Abstract
Dual-specificity phosphatase 5 (DUSP5), which specifically inactivates the extracellular signal-regulated kinase (ERK) 1/2 within the mitogen-activated protein kinase (MAPK) signaling, has recently been considered to be a tumor suppressor. However, its role in prostate cancer is still elusive. In this study, we performed immunohistochemistry analysis on human tissue microarray (TMA) to detect the DUSP5 protein expression pattern. The results indicated that DUSP5 was down-regulated in the human prostate cancer relative to the adjacent benign tissues (IRS: PCa = 4.29 ± 1.72 versus Benign = 4.89 ± 1.58, P = 0.04). In addition, when we linked the DUSP5 protein levels to the clinicopathological features of the patients, we found that the downregulation of DUSP5 was significantly associated with advanced pathological stage (P = 0.004) and high Gleason score (P = 0.009). Moreover, we attempted to validate these findings and investigate the prognostic value of DUSP5 in a publicly available microarray-based Taylor Dataset. Statistic analysis demonstrated that the downregulation of DUSP5 was closely correlated with high Gleason score (P = 0.011), positive metastasis (P < 0.001) and biochemical recurrence (BCR) (P = 0.016). More importantly, Kaplan-Meier analysis revealed that significant differences between patients with high and low DUSP5 expression level in regard to the BCR-free survival of overall (P = 0.009), non-metastatic (P = 0.006) and patients with Gleason score 7 (P = 0.044). Multivariate analysis by Cox regression indicated that DUSP5 could be an independent predictor for the risk of BCR (HR: 0.41, 95% CI: 0.2-0.82; P = 0.012). In summary, our findings disclose that DUSP5 may be an important tumor suppressor that inhibits the progression of PCa. The downregulation of DUSP5 may accurately predict poor prognosis in PCa patients.
Keywords: Prostate cancer, dual-specificity phosphatase 5, tumor suppressor, prognosis
Introduction
Prostate cancer (PCa) represents the second most commonly diagnosed malignancy and the sixth leading cause for cancer related death among men worldwide [1]. The management of prostate cancer is still challenging due to its highly variable natural history. Some indolent cases can be well managed with close follow-up. Studies have reported that 48 cases of prostate cancer were treated only to prevent one death from prostate cancer [2]. On the other hand, aggressive prostate cancer needs active radical treatment along with adjuvant treatment. Thus, it is critical to accurately stratify patients into low-risk and high-risk cases. The traditional methods, including TNM stage, serum prostate-specific antigen (PSA), surgical margin status, lymph node status and Gleason score, in various combinations, have been commonly used to predict the prognosis of PCa patients [3-5]. However, various models based on these factors usually achieve 75-85% accuracy rate for prognosis prediction [6,7]. Especially, growing evidences have shown that PSA screening worldwide have led to indiscriminate over treatment of indolent prostate cancer [8], which is a serious problem because radical treatment is associated with a high morbidity. Therefore, it is urgent to better understand the molecular mechanism underlying the process of PCa progression and develop more accurate predictors to evaluate PCa clinical outcomes.
Dual-specificity phosphatases (DUSPs) are a family of protein tyrosine phosphatases, which inactivate their target kinases by dephosphorylating both the phosphoserine/threonine and phosphotyrosine residues [9]. DUSPs negatively regulate members of the mitogen-activated protein (MAP) kinase superfamily (MAPK/ERK, SAPK/JNK, p38), which serve to integrate information from extracellular signals to the effectors that control diverse cancer-related processes such as proliferation, differentiation, migration and apoptosis [10-14]. The DUSP5 protein product belongs to the DUSP family of phosphatases, which reported to specifically control the extracellular-signal regulated kinase (ERK) [15]. DUSP5 has recently been demonstrated to be a tumor suppressor. So-Hyun Shin et al. reported that the downregulation of DUSP5 in human gastric cancer caused increased maintenance of phosphorylated ERK1/2, which significantly associated with shortened survival of the patients. Moreover, re-expression of DUSP5 remarkably repressed the growth and colony-forming ability of DUSP5-silenced gastric cancer cell lines [16]. DUSP5 also been proven to be the target gene of the typical tumor suppressor P53 and execute its tumor suppressive role via dephosphorylating Erk1/2 in glioblastoma, osteosarcoma and lung cancer [17]. Therefore, DUSP5 is a critical tumor suppressor through negatively controlling the MAPK signaling. However, to date, the role of DUSP5 in human PCa is still unclear.
Here, using a human PCa tissue microarray combined with a publicly available dataset, we showed that DUSP5 was down-regulated in the human PCa. Down-regulation of DUSP5 was closely associated with advanced pathological stage, high Gleason score, positive metastatic outcome and shorter biochemical recurrence (BCR)-free survival. Our study firstly indicated that DUSP5 may inhibit the progression of PCa and serve a critical predictor for the clinical outcome of PCa, which could help doctors make the optimal therapeutic decision.
Materials and methods
Patients and tissue samples
Tissue microarray (TMA, n = 178) including 97 primary PCa tissues and 81 adjacent non-cancerous prostate tissues were obtained from Shanghai Outdo Biotech Co, LTD (Cat No: HPro-Ade180PG-01), along with the detailed clinical information. Patients with known chemotherapy or radiotherapy before the surgery were excluded from the study. The Taylor dataset is a publicly available dataset including 150 primary PCa tissues and 29 adjacent non-cancerous prostate tissues with mRNA microarray expression data [18]. The detailed information on the clinical features of all patients in this study was classified as Table 1.
Table 1.
Clinical features of all patients
| Clinical features | Experiment type | |
|---|---|---|
|
| ||
| Immunohistochemistry | Taylor dataset | |
| Prostate cancer (Cases) | 97 | 150 |
| Mean age | 70.71 ± 8.00 | 58.34 ± 7.07 |
| ≤ 66 | 26 | 125 |
| > 66 | 71 | 25 |
| Serum PSA levels (ng/ml) | ||
| < 4 | - | 24 |
| ≥ 4 | - | 123 |
| Gleason score | ||
| ≤ 6 | 26 | 41 |
| 7 | 43 | 76 |
| ≥ 8 | 28 | 22 |
| Metastasis | 0 | 28 |
| Adjacent benign tissue (Cases) | 81 | 29 |
Note: the “-” means that there are lack of relative information of patients in our cohort. All 150 patients in the Taylor dataset were given a follow-up exam ranging from 1 to 175 months (median, 55 months). For the analysis of survival and follow-up, the date of prostatectomy was used as the beginning of the follow-up period. The primary analysis endpoint for the cohort of patients was time to biochemical recurrence. Other analysis endpoint was overall survival. All the patients who died from diseases other than PCa or unexpected events were excluded from the cohort.
Immunohistochemistry analysis
The specimens were fixed in 10% neutral buffered formalin and subsequently embedded in paraffin. The paraffin-embedded tissues were cut at 4 μm and then deparaffinized with xylene and rehydrated for further peroxidase (DAB) immunohistochemistry staining employing DAKO EnVision System (Dako Diagnostics, Switzerland). Briefly, following a brief proteolytic digestion and a peroxidase blocking of tissue slides, the slides were incubated overnight with the primary antibody against DUSP5 (rabbit polyclonal antibody, bs-13036R, BEIJING BIOSYNTHESIS BIOTECHNOLOGY Co. Ltd., China) at a dilution of 1:200, at 4°C. After washing, peroxidase labeled polymer and substrate-chromogen were then employed in order to visualize the staining of the interested protein. In each immunohistochemistry run, negative controls were carried out by omitting the primary antibody.
Following a hematoxylin counterstaining, immunostaining was scored by two independent experienced pathologists, who were blinded to the clinicopathological data and clinical outcomes of the patients. The scores of the two pathologists were compared and any discrepant scores were trained through re-examining the stainings by both pathologists to achieve a consensus score. The immunolabeling of cancer cells was evaluated. The number of positive-staining cells in ten representative microscopic fields was counted and the percentage of positive cells was also calculated. According to the antibody specification sheet, cytoplasmic staining was regarded as positive signals. Given the heteogenicity of the staining of the target proteins, tumor specimens were scored in a semi-quantitative manner. The percentage scoring of immunoreactive tumor cells was as follows: 0 (0-5%), 1 (6-25%), 2 (26-50%), 3 (51-75%), and 4 (> 75%). The staining intensity was visually scored and stratified as follows: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). A final immunoreactivity scores (IRS) of DUSP5 was obtained for each case by adding the percentage and the intensity score.
Statistical analysis
The version13.0 SPSS for Windows (SPSS Inc, IL, USA) and SAS 9.1 (SAS Institute, Cary, NC) softwares were used for statistical analysis. Continuous variables were expressed as X̅ ± s. Statistical analysis was performed independently by two biostatisticians with Fisher’s exact test for any 2 × 2 tables and Pearson χ2 test for non-2 × 2 tables. Independent-Samples T tests and One-Way ANOVA were performed to examine the associations between DUSP5 expression and clinicopathological characters of PCa patients in Taylor dataset. Kaplan-Meier method was used for the survival analysis and Cox regression analysis was used for the univariate and multivariate analysis. Differences were considered statistically significant when the p value was less than 0.05.
Results
DUSP5 protein is down-regulated in human PCa clinical specimens
Firstly, the immunohistochemical staining using the antibody that specifically recognizes DUSP5 was employed to detect the expression pattern and subcellular localization of DUSP5 protein in 97 PCa and 81 adjacent non-cancerous prostate tissues (Figure 1A). As shown in the Figure 1C-E, with the exception of strong intensity, we found that this antibody stained the cytoplasm and cellular membrane of PCa and benign glandular epithelium cells, giving evenly distributed staining pattern with intermediate, weak and negative intensities. Interestingly, the expression level of DUSP5 protein in PCa tissues was significantly lower than that in adjacent benign prostate tissues (IRS: PCa = 4.29 ± 1.72 versus Benign = 4.89 ± 1.58, P = 0.04) (Figure 1B).
Figure 1.
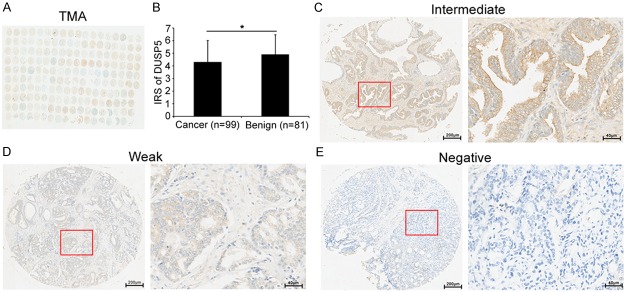
Immunohistochemical staining for DUSP5 in PCa and adjacent non-cancerous prostate tissues in our TMA samples. (A) A full view of the immunohistochemistry staining for DUSP5 in our TMA cohort. (B) The immunoreactivity scores (IRS) of DUSP5 in prostate cancer were lower than that in adjacent benign prostate tissues (IRS: PCa = 4.29 ± 1.72 versus Benign = 4.89 ± 1.58, P = 0.04). Data were presented as Mean ± SEM. *P < 0.05. (C-E) The immunohistochemistry staining indicated that DUSP5 immunostainings mainly occurred in the cytoplasm and cellular membrane of PCa and benign glandular epithelium cells. The intensity of DUSP5 immunostainings was intermediate (C), weak (D) and negative (E), with the exception of strong (Left panel: magnification × 40; right panel: magnification × 200).
We further investigated whether the DUSP5 protein level associated with the clinicopathological features of PCa in our cohort. When the DUSP5 protein expression level in PCa was divided into high level (IRS ≥ 5) and low level (IRS < 5), we found that DUSP5 low expression was significantly associated with the advanced pathological stage (P = 0.004) and high Gleason score (P = 0.009) (Table 2). These findings indicated that DUSP5 may act as a suppressive role in the progression of PCa.
Table 2.
Associations of DUSP5 expression with the clinicopathological characteristics of PCa in two cohorts
| Clinical feature | Tissue microarray in our cohort | Taylor dataset | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Case | Low (n = 65) | High (n = 113) | P | Case | X̅ ± s | P | |
| DUSP5 expression level | |||||||
| Benign | 81 | 23 (35.4) | 58 (51.3) | 0.04 | 29 | 8.49 ± 0.38 | 0.9 |
| Cancer | 97 | 42 (64.6) | 55 (48.7) | 150 | 8.48 ± 0.5 | ||
| Age (years) | |||||||
| < 66 | 26 | 11 (26.2) | 15 (27.3) | 0.9 | 125 | 8.39 ± 0.47 | 0.373 |
| ≥ 66 | 71 | 31 (73.8) | 40 (72.7) | 25 | 8.49 ± 0.51 | ||
| Preoperation PSA (ng/ml) | |||||||
| < 4 | - | - | - | - | 24 | 8.51 ± 0.38 | 0.665 |
| ≥ 4 | - | - | - | 123 | 8.46 ± 0.53 | ||
| Gleason score | |||||||
| ≤ 6 | 26 | 9 (21.4) | 17 (31.5) | 0.009 | 41 | 8.53 ± 0.47 | 0.011 |
| 7 | 44 | 15 (35.7) | 29 (53.7) | 76 | 8.59 ± 0.46 | ||
| ≥ 8 | 26 | 18 (42.9) | 8 (14.8) | 22 | 8.24 ± 0.49 | ||
| Pathological Stage | |||||||
| < T3A | 70 | 24 (57.1) | 46 (83.6) | 0.004 | 86 | 8.52 ± 0.44 | 0.604 |
| ≥ T3A | 29 | 18 (42.9) | 9 (16.4) | 55 | 8.48 ± 0.56 | ||
| Metastasis | |||||||
| No | - | - | - | - | 122 | 8.55 ± 0.46 | < 0.001 |
| Yes | - | - | - | 28 | 8.17 ± 0.58 | ||
| Overall survival | |||||||
| Alive | - | - | - | - | 131 | 8.50 ± 0.49 | 0.271 |
| Die | - | - | - | 19 | 8.36 ± 0.6 | ||
| PSA failure | |||||||
| Negative | - | - | - | - | 104 | 8.57 ± 0.46 | 0.016 |
| Positive | - | - | - | 36 | 8.34 ± 0.52 | ||
Note: the “-” means there are lack of relative information of patients in our cohort.
Decreased expression of DUSP5 associates with the aggressive progression and poor prognosis of human PCa in Taylor dataset
To validate the results of our cohort, a publicly available dataset (Taylor dataset) consisting of 150 primary PCa tissues and 29 adjacent non-cancerous prostate tissues with mRNA microarray expression data [18] was used. The data shown in Table 2 revealed that DUSP5 downregulation was frequently found in PCa tissues with high Gleason score (P = 0.011), positive metastasis failure (P < 0.001) and biochemical recurrence (P = 0.016). However, there were no significant differences of DUSP5 expression in tissue type (cancer versus benign, P = 0.9), preoperation PSA level (< 4 versus ≥ 4, P = 0.665) and pathological stage (< T3A versus ≥ T3A, P = 0.604).
Furthermore, the Kaplan-Meier method was performed to evaluate the prognostic value of DUSP5 expression in PCa. The mean of DUSP5 microarray expression level in all PCa tissues of the Taylor dataset was used as a cutoff to divide the PCa tissues of each group into high and low DUSP5 expression groups. Pairwise comparisons showed a significant difference in the BCR-free survival (Figure 2A) and non-metastatic BCR-free survival (Figure 2B) between patients with high and low DUSP5 expression. Of note, the DUSP5 expression could stratify the patients with Gleason score 7 into high risks and low risks of BCR (Figure 2C). However, the data revealed that there were no significant differences of overall, non-metastatic and patients with Gleason score 7 survival between high and low DUSP5 expression group (Figure 2D-F).
Figure 2.
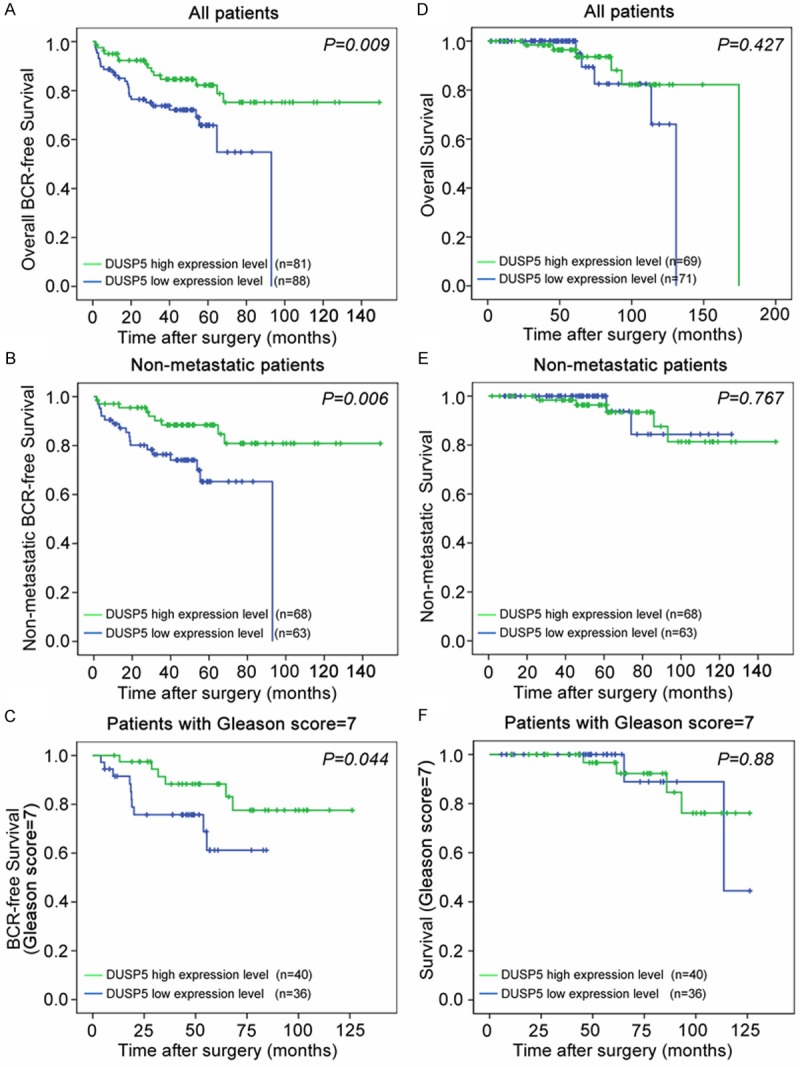
Decreased expression of DUSP5 predicts poor prognosis of PCa patients. A-C. Kaplan-Meier method was performed to evaluate the difference of overall, non-metastatic and patients with Gleason score 7 BCR-free survival between high and low expression levels of DUSP5. D-F. Kaplan-Meier method was performed to evaluate the difference of overall, non-metastatic and patients with Gleason score 7 survival between high and low expression levels of DUSP5. The mean of DUSP5 microarray expression level in PCa tissues of the Taylor dataset was used as a cutoff to divide the PCa tissues of each group into high and low DUSP5 expression groups.
DUSP5 expression serves as an independent predictor for BCR risk of PCa patients
We continued to examine whether DUSP5 expression serves as an independent predictor for BCR risk of PCa patients in the Taylor dataset by performing univariate and multivariate analysis. The results showed that DUSP5 expression level (HR: 0.41, 95% CI: 0.2-0.82; P = 0.012) along with pathological tumor stage (HR: 3.37, 95% CI: 1.61-7.03; P = 0.001) and Gleason score (HR: 5.72, 95% CI: 3.19-10.27; P < 0.001) could serve as independent predictors for BCR risk (Table 3), which suggested that DUSP5 expression could be an independent predictor for BCR risk of PCa patients.
Table 3.
Prognostic value of DUSP5 expression for the biochemical recurrence-free survival in univariate and multivariate analysis by Cox Regression
| Univaviable analysis | Multivaviable analysis | |||
|---|---|---|---|---|
|
|
||||
| HR (95% CI) | P | HR (95% CI) | P | |
| Preoperative PSA | 1.23 (0.52-2.93) | 0.642 | 0.81 (0.33-1.98) | 0.644 |
| Clinical tumor stage | 1.1 (0.578-2.018) | 0.81 | 0.78 (0.4-1.51) | 0.46 |
| Pathological tumor stage | 5.81 (2.95-11.42) | < 0.001 | 3.37 (1.61-7.03) | 0.001 |
| Gleason score | 7.67 (4.38-13.42) | < 0.001 | 5.72 (3.19-10.27) | < 0.001 |
| DUSP5 | 0.42 (0.22-0.82) | 0.011 | 0.41 (0.2-0.82) | 0.012 |
HR, Hazard ratio; CI, confidence interval; Preoperative PSA (ng/ml), between ≤ 4 and > 4; Clinical tumor stage, between < T2A and ≥ T2A; Pathological tumor stage, between T2A-T2C and T3A-T4; Gleason score, among Gleason score ≤ 6, 7 and ≥ 8; DUSP5, continuous DUSP5 expression levels.
Discussion
Prostate cancer remains a serious problem, because it is still difficult to accurately stratify the patients into aggressive and indolent cases according to the commonly-used clinicopathologic factors, which leads to indiscriminate over treatment and high morbidity. Thus, it is critical to investigate the molecular mechanism by which progression and metastasis of PCa appear, so that the optimum strategy can be decided. In this study, we reported that DUSP5 was down-regualted in the human PCa and the downregulation of DUSP5 were significantly associated with aggressive clinicopathologic features of PCa. Moreover, the DUSP5 expression level could serve as an independent factor for predicting the BCR risk of PCa. These findings demonstrated that DUSP5 may have a suppressive role in the progression of PCa and could predict the natural history of PCa more accurately combined with the traditional factors.
Gene dysregulation are common events in PCa. Many of them have been proven to have critical biofunctions in PCa. For example, ERG and TMPRSS2 fusions could be detected in approximately 50% of PCa and the upregulation of ERG promote genes transcription mediating the initiation and progression of PCa [19]. Here, we analyzed the DUSP5 protein levels in a human tissue microarray including 97 primary PCa and 81 adjacent non-cancerous prostate tissues. The results demonstrated that DUSP5 was frequently down-regulated in PCa. However, when we continued to validate this result in another cohort which is a publicly available dataset, we found that there were no significant difference between tumor and benign tissues in relation to DUSP5 mRNA microarray expression data. The mechanism for the downregulation of DUSP5 in cancer could be the hypermethylation of its promoter [16]. The transcription of DUSP5 was reported to be regulated by VEGF [20]. The definite mechanism by which DUSP5 was down-regulated in PCa was needed to be further disclosed.
In the present study, we also investigate the correlations between the DUSP5 protein levels and the clinicopathological features in the TMA. The results indicated that the downregulation of DUSP5 was dramatically associated with advanced pathological stage and high Gleason score. More importantly, these findings were further validated in the Taylor dataset. The results based on the Taylor dataset showed DUSP5 downregulation was associated with positive metastasis and BCR in addition. These findings together suggested that DUSP5 functioned as a tumor suppressor and inhibit the progression of PCa. DUSP5 belongs to dual-specificity phosphatases family and specifically inactivates the ERK [15], which is one of three major groups of mitogen-activated protein kinase (MAPK) family [21]. Abnormal MAPK signalling has been demonstrated to be involved in the processes critical to the development and progression of human cancer [15]. Moreover, growing studies demonstrated that DUSP5 is a tumor suppressor. Rushworth LK et al. reported that DUSP5 suppressed skin cancer by inhibiting the Ras/ERK kinase signaling [22]. In gastric cancer (GC), DUSP5 caused arrest in the transition from G1 to S phase in the cell cycle through dephosphorylating ERK1/2 in the nucleus of GC cells, which decreased the growth and colony-forming ability of the GC cell lines [16]. Furthermore, DUSP5 could inhibit the vascular development, which is a critical event in the process of tumor metastasis. Bellou S et al. reported that DUSP5 could dephosphorylate VEGF-phosphorylated ERK1/2 inhibiting proliferation of endothelial cells [20]. Another group presented that DUSP5 counteracted the function of a serine threonine kinase, Snrk-1, which promotes angioblast development [23]. Taken together, DUSP5 may participate in various cancer-related biological processes via negatively targeting ERK signaling, by which DUSP5 inhibits PCa progression.
To accurately stratify the cases and predict the prognosis is critical to the management of PCa patients. In our study, survival analysis on the Taylor dataset showed that DUSP5 downregulation was closely correlated with shorter overall BCR-free survival and non-metastatic BCR-free survival. Besides, DUSP5 expression could be an independent predictor for the risk of BCR. However, the data revealed that there were no significant differences in overall survival and non-metastatic survival between high and low DUSP5 expression group. The mortalities from other reasons during the long follow-up, such as cardiovascular diseases, may contribute to this result. Cases with Gleason score 7, which are considered to have moderate risk of metastasis or death, commonly turned out aggressive progression. Specially, in our study, we demonstrated that DUSP5 expression level could also stratify the cases with Gleason score 7 into high risk and low risk of BCR, which offered a helpful clue to the doctors.
In conclusion, although the results here require further validation in a multi-institutional cohort and the DUSP5 molecular biological function in PCa needs investigation in the further. The present study extends our knowledge on the prognostic value of DUSP5 in PCa. DUSP5 may become an important molecular node for chemotherapy during the management of PCa.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81170699, 81272813), Projects of Guangdong Key Laboratory of Clinical Molecular Medicine and Diagnostics, Guangzhou Medical Key Subject Construction Project.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Maattanen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A, Investigators E. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 3.Kattan MW, Eastham J. Algorithms for prostate-specific antigen recurrence after treatment of localized prostate cancer. Clin Prostate Cancer. 2003;1:221–226. doi: 10.3816/cgc.2003.n.003. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ Jr, Dotan ZA, DiBlasio CJ, Reuther A, Klein EA, Kattan MW. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J. Clin. Oncol. 2005;23:7005–7012. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kattan MW, Vickers AJ, Yu C, Bianco FJ, Cronin AM, Eastham JA, Klein EA, Reuther AM, Edson Pontes J, Scardino PT. Preoperative and postoperative nomograms incorporating surgeon experience for clinically localized prostate cancer. Cancer. 2009;115:1005–1010. doi: 10.1002/cncr.24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–523. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 7.Capitanio U, Briganti A, Gallina A, Suardi N, Karakiewicz PI, Montorsi F, Scattoni V. Predictive models before and after radical prostatectomy. Prostate. 2010;70:1371–1378. doi: 10.1002/pros.21159. [DOI] [PubMed] [Google Scholar]
- 8.D’Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J. Clin. Oncol. 2003;21:2163–2172. doi: 10.1200/JCO.2003.01.075. [DOI] [PubMed] [Google Scholar]
- 9.Theodosiou A, Ashworth A. MAP kinase phosphatases. Genome Biol. 2002;3:REVIEWS3009. doi: 10.1186/gb-2002-3-7-reviews3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 11.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 12.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 13.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 14.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 15.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–261. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 16.Shin SH, Park SY, Kang GH. Down-regulation of dual-specificity phosphatase 5 in gastric cancer by promoter CpG island hypermethylation and its potential role in carcinogenesis. Am J Pathol. 2013;182:1275–1285. doi: 10.1016/j.ajpath.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Ueda K, Arakawa H, Nakamura Y. Dual-specificity phosphatase 5 (DUSP5) as a direct transcriptional target of tumor suppressor p53. Oncogene. 2003;22:5586–5591. doi: 10.1038/sj.onc.1206845. [DOI] [PubMed] [Google Scholar]
- 18.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 20.Bellou S, Hink MA, Bagli E, Panopoulou E, Bastiaens PI, Murphy C, Fotsis T. VEGF autoregulates its proliferative and migratory ERK1/2 and p38 cascades by enhancing the expression of DUSP1 and DUSP5 phosphatases in endothelial cells. Am J Physiol Cell Physiol. 2009;297:C1477–1489. doi: 10.1152/ajpcell.00058.2009. [DOI] [PubMed] [Google Scholar]
- 21.Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- 22.Rushworth LK, Kidger AM, Delavaine L, Stewart G, van Schelven S, Davidson J, Bryant CJ, Caddye E, East P, Caunt CJ, Keyse SM. Dual-specificity phosphatase 5 regulates nuclear ERK activity and suppresses skin cancer by inhibiting mutant Harvey-Ras (HRasQ61L)-driven SerpinB2 expression. Proc Natl Acad Sci U S A. 2014;111:18267–72. doi: 10.1073/pnas.1420159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pramanik K, Chun CZ, Garnaas MK, Samant GV, Li K, Horswill MA, North PE, Ramchandran R. Dusp-5 and Snrk-1 coordinately function during vascular development and disease. Blood. 2009;113:1184–1191. doi: 10.1182/blood-2008-06-162180. [DOI] [PMC free article] [PubMed] [Google Scholar]


