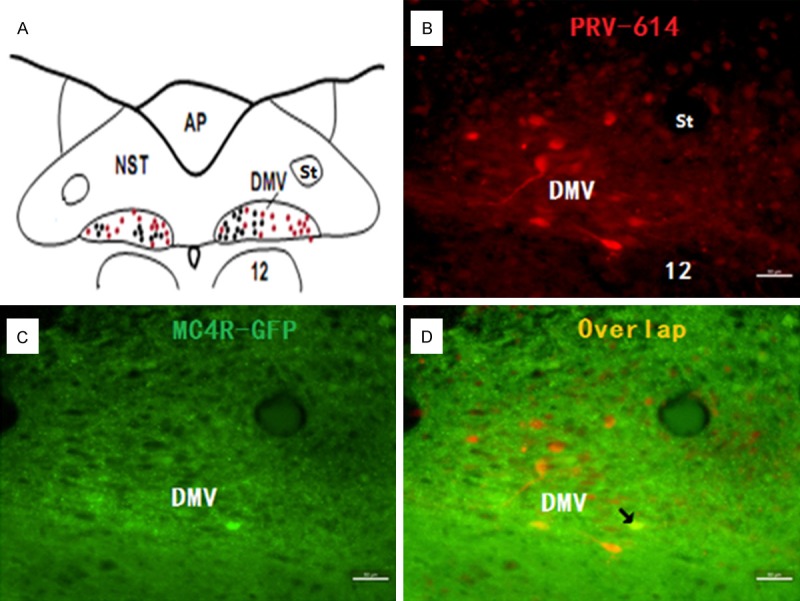
PRV-614/MC4R-GFP double-labeled neurons in the dorsal motor nucleus of the vagus nerve (DMV). Image (A) taken from Toshiko Tsumori (Brain Res 2014) indicates the approximate location from which the images were digitized. Images (B, D) were taken from an animal after injections of PRV-614. Image (B) showed neurons infected with PRV-614, which send transsynaptic projections to the stomach; Image (C) showed MC4R-GFP positive neurons in the DMV; Image (D) showed overlaid images of (B) plus (C). Arrows indicate double-labeled neurons. DMV, dorsal motor nucleus of the vagus nerve; 12, hypoglossal nucleus. Scale bars, 50 μm.
Identification of stomach-related mouse DMV and IML neurons with PRV-614
A retrogradely transported PRV-614, a PRV construct isogenic with PRV Bartha, which expresses red fluorescent protein (RFP), was used to identify stomach-related neurons in the DMV and IML. A set of experiments was conducted to determine the time course of PRV-614 labeling in mice to reveal sufficient labeling of preautonomic stomach-related neurons in the DMV and IML. At 1 and 2 d post-inoculation, we did not observe RFP labeling in either the IML or the DMV. The infection of DMV and IML neurons detected at 5 d after inoculation of the stomach with PRV-614 (Figures 1, 2 and 3). The infection stage following inoculation of the stomach with PRV-614 in mice was consistent with previously published results using PRV Bartha or PRV-614 [6,9].
Figure 3.
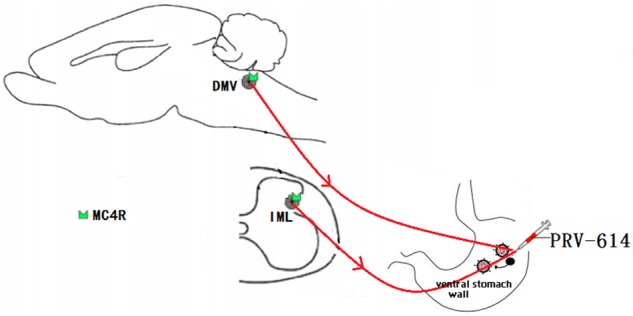
Summary diagram showed that the melanocortinergic pathway between the sympathetic and parasympathetic circuitry involved in gastric function. Dorsal motor nucleus of the vagus nerve (DMV) and intermediolateral column of spinal cord are all participated in the modulation of the gastric activities. It is speculated that IML sympathetic preganglionic neurons control gastric activity by the melanocortinergic-sympathetic signals whereas DMV parasympathetic preganglionic neurons control gastric activity by the melanocortinergic-parasympathetic signals. IML, intermediolateral column; MC4R, the melanocortin-4 receptor; PRV-614, pseudorabies virus-614. Some drawings were taken from HB Xiang (Brain 2013) and L Banihashemi (Neuroscience 2010).
Discussion
In this study, we report the distribution of MC4R-GFP in the two main autonomic centers of the CNS. Two major findings have emerged from this investigation: 1) PRV-614 and MC4R-GFP are expressed in the DMV and IML neurons; 2) Stomach-related DMV and IML neurons express MC4R.
By using PRV-614, we identified stomach-related neurons in the IML and DMV. This approach allowed us to determine expression of MC4R at stomach-related preautonomic neurons. Our results further support the viral fluorescent transneuronal tracer, PRV-614 as a useful tool for labeling a specific neuronal population that have direct or indirect projections to the visceral organs. PRV-614 is used to study neurons of the CNS that have projections to the visceral organs including the kidney, stomach and liver [21-24]. Results from previous work suggested that the spinal IML contained sympathetic preganglionic neurons (SPNs) [6,25]. Our findings demonstrate that PRV-614 and MC4R appear to be co-expressed in the IML of spinal cord, suggesting that MC4R expression in sympathetic circuitry involved in gastric function. This neuroanatomical evidence may underlie a direct central modulation of the sympathetic outflow to stomach by the melanocortins through the MC4R signaling. Our data are also consistent with the prior demonstrations that MC4R expression exist spinal primary afferent neurons [26] and underscoring the role of the melanocortinergic signaling in modulating nociceptive peripheral sensory modalities [27].
It is well known that DMV plays an important role in the central regulation of the parasympathetic nervous system [28]. Our study shows that PRV-614 and MC4R appear to be co-expressed in DMV neurons in the brainstem. Zhang et al reported Gastrointestinal-related neurons in the dorsal vagal complex including DMV in rats [29]. Although immunohistochemical distribution of MC4R in mouse brainstem has been shown previously [5,11,30], this is the first study demonstrating MC4R co-expression with stomach-related PRV-614 in the same DMV neurons, suggesting that MC4R signaling plays a pivotal role in the central regulation of gastric function via the regulation of parasympathetic nervous system. Our data are consistent with previous findings showing MC4R expression in different classes of vagal afferent neurons [27].
In conclusion, our findings revealed MC4R expression at stomach-related preautonomic IML and DMV neurons providing anatomical background for both melanocortinergic signaling and MC4R activation, which potentially can contribute to the central control of the gastric function. However, to establish the role of MC4R in the regulation of gastric function requires further functional investigation.
Acknowledgements
We gratefully acknowledge Dr. Lynn Enquist for kindly providing us with PRV-614 and Dr. Joel Elmquist (UT Southwestern Medical Center) for providing the MC4R-GFP transgenic mice. PRV-614 was generated by the Enquist laboratory at Princeton University and was made available through the Center for Neuroanatomy with Neurotropic Viruses (NIH P40 OD010996).
Disclosure of conflict of interest
None.
References
- 1.Zhang X, Fogel R, Renehan WE. Stimulation of the paraventricular nucleus modulates the activity of gut-sensitive neurons in the vagal complex. Am J Physiol. 1999;277:G79–90. doi: 10.1152/ajpgi.1999.277.1.G79. [DOI] [PubMed] [Google Scholar]
- 2.Tache Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:G173–177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- 3.Grabauskas G, Zhou SY, Das S, Lu Y, Owyang C, Moises HC. Prolactin-releasing peptide affects gastric motor function in rat by modulating synaptic transmission in the dorsal vagal complex. J Physiol. 2004;561:821–839. doi: 10.1113/jphysiol.2004.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz MT, Murphy EC, Sahibzada N, Verbalis JG, Gillis RA. A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am J Physiol Regul Integr Comp Physiol. 2007;292:R291–307. doi: 10.1152/ajpregu.00863.2005. [DOI] [PubMed] [Google Scholar]
- 5.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye DW, Liu C, Tian XB, Xiang HB. Identification of neuroanatomic circuits from spinal cord to stomach in mouse: Retrograde transneuronal viral tracing study. Int J Clin Exp Pathol. 2014;7:5343–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Banihashemi L, Rinaman L. Repeated brief postnatal maternal separation enhances hypothalamic gastric autonomic circuits in juvenile rats. Neuroscience. 2010;165:265–277. doi: 10.1016/j.neuroscience.2009.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao H, Glatzer NR, Williams KW, Derbenev AV, Liu D, Smith BN. Morphological and electrophysiological features of motor neurons and putative interneurons in the dorsal vagal complex of rats and mice. Brain Res. 2009;1291:40–52. doi: 10.1016/j.brainres.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinaman L, Schwartz G. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci. 2004;24:2782–2786. doi: 10.1523/JNEUROSCI.5329-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J Neurosci. 2005;25:9102–9111. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson J, Cruz MT, Majumdar U, Lewin A, Kingsbury KA, Dezfuli G, Vicini S, Verbalis JG, Dretchen KL, Gillis RA, Sahibzada N. Melanocortin signaling in the brainstem influences vagal outflow to the stomach. J Neurosci. 2013;33:13286–13299. doi: 10.1523/JNEUROSCI.0780-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J. Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Comp Neurol. 2010;518:6–24. doi: 10.1002/cne.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng L, Liu TT, Ye DW, Qiu Q, Xiang HB, Cheung CW. Stimulation of the dorsal portion of subthalamic nucleus may be a viable therapeutic approach in pharmacoresistant epilepsy: A virally mediated transsynaptic tracing study in transgenic mouse model. Epilepsy Behav. 2014;31C:114–116. doi: 10.1016/j.yebeh.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Hong Q, Fang G, Liu TT, Guan XH, Xiang HB, Liu Z. Posterior pedunculopontine tegmental nucleus may be involved in visual complaints with intractable epilepsy. Epilepsy Behav. 2014;34C:55–57. doi: 10.1016/j.yebeh.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Ke B, Liu TT, Liu C, Xiang HB, Xiong J. Dorsal subthalamic nucleus electrical stimulation for drug/treatment-refractory epilepsy may modulate melanocortinergic signaling in astrocytes. Epilepsy Behav. 2014;36:6–8. doi: 10.1016/j.yebeh.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Qiu Q, Li RC, Ding DF, Liu C, Liu TT, Tian XB, Xiang HB, Cheung CW. Possible mechanism of regulating glucose metabolism with subthalamic nucleus stimulation in parkinson’s disease: a virally mediated trans-synaptic tracing study in transgenic mice. Parkinsonism Relat Disord. 2014;20:468–470. doi: 10.1016/j.parkreldis.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang HB, Liu C, Liu TT, Xiong J. Central circuits regulating the sympathetic outflow to lumbar muscles in spinally transected mice by retrograde transsynaptic transport. Int J Clin Exp Pathol. 2014;7:2987–2997. [PMC free article] [PubMed] [Google Scholar]
- 19.Pan XC, Song YT, Liu C, Xiang HB, Lu CJ. Melanocortin-4 receptor expression in the rostral ventromedial medulla involved in modulation of nociception in transgenic mice. J Huazhong Univ Sci Technolog Med Sci. 2013;33:195–198. doi: 10.1007/s11596-013-1096-9. [DOI] [PubMed] [Google Scholar]
- 20.Ye DW, Liu C, Liu TT, Tian XB, Xiang HB. Motor cortex-periaqueductal gray-spinal cord neuronal circuitry may involve in modulation of nociception: a virally mediated transsynaptic tracing study in spinally transected transgenic mouse model. PLoS One. 2014;9:e89486. doi: 10.1371/journal.pone.0089486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang HB, Liu C, Guo QQ, Li RC, Ye DW. Deep brain stimulation of the pedunculopontine tegmental nucleus may influence renal function. Med Hypotheses. 2011;77:1135–1138. doi: 10.1016/j.mehy.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Card JP, Kobiler O, McCambridge J, Ebdlahad S, Shan Z, Raizada MK, Sved AF, Enquist LW. Microdissection of neural networks by conditional reporter expression from a Brainbow herpesvirus. Proc Natl Acad Sci U S A. 2011;108:3377–3382. doi: 10.1073/pnas.1015033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Card JP, Kobiler O, Ludmir EB, Desai V, Sved AF, Enquist LW. A dual infection pseudorabies virus conditional reporter approach to identify projections to collateralized neurons in complex neural circuits. PLoS One. 2011;6:e21141. doi: 10.1371/journal.pone.0021141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley S, Pinto S, Segal J, Perez CA, Viale A, DeFalco J, Cai X, Heisler LK, Friedman JM. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc Natl Acad Sci U S A. 2010;107:7024–7029. doi: 10.1073/pnas.1002790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badoer E. Role of the hypothalamic PVN in the regulation of renal sympathetic nerve activity and blood flow during hyperthermia and in heart failure. Am J Physiol Renal Physiol. 2010;298:F839–846. doi: 10.1152/ajprenal.00734.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 27.Gautron L, Lee CE, Lee S, Elmquist JK. Melanocortin-4 receptor expression in different classes of spinal and vagal primary afferent neurons in the mouse. J Comp Neurol. 2012;520:3933–3948. doi: 10.1002/cne.23137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zsombok A, Gao H, Miyata K, Issa A, Derbenev AV. Immunohistochemical localization of transient receptor potential vanilloid type 1 and insulin receptor substrate 2 and their co-localization with liver-related neurons in the hypothalamus and brainstem. Brain Res. 2011;1398:30–39. doi: 10.1016/j.brainres.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Cui J, Tan Z, Jiang C, Fogel R. The central nucleus of the amygdala modulates gut-related neurons in the dorsal vagal complex in rats. J Physiol. 2003;553:1005–1018. doi: 10.1113/jphysiol.2003.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


