Abstract
Objective: This study is to investigate the risk factors of portal venous system thrombosis (PVT) in patients with cirrhotic portal hypertension after splenectomy and to establish a Logistic regression prediction model. Methods: A total of 119 patients with cirrhotic portal hypertension were enrolled. Their clinical data was retrospectively analyzed. They were divided into PVT group (n = 18) and non-PVT group (n = 101). One-way analysis and multivariate Logistic regression analysis were performed to analyze the independent risk factors of PVT. Logistic regression prediction model was established. The receiver operating characteristic curve was generated and correlation analysis was conducted. Results: Platelet count (PLT), mean platelet volume (MPV) and D-Dimer were independent risk factors affecting PVT. Anticoagulation therapy (UAT) and usage of reducing portal pressure therapy (URPT) were independent protective factors of PVT. Logistic regression prediction model was expressed as Logit P = -9.165 + 0.664 × PLT (× 1011/L) + 0.413 × MPV (fL) + 0.662 × D-Dimer (mg/L) -1.674 × UAT (Yes = 1, No = 0) -1.518 × URPT (Yes = 1, No = 0). And, the cut-off value of Logit P was -1.14. The area under the receiver operating characteristic curve and the accuracy were 0.865 and 84.03%. The cut-off value of PLT, MPV and D-Dimer were 4.42 × 1011/L, 13.30 fL and 2.55 mg/L, respectively. MPV and D-Dimer were positively correlated. Conclusion: PLT, MPV and D-Dimer are independent risk factors while UAT and URPT are independent protective factors of PVT. Logistic regression prediction model can predict PVT with a high sensitivity, specificity and accuracy. It provides theoretical foundation and cut-off value for predicting PVT after splenectomy.
Keywords: Cirrhosis, portal hypertensive, splenectomy, portal venous system thrombosis, the independent risk factors, the logistic regression predict model
Introduction
Splenectomy is a conventional surgery for the treatment of portal hypertension, hypersplenism, and upper gastrointestinal bleeding. It can significantly improve the prognosis of patients and increase the survival of patients with liver cirrhosis [1-6]. However, splenectomy has many complications. Portal vein thrombosis (PVT) is one of the common and serious complications of splenectomy [7]. If PVT is not timely diagnosed and treated, patient’s prognosis or even life will be seriously affected. Liver obstruction caused by PVT, liver fibrosis and nodular regeneration of liver cells may aggravate portal hypertension, leading to serious complications such as upper gastrointestinal bleeding, intractable ascites, jaundice, ischemic necrosis of the small intestine and hepatic encephalopathy [8].
The application of color Doppler ultrasound, CT and MRI in clinical practice has improved the detection rate of PVT [9]. It was reported [10,11] that the natural incident rate of PVT in patients with liver cirrhosis was 6.6%. The incidence rate of PVT after splenectomy was 18.9%-57% and sometimes was up to 91.1%, significantly higher than the natural incident rate of PVT without surgery. These results indicate that the incidence of PVT in patients underwent splenectomy is relatively high.
The incidence of PVT is occult. The early symptoms of PVT are mild and difficulty to be detected. Thus, the period for treatment of early stage thrombosis with thrombolytic therapy is easily missed. Once there are symptoms of serious complications, ideal interventions for the treatment of PVT are rarely available [12]. Currently, there are no commonly accepted standards to evaluate the risk factors and prevention strategies for PVT [13,14]. Therefore, it is very important to analyze the risk factors of PVT and to establish a Logistic regression prediction model to predict the incidence of PVT. In this study, we collected the clinical data of 119 cases of patients with liver cirrhosis. They had hypersplenism or esophageal varices and were hospitalized in the period from 2009-01-01 to 2013-12-31. They all underwent splenectomy. Their clinical data was retrospectively analyzed to evaluate the independent factors of PVT and to predict the incidence of PVT.
Materials and methods
Subjects
A total of 119 patients with liver cirrhosis and portal hypertension were enrolled in this study. They received splenectomy at Department of General Surgery, the First Affiliated Hospital of Xinjiang Medical University and during the period from 2009-01-01 to 2013-12-31. Their clinical data was complete. Before surgery, all patients had varying degrees of hypersplenism and esophageal varices. No patient had PVT before surgery, as revealed by color Doppler ultrasound or abdominal computed tomography angiography (CTA) examination. At 7 to 9 weeks after surgery, the occurrence of PVT was detected by color Doppler ultrasound or abdominal CTA examination. According to the examination results, patients were divided into PVT group (n = 18) and non-PVT group (n = 101).
Exclusion criteria: liver cirrhosis patients associated with other malignancies; liver cirrhosis patients associated with abdominal trauma (such as splenic and hepatic rupture) which required splenectomy to prevent hemorrhagic shock; liver cirrhosis patients associated with congenital malformations of portal veins (such as vascular intimal cavernous degeneration of portal vein and Budd-Chiari syndrome); liver cirrhosis patients associated with preoperative PVT; liver cirrhosis patients associated with severe underlying diseases (such as cardiovascular diseases and respiratory diseases, etc.); patients with a previous history of pulmonary embolism or deep vein thrombosis; patients with incomplete clinical data.
Prior written and informed content was obtained from every patient. This study was approved by the ethical committee of Xinjiang Medical University.
Data collection
The following data were recorded: gender, age, etiology, combination with diabetes, history of upper gastrointestinal bleeding, degree of esophageal and gastric varices, preoperative platelet and white blood cell count, grade of preoperative hepatic functional reserve, surgical approach, the operative time, blood loss, other serious postoperative complications (such as pulmonary complications, abdominal bleeding, subphrenic infection, abdominal infection, sepsis, pancreatic leakage, upper gastrointestinal bleeding, septic shock, or multiple organ failure), postoperative anticoagulation, hemostasis, use of drugs to lower portal pressure and biochemical data at 7 to 10 days after surgery.
At 7 to 10 days after surgery (in which period that formation of PVT was at a peak period), the biochemical indexes of platelet count (PLT), mean platelet volume (MPV), platelet distribution width (PDW) and D-Dimer were measured and collected. The higher value was used if more than two sets of test results were obtained. The biochemical test results nearest to surgery were defined as baseline values. Hypersplenism was defined when the preoperative platelet count < 80 × 109/L or there were varying degrees of enlarged spleen. The degree of hypersplenism was defined as follows: mild hypersplenism, preoperative platelet count 60-80 × 109/L; moderate hypersplenism, preoperative platelet count 40-60 × 109/L; severe hypersplenism, preoperative platelet count < 40 × 109/L.
Statistical analysis
The data was analyzed with IBM SPSS (version 19, SPSS Statistics/IBM Corp, Chicago, Illinois, USA). Measurement data was expressed as mean ± standard deviation. Count data was presented as the number of cases (n) and percentage (%). The t test and chi-square test was performed to analyze the differences between PVT group and non-PVT group. Multivariate Logistic regression analysis was conducted among multiple factors. Logistic regression prediction model was established and Logit P value was calculated. Receiver operating characteristic curve (ROC curve) was generated. P-P scatter plot was plotted after bivariate linear correlation analysis. P < 0.05 was considered as significant difference.
Results
General information of subjects
The general information of subjects was listed as follows. Among the 119 patients, there were 68 cases (57.1%) of male patients and 51 cases (42.9%) of female patients. They aged 30 to 70 years, with an average age of (50 ± 11) years. For the types of liver cirrhosis, there were 52 cases (43.7%) with liver cirrhosis caused by hepatitis B, 21 cases (17.6%) with liver cirrhosis caused by hepatitis C, 3 cases (2.5%) with alcoholic liver cirrhosis, 5 cases (4.2%) with primary biliary cirrhosis, and 38 cases (32.0%) with cryptogenic cirrhosis patients. For surgical treatment, 13 patients (10.9%) had simple splenectomy, 47 patients (39.5%) received splenectomy and portal-azygous disconnection, and 59 patients (49.6%) underwent splenectomy and pericardial devascularization.
Relationship between clinical features and occurrence of PVT
To determine the relationship between clinical features of patients and occurrence of PVT, clinical features of patients were compared between patients with PVT and without PVT. According to the occurrence of PVT, patients were divided into PVT group (n = 18) and non-PVT group (n = 101). As shown in Table 1, there were significant differences in clinical features of intraoperative blood loss, serious postoperative complications, PLT, MPV, D-Dimer, UAT, and URPT between PVT group and non-PVT group (P < 0.05). No significant difference was found in other clinical features between these two groups. These results suggest that the clinical features of intraoperative blood loss, serious postoperative complications, PLT, MPV, D-Dimer, UAT, and URPT may be related with the occurrence of PVT.
Table 1.
Comparison of clinical features between PVT group and non-PVT group
| Clinical features | PVT group (n = 18) | Non-PVT group (n = 101) | P | |
|---|---|---|---|---|
| Gender | Male/Female | 11 (61)/7 (39) | 57 (56)/44 (44) | 0.712 |
| Age (year) | 46.05 ± 10.73 | 50.13 ± 10.77 | 0.141 | |
| Child-pugh score | Child-pugh A | 6 (33) | 25 (25) | 0.636 |
| Child-pugh B | 12 (67) | 76 (75) | ||
| Upper gastrointestinal hemorrhage | Yes/No | 13 (72)/5 (28) | 53 (52)/48 (48) | 0.120 |
| Diabetes | Yes/No | 3 (17)/15 (83) | 10 (10)/91 (90) | 0.662 |
| Diameter of portal vein (cm) | 1.56 ± 0.27 | 1.43 ± 0.31 | 0.114 | |
| Esophageal and gastric varices | Degree II | 6 (33) | 51 (50) | 0.179 |
| Degree III | 12 (67) | 50 (50) | ||
| Degree of hypersplenism | Mild | 7 (39) | 34 (34) | 0.805 |
| Moderate | 6 (33) | 42 (42) | ||
| Severe | 5 (28) | 25 (24) | ||
| Operation time (min) | 301 ± 64 | 294 ± 42 | 0.535 | |
| Intraoperative blood loss (ml) | 505 ± 53 | 475 ± 58 | 0.042 | |
| Serious postoperative complications | Yes/No | 8 (44)/10 (56) | 15 (15)/86 (85) | 0.009 |
| UAT | Yes/No | 7 (39)/11 (61) | 76 (75)/25 (25) | 0.002 |
| hemostasis | Yes/No | 14 (78)/4 (22) | 75 (74)/26 (26) | 0.982 |
| URPT | Yes/No | 9 (50)/9 (50) | 74 (73)/27 (27) | 0.048 |
| PLT (×1011/L) | 4.21 ± 0.96 | 3.62 ± 0.95 | 0.016 | |
| MPV (fL) | 12.93 ± 1.52 | 11.35 ± 1.86 | 0.001 | |
| PDW (%) | 16.93 ± 3.19 | 15.72 ± 2.43 | 0.066 | |
| D-Dimer (mg/L) | 2.98 ± 1.34 | 2.13 ± 0.92 | 0.017 |
Note: UAT, anticoagulation therapy; URPT, usage of reducing portal pressure therapy; PLT, platelet count; MPV, mean platelet volume; PDW, platelet distribution width.
Analysis of independent factors for PVT
To analyze the independent factors for PVT, multivariate Logistic regression analysis was performed. The analyzed factors were intraoperative blood loss, serious postoperative complications, PLT, MPV, D-Dimer, UAT, and URPT. As shown in Table 2, the factors of PLT, MPV, D-Dimer, UAT, and URPT were significantly different between PVT group and non-PVT group (P < 0.05). Among these factors, PLT, MPV, and D-Dimer were independent risk factors for postoperative PVT occurrence, with OR values > 1. UAT and URPT were independent protective factors for postoperative PVT occurrence, with OR values < 1. These results indicate that PLT, MPV, D-Dimer, UAT, and URPT are independent factors for PVT.
Table 2.
Multivariate logistic regression analysis
| Variables | Regression coefficient (β value) | Standard error (SE) | Wald value | Degree of freedom | P | OR | 95% confidence interval (CI) |
|---|---|---|---|---|---|---|---|
| PLT (×1011/L) | 0.664 | 0.308 | 4.65 | 1 | 0.031 | 1.94 | 1.06 ~ 3.55 |
| MPV (fL) | 0.413 | 0.208 | 3.97 | 1 | 0.046 | 1.51 | 1.01 ~ 2.27 |
| D-Dimer (mg/L) | 0.662 | 0.282 | 5.53 | 1 | 0.019 | 1.94 | 1.12 ~ 3.37 |
| UAT | -1.647 | 0.678 | 5.91 | 1 | 0.015 | 0.19 | 0.05 ~ 0.73 |
| URPT | -1.518 | 0.703 | 4.66 | 1 | 0.031 | 0.22 | 0.06 ~ 0.87 |
| Constant | -9.165 | 3.264 | 7.88 | 1 | 0.005 | 0.00 |
Prediction of PVT with Logistic regression prediction model
To obtain an accurate cut-off value and prediction model for predicting independent factors of PVT after splenectomy, Logistic regression prediction model was established. Based on the results of multivariate Logistic regression analysis, the Logistic regression prediction model was established, which was Logit P = -9.165 + 0.664 × PLT (× 1011/L) + 0.413 × MPV (fL) + 0.662 × D-Dimer (mg/L) -1.674 × UAT (yes = 1, no = 0) -1.518 × URPT (yes = 1, no = 0). The values of Logit P were between -5.3 and 2.8. The bigger Logit P value was, the higher the risk for PVT was. Then, ROC curve was generated based on the Logistic regression prediction model (Figure 1). The area under ROC curve (AUROC) of Logit P, PLT, MPV and D-Dimer was shown in Table 3. The AUROC for Logit P was 0.865, higher than that of PLT, MPV and D-Dimer. The cut-off value for Logit P, PLT, MPV and D-Dimer was -1.14, 4.42 × 1011/L, 13.30 fL, and 2.55 mg/L, respectively. Base on the cut-off value of Logit P, PVT was formed when Logit P ≥ -1.14 and PVT was not formed when Logit P < -1.14. As shown in Table 4, there were 29 patients with Logit P ≥ -1.14. Among these 29 patients, 14 patients had postoperative PVT. The positive prediction value was 48.28% and the sensitivity was 77.78%. Among the 18 cases with PVT, 14 cases were with Logit P ≥ -1.14. There were 90 patients with Logit P < -1.14. And, 86 patients did not have postoperative PVT. The negative prediction value was 95.56%, the specificity was 85.15%, and the total accuracy was 84.03%. Among the 101 cases without PVT, 86 cases were with Logit P < -1.14. These results suggest that Logistic regression prediction model has good sensitivity, specificity and accuracy in predicting PVT.
Figure 1.
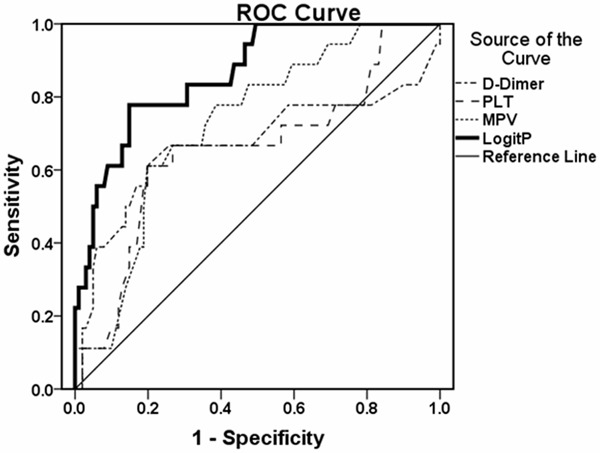
ROC curve. ROC curve was generated based on the Logistic regression prediction model (Figure 1). The area under ROC curve of Logit P, PLT, MPV and D-Dimer was calculated.
Table 3.
AUROC of each predictive factor
| Predictive factors | AUROC | Cut off value |
|---|---|---|
| Logit P | 0.865 (0.780 ~ 0.949) | -1.14 |
| PLT (×1011/L) | 0.655 (0.506 ~ 0.804) | 4.42 |
| MPV (fL) | 0.727 (0.613 ~ 0.842) | 13.30 |
| D-Dimer (mg/L) | 0.670 (0.499 ~ 0.842) | 2.55 |
Note: AUROC, area under the receiver operating characteristic curve.
Table 4.
Prediction of PVT with Logit P
| Cut-off value | Cases | PVT | Sensitivity (%) | Specificity (%) | Positive prediction value (%) | Negative prediction value (%) | Accuracy (%) | AUROC | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Yes | No | ||||||||
| ≥ -1.14 | 29 | 14 | 15 | - | - | - | - | - | (0.780 ~ 0.949) |
| < -1.14 | 90 | 4 | 86 | - | - | - | - | - | - |
| 119 | 18 | 101 | 77.78 | 85.15 | 48.28 | 95.56 | 84.03 | 0.865 | |
Note: -, not indicated.
Linear correlation analysis
To further analyze the relationship among the independent factors of PLT, MPV, D-Dimer, UAT, and URPT, bivariate linear correlation analysis was performed. There was positive correlation between MPV and D-Dimer (r = 0.230, P < 0.05) (Figure 2). However, no correlation was found among other factors (data not shown). These results indicate that MPV is positively correlated with D-Dimer.
Figure 2.
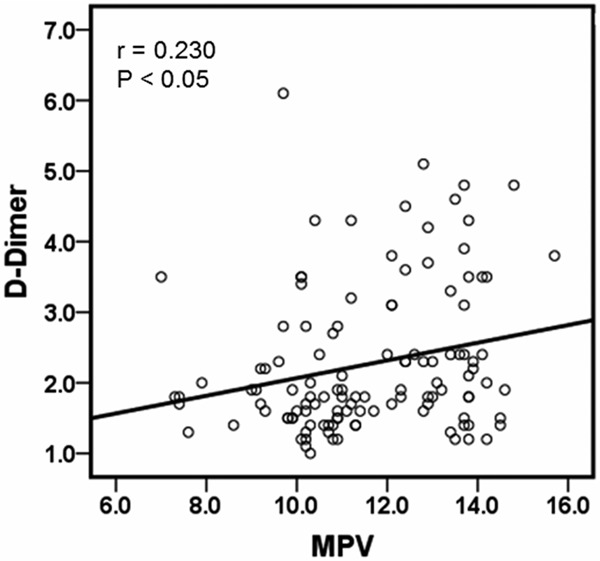
Linear correlation analysis between MPV and D-Dimer. Bivariate linear correlation analysis was performed to analyze the correlation between MPV and D-Dimer. P-P scatter plot was plotted after bivariate linear correlation analysis.
Discussion
Studies [15-17] find that MPV is closely related with platelet activity. Platelets with larger volumes contain more dense granules, a- particles and highly active proteases than small platelets. When platelets with larger volumes are activated, they can release more thrombotic precursor material, thus increasing the risk of thrombosis. Enlarged MPV is considered to be a sign of increased platelet activity. And, enlarged MPV reflects the level of vascular injury and inflammation in the body. Vascular endothelial cell injury can release thromboxane and endothelin, which activates the extrinsic coagulation system to form thrombosis. Under normal circumstances, the aging platelets and red blood cells can be removed by the spleen. After splenectomy, platelets increase rapidly in the short term and the blood is in a hypercoagulable state, thereby increasing the risk of thrombosis. D-Dimer is a specific marker of the fibrinolytic process. It is reported that [18-20] D-Dimer can be used as a very important and sensitive diagnostic indicator of thrombosis. However, the correlation among occurrence of PVT, enlarged MPV and PVT after splenectomy is rarely studied.
In this study, intraoperative blood loss and other serious postoperative complications were significantly different between PVT group and non-PVT group, as shown by univariate analysis. However, the multivariate Logistic regression analysis showed no significant difference, suggesting that they were independent risk factors. PLT, MPV, and D-Dimer were independent risk factors of PVT after splenectomy. At 7 to 10 days after splenectomy, the occurrence of PVT would increase when PLT ≥ 4.42 × 1011/L, MPV ≥ 13.30 fL, and D-Dimer ≥ 2.55 mg/L. To the best of our knowledge, this is the first study to report MPV as an independent risk factor for PVT. Our results also showed that there was a positive correlation between MPV and D-Dimer. This may be because that MPV is enlarged and risk of thrombosis is increased after splenectomy. Thus, as a thrombolytic product and an important indicator of thrombosis, D-Dimer will increase accordingly.
Multivariate Logistic regression analysis found that UAT was an independent protective factor for PVT. This result was consistent with previous reports [21-23]. For example, Rosario et al [21] considered that the low molecular weight heparin was valuable in anticoagulation prophylaxis of people at higher risk for thrombosis. The selective anticoagulant activity and high bioavailability of low molecular weight heparin has been widely used in clinical treatment of thrombosis and prevention of thrombotic disease. And, due to the high selectivity of the low molecular weight heparin, the risk of bleeding is greatly reduced compared with unfractionated heparin. Furthermore, we reported for the first time that URPT was also an independent protective factor for PVT within 2 month after surgery. This result suggests that using drugs to lower the pressure of portal vein is necessary and possible.
In conclusion, Logit P ≥ -1.14 indicates a higher risk of PVT after splenectomy. The high sensitivity, specificity and accuracy of the prediction model suggest that the prediction model is of certain value in predicting PVT. Our study provides a good prediction model and cut-off value for the prevention of thrombosis after splenectomy. The predictive value of this prediction model needs to be further verified.
Acknowledgements
This work was supported by the Science and Technology Support Foundation of Xinjiang Uygur Autonomous Region (No. 201141137) and the National Natural Science Foundation of China (No. 81360138).
Disclosure of conflict of interest
None.
References
- 1.Shimada M, Hashizume M, Shirabe K, Takenaka K, Sugimachi K. A new surgical strategy for cirrhotic patients with hepatocellular carcinoma and hypersplenism. Performing a hepatectomy after a laparoscopic splenectomy. Surg Endosc. 2000;14:127–30. doi: 10.1007/s004649900082. [DOI] [PubMed] [Google Scholar]
- 2.Kercher KW, Carbonell AM, Heniford BT, Matthews BD, Cunningham DM, Reindollar RW. Laparoscopic splenectomy reverses thrombocytopenia in patients with hepatitis C cirrhosis and portal hypertension. J Gastrointest Surg. 2004;8:120–6. doi: 10.1016/j.gassur.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi PH, Mehia C, Joachim Reimers H, Solomon HS, Bacon BR. Splenectomy for thrombocytopenia in patients with hepatitis C cirrhosis. J Clin Gastroenterol. 2006;40:740–4. doi: 10.1097/00004836-200609000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Yoshizumi T, Taketomi A, Soejima Y, Ikegami T, Uchiyama H, Kayashima H, Harada N, Yamashita Y, Kawanaka H, Nishizak T, Maehara Y. The beneficial role of simultaneous splenectomy in living donor liver transplantation in patients with small-for-size graft. Transpl Int. 2008;21:833–42. doi: 10.1111/j.1432-2277.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 5.Hirooka M, Ishida K, Kisaka Y, Uehara T, Watanabe Y, Hiasa Y, Michitaka K, Onji M. Efficacy of splenectomy for hypersplenic patients with advanced hepatocellular carcinoma. Hepatol Res. 2008;38:1172–7. doi: 10.1111/j.1872-034X.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 6.Morihara D, Kobayashi M, Ikeda K, Kawamura Y, Saneto H, Yatuji H, Hosaka T, Sezaki H, Akuta N, Suzuki Y, Suzuki F, Kumada H. Effectiveness of combination therapy of splenectomy and long-term interferon in patients with hepatitis C virus-related cirrhosis and thrombocytopenia. Hepatol Res. 2009;39:439–47. doi: 10.1111/j.1872-034X.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 7.Mei B, Liu FL, Chen XP, Chen YF. Prevention of low molecular weight heparins, which was early administrated after devascularization operation, on thrombosis of portal vein. Chinese J Exp Surg. 2005;22:1467–1468. [Google Scholar]
- 8.Ni XL, Gu DY, Hu GH. Effects of alprostadil in the prevention of portal vein thrombosis after splenectomy and devascularization: a historical control study. Chinese J Hepatobiliary Surg. 2014;20:351–354. [Google Scholar]
- 9.Ni SY, Fan YH, Cai LJ, Lv B. Extensive Portal Vein Thrombosis 1 Year after Splenectomy in A Cirrhotic Patient. Chinese J Gastroenterol. 2012;17:447–448. [Google Scholar]
- 10.de Cleva R, Herman P, Saad WA, Pugliese V, Zilberstein B, Rodrigues JJ, Laudanna AA. Postoperative portal vein thrombosis in patients with hepatosplenic mansonic schistosomiasis: relationship with intraoperative portal pressure and flow. A prospective study. Hepatogastroenterology. 2005;52:1529–33. [PubMed] [Google Scholar]
- 11.Ferreira FG, Chin EW, Santos M de F, de Carvalho DL, De Capua Junior A. Portal congestion and thrombosis after esophagogastric devascularization and splenectomy. Rev Assoc Med Bras. 2005;51:233–6. doi: 10.1590/s0104-42302005000400021. [DOI] [PubMed] [Google Scholar]
- 12.Winslow ER, Brunt LM, Drebin JA, Soper NJ, Klingensmith ME. Portal vein thrombosis after splenectomy. Am J Surg. 2002;184:631–5. doi: 10.1016/s0002-9610(02)01095-4. discussion 635-6. [DOI] [PubMed] [Google Scholar]
- 13.Francoz C, Belghiti J, Vilgrain V, Sommacale D, Paradis V, Condat B, Denninger MH, Sauvanet A, Valla D, Durand F. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54:691–7. doi: 10.1136/gut.2004.042796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanazaki K, Kajikawa S, Adachi W, Amano J. Portal vein thrombosis may be a fatal complication after synchronous splenectomy in patients with hepatocellular carcinoma and hypersplenism. J Am Coll Surg. 2000;191:341–2. doi: 10.1016/s1072-7515(00)00364-1. [DOI] [PubMed] [Google Scholar]
- 15.Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, Mohler ER, Reilly MP, Berger JS. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–56. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 17.Margetic S. Inflammation and haemostasis. Biochem Med (Zagreb) 2012;22:49–62. [PMC free article] [PubMed] [Google Scholar]
- 18.Stamou KM, Toutouzas KG, Kekis PB, Nakos S, Gafou A, Manouras A, Krespis E, Katsaragakis S, Bramis J. Prospective study of the incidence and risk factors of postsplenectomy thrombosis of the portal, mesenteric, and splenic veins. Arch Surg. 2006;141:663–9. doi: 10.1001/archsurg.141.7.663. [DOI] [PubMed] [Google Scholar]
- 19.Shorr AF, Thomas SJ, Alkins SA, Fitzpatrick TM, Ling GS. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest. 2002;121:1262–8. doi: 10.1378/chest.121.4.1262. [DOI] [PubMed] [Google Scholar]
- 20.Kinasewitz GT, Yan SB, Basson B, Comp P, Russell JA, Cariou A, Um SL, Utterback B, Laterre PF, Dhainaut JF. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569] . Crit Care. 2004;8:R82–90. doi: 10.1186/cc2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vecchio R, Cacciola E, Cacciola RR, Marchese S, Intagliata E. Portal vein thrombosis after laparoscopic and open splenectomy. J Laparoendosc Adv Surg Tech A. 2011;21:71–5. doi: 10.1089/lap.2010.0325. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Kopac D, Brisebois R, Sample C, Shapiro AM. Randomized controlled trial to investigate the impact of anticoagulation on the incidence of splenic or portal vein thrombosis after laparoscopic splenectomy. Can J Surg. 2011;54:227–31. doi: 10.1503/cjs.049909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Castro KI, Simioni P, Burra P, Senzolo M. Anticoagulation for the treatment of thrombotic complications in patients with cirrhosis. Liver Int. 2012;32:1465–76. doi: 10.1111/j.1478-3231.2012.02839.x. [DOI] [PubMed] [Google Scholar]


