Abstract
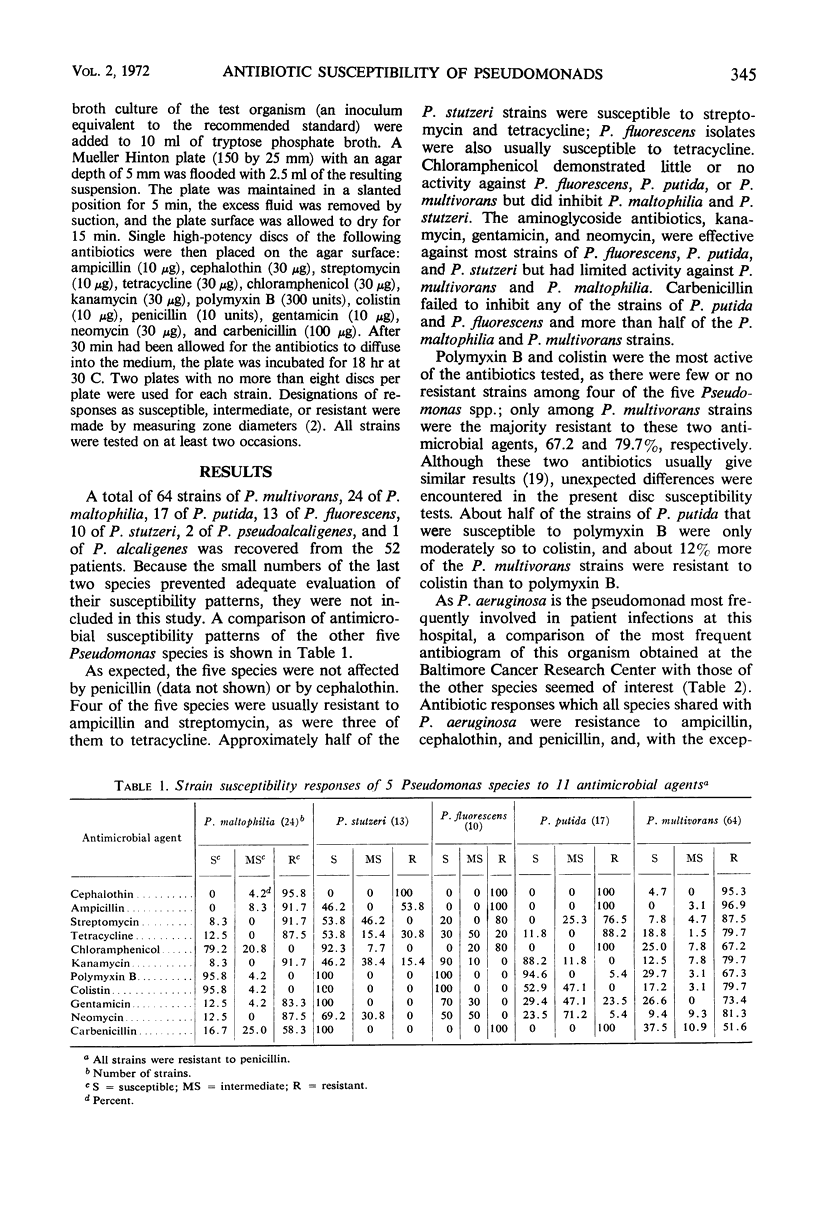
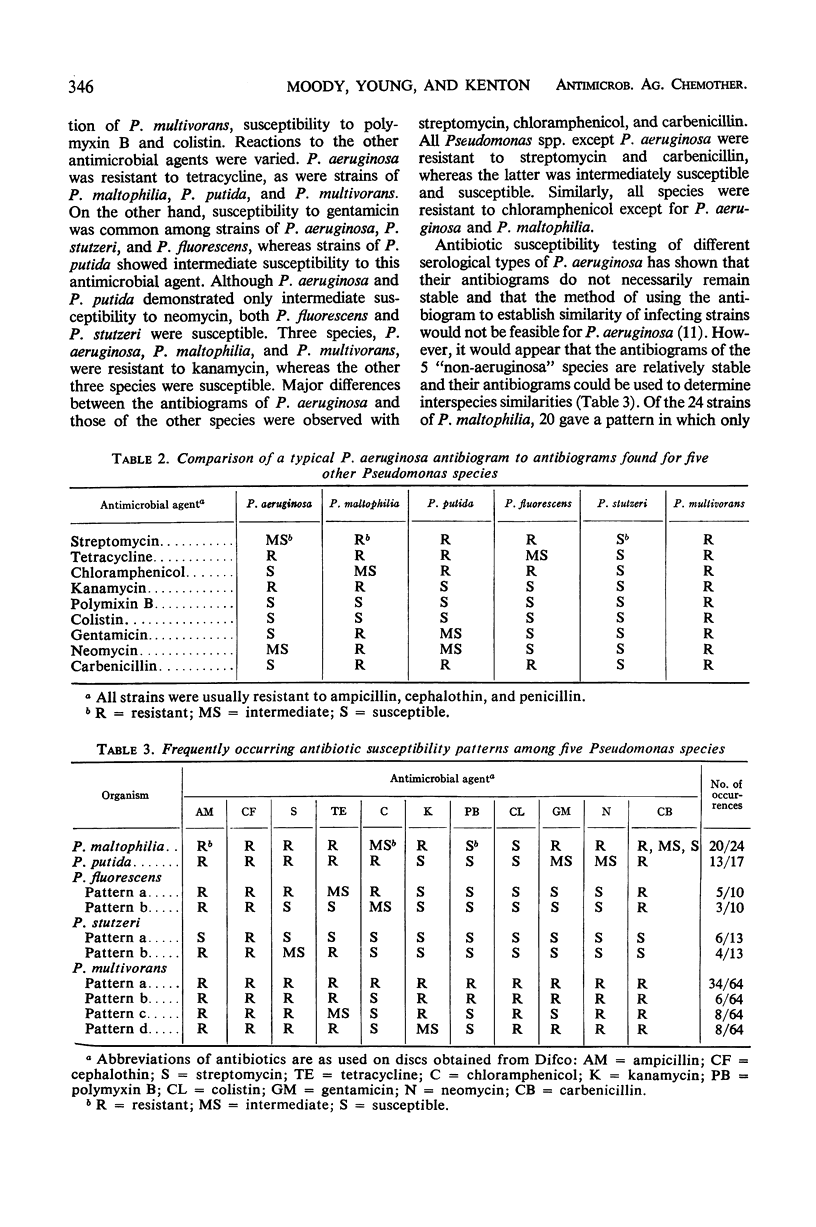
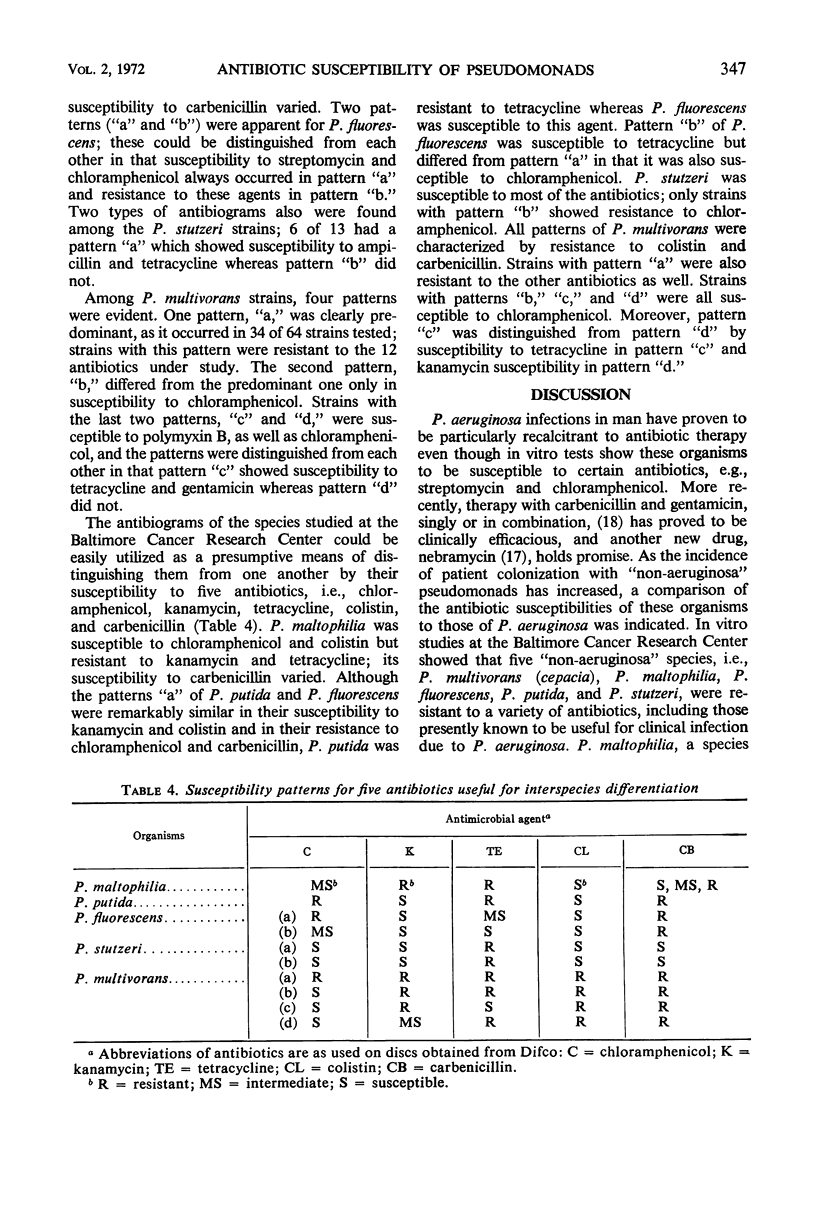
The increase in occurrence of infections due to opportunistic gram-negative bacilli in patients with impaired host defenses emphasizes the need for information on the antibiotic susceptibility of the organisms that colonize such patients. During a 20-month period, more than 100 pseudomonads which were not Pseudomonas aeruginosa were recovered from cancer patients at the Baltimore Cancer Research Center. These included P. fluorescens, P. putida, P. multivorans (cepacia), P. maltophilia, P. stutzeri, P. alcaligenes, and P. pseudoalcaligenes. Susceptibility tests with 12 antibiotics indicated that the intraspecies antibiograms for many of these species were more uniform than those of P. aeruginosa. The stability of susceptibility patterns allowed the antibiograms to be used as aids in the preliminary differentiation of these organisms. Variable antibiogram patterns were noted among certain species, i.e., P. fluorescens, P. stutzeri, and P. multivorans, whereas each of the other species had essentially one pattern. These in vitro studies showed that some of the Pseudomonas species other than P. aeruginosa were resistant to a number of antibiotics. Among these were antibiotics that are in general use for P. aeruginosa infections. Such differences in antibiotic susceptibilities emphasize the necessity for careful speciation of this group of microorganisms to assure proper epidemiological documentation of colonization and infection, as well as to ensure therapy with an antimicrobial agent to which the organism is susceptible in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassett D. C., Stokes K. J., Thomas W. R. Wound infection with Pseudomonas multivorans. A water-borne contaminant of disinfectant solutions. Lancet. 1970 Jun 6;1(7658):1188–1191. doi: 10.1016/s0140-6736(70)91783-6. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Dailey R. H., Benner E. J. Necrotizing pneumonicitis due to the pseudomonad "eugonic oxidizer--group I". N Engl J Med. 1968 Aug 15;279(7):361–362. doi: 10.1056/NEJM196808152790706. [DOI] [PubMed] [Google Scholar]
- Finland M. Changing ecology of bacterial infections as related to antibacterial therapy. J Infect Dis. 1970 Nov;122(5):419–431. doi: 10.1093/infdis/122.5.419. [DOI] [PubMed] [Google Scholar]
- Gardner P., Griffin W. B., Swartz M. N., Kunz L. J. Nonfermentative gram-negative bacilli of nosocomial interest. Am J Med. 1970 Jun;48(6):735–749. doi: 10.1016/s0002-9343(70)80009-2. [DOI] [PubMed] [Google Scholar]
- Gilardi G. L. Antimicrobial susceptibility as a diagnostic aid in the identification of nonfermenting gram-negative bacteria. Appl Microbiol. 1971 Nov;22(5):821–823. doi: 10.1128/am.22.5.821-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi G. L. Characterization of Pseudomonas species isolated from clinical specimens. Appl Microbiol. 1971 Mar;21(3):414–419. doi: 10.1128/am.21.3.414-419.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi G. L. Pseudomonas maltophilia infections in man. Am J Clin Pathol. 1969 Jan;51(1):58–61. doi: 10.1093/ajcp/51.1.58. [DOI] [PubMed] [Google Scholar]
- Hardy P. C., Ederer G. M., Matsen J. M. Contamination of commercially packaged urinary catheter kits with the pseudomonad EO-1. N Engl J Med. 1970 Jan 1;282(1):33–35. doi: 10.1056/NEJM197001012820108. [DOI] [PubMed] [Google Scholar]
- Mitchell R. G., Hayward A. C. Postoperative urinary-tract infections caused by contaminated irrigating fluid. Lancet. 1966 Apr 9;1(7441):793–795. doi: 10.1016/s0140-6736(66)91866-6. [DOI] [PubMed] [Google Scholar]
- Moody M. R., Young V. M., Schimpff S., Kenton D. M. Relationship of Pseudomonas aeruginosa immunotypes to antibiotic susceptibility patterns. Antimicrob Agents Chemother (Bethesda) 1970;10:249–253. [PubMed] [Google Scholar]
- Pedersen M. M., Marso E., Pickett M. J. Nonfermentative bacilli associated with man. 3. Pathogenicity and antibiotic susceptibility. Am J Clin Pathol. 1970 Aug;54(2):178–192. doi: 10.1093/ajcp/54.2.178. [DOI] [PubMed] [Google Scholar]
- Phillips I., Eykyn S., Curtis M. A., Snell J. J. Pseudomonas cepacia (multivorans) septicaemia in an intensive-care unit. Lancet. 1971 Feb 20;1(7695):375–377. doi: 10.1016/s0140-6736(71)92212-4. [DOI] [PubMed] [Google Scholar]
- Pickett M. J., Pedersen M. M. Characterization of saccharolytic nonfermentative bacteria associated with man. Can J Microbiol. 1970 May;16(5):351–362. doi: 10.1139/m70-062. [DOI] [PubMed] [Google Scholar]
- Pickett M. J., Pedersen M. M. Nonfermentative bacilli associated with man. II. Detection and identification. Am J Clin Pathol. 1970 Aug;54(2):164–177. doi: 10.1093/ajcp/54.2.164. [DOI] [PubMed] [Google Scholar]
- Pickett M. J., Pedersen M. M. Salient features of nonsaccharolytic and weakly saccharolytic nonfermentative rods. Can J Microbiol. 1970 Jun;16(6):401–409. doi: 10.1139/m70-069. [DOI] [PubMed] [Google Scholar]
- Preston D. A., Wick W. E. Preclinical assessment of the antibacterial activity of nebramycin factor 6. Antimicrob Agents Chemother (Bethesda) 1970;10:322–327. [PubMed] [Google Scholar]
- SORRELL W. B., WHITE L. V. Acute bacterial endocarditis caused by a variant of the genus Herrellea. Am J Clin Pathol. 1952 Feb;23(2):134–138. doi: 10.1093/ajcp/23.2.134. [DOI] [PubMed] [Google Scholar]
- Schimpff S., Satterlee W., Young V. M., Serpick A. Empiric therapy with carbenicillin and gentamicin for febrile patients with cancer and granulocytopenia. N Engl J Med. 1971 May 13;284(19):1061–1065. doi: 10.1056/NEJM197105132841904. [DOI] [PubMed] [Google Scholar]
- Seppälä M., Räihä N. C., Tamminen V. Ethanol elimination in a mother and her premature twins. Lancet. 1971 Jun 5;1(7710):1188–1189. doi: 10.1016/s0140-6736(71)91702-8. [DOI] [PubMed] [Google Scholar]
- Speller D. C., Stephens M. E., Viant A. C. Hospital infection by Pseudomonas cepacia. Lancet. 1971 Apr 17;1(7703):798–799. doi: 10.1016/s0140-6736(71)91236-0. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Sutter V. L. Identification of Pseudomonas species isolated from hospital environment and human sources. Appl Microbiol. 1968 Oct;16(10):1532–1538. doi: 10.1128/am.16.10.1532-1538.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd Antimicrobial susceptibility of enterobacteriaceae and nonfermenting gram-negative bacilli. Mayo Clin Proc. 1969 Nov;44(11):811–824. [PubMed] [Google Scholar]



