Abstract
To assess the clinical efficacy and safety of Silibinin in preventing drug-induced liver injury (DILI) in the general population (high-risk patients with non-drug induced liver injury). Method: A prospective, multi-center, randomized, open-label and controlled trial was conducted with 568 patients undergoing primary treatment of pulmonary tuberculosis. The study included 277 patients in experimental group and 291 patients in control group. The patients in the two group were treated with conventional 2HREZ (S)/4HR for tuberculosis (TB), and additional Silibinin capsules (oral administration of 70 mg/time, 3 times/day for 8 weeks in experimental group. Outcomes of liver function, interruption of anti-TB treatment and therapeutic results, as well as adverse reactions were observed and analyzed. Results: At 2, 4 and 8 weeks of treatment, the incidences of liver injury in experimental group were 3.97%, 1.44% and 2.17%, respectively; the incidences in control group were 4.12%, 4.12% and 2.41%, respectively. Statistical analysis showed that there was no difference in the incidence between the two groups at each treatment period (P>0.05). At 8 weeks, the numbers of patients diagnosed of DILI were 18 (7.22%) and 27 (9.28%) in experimental and control groups, respectively (P>0.05). 34.30% and 27.49% of the patients in experimental and control groups had transient abnormal liver function or symptoms, respectively; similar percentages (3.25% and 6.19%) of the patients in two groups have liver function injury and symptoms, and were suspended for anti-TB treatment (P>0.05). The incidence of anorexia and nausea symptoms was lower in experimental group than in control group, and the differences were significant at 4 and 8 weeks (P<0.05). 8 weeks after the treatment, 98.30% of the sputum smear culture were negative in experimental group, which was significantly higher (P<0.01) than that in control group (92.98%). Conclusion: Preventive hepatoprotective therapy in the general population may reduce drug discontinuation rate, improve patient’s compliance and outcomes of anti-TB treatment.
Keywords: Pulmonary tuberculosis, Silibinin, drug-induced liver injury
Introduction
Anti-tuberculosis (TB) drug-induced liver injury (DILI) is reported to be between 2% and 30% in different countries. The difference may be resulted from a number of factors, such as race, socioeconomic status, geographic location, diagnosis standard used to assess DILI and prevalence rate of viral hepatitis. China is reported to be a high DILI country (8-30%) [1]. Whether prophylactic treatment should be given during anti-TB treatment has been controversial with little evidence from evidence-based medicine. A systematic literature review on this topic shows that the methodologies used in published studies are poor in quality with relatively small sample size. In these studies, placebo is often not included and the experiments are not blinded. The reviewed articles are low in quality and have publication bias. No reports have made subgroup analysis between specific groups and the general population. In addition, these conclusion is not clear if there is a better preventive effect for the general population to use prophylactic treatment during anti-TB treatment [2].
In an attempt to resolve the controversial, we conducted an open-label, randomized and multi-center clinical trial to evaluate the safety and efficacy of prophylactic treatment in preventing drug-induced liver injury. This study would allow to objectively assessing factors determining the therapeutic efficacy of liver protective drugs.
Methods
Experimental design and ethics
The trial was an open-label, randomized and multi-center clinical study with one group of control and conducted at Shanghai Pulmonary Hospital (lead hospital), Guangzhou Chest Hospital, Haihe Hospital (Tianjin), Nanjing Chest Hospital, Longtan Hospital (Guangxi), Shandong Provincial Chest Hospital, Fuzhou Pulmonary Hospital (Fujian), Wuhan TB Prevention Institute, Jilin Hospital of Infectious Diseases, Anhui Provincial Chest Hospital, Suzhou Fifth People’s Hospital, and Wuxi Hospital of Infectious Diseases.
The study has been approved and registered by the Ethics Committees, all protocol followed the national guideline of quality management for drug clinical trial, and all works were undertaken following the provisions of the Declaration of Helsinki Patients were fully informed about the nature of the study, the nature of the disease, drug characteristics, treatment methods and the risk involved in participating in the study. The informed consent was obtained from every participant.
Inclusion criteria
Participants were included if they were (1) aged 18 to 65 years regardless of gender, (2) diagnosed of TB (according to diagnosis standard for pulmonary TB issued by Ministry of Health on January 16, 2008) [3] and having primary pulmonary TB, (3) without previous history of using anti-TB drugs, (4) normal for serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AKP), total bilirubin (TBiL), and direct bilirubin (DBiL) testing.
Exclusion criteria
Patients were excluded if they (1) had basic liver diseases (such as alcoholic liver disease, autoimmune liver disease, Schistosoma liver disease, non-alcoholic fatty hepatitis, hepatitis B virus or hepatitis C virus); (2) had severe heart, brain, kidney, gastrointestinal diseases and systemic diseases; (3) were positive for HIV antibody; (4) were taking medications that could affect curative effect in the study; (5) were allergic or intolerant to Silibinin; (6) were unable to express their complaints (such as patients with serious psychosis and neurosis); (7) were not compliant; (8) were pregnant, lactating or at childbearing age to get pregnant; (9) participated in other clinical trials within three months prior to the study; and (10) had other conditions that the physicians believed not suitable for the study.
Rejection criteria
Participants were rejected if they (1) were found violating inclusion and exclusion criteria; (2) did not take the drug after enrollment; (3) did not take the drug as instructed and were poor in compliance; (4) took other drugs that could affect the outcome of this study; (5) produced drug-resistant Mycobacterium tuberculosis in sputum culture and needed to change the anti-TB treatment plan; and (6) had serious adverse events and needed to stop the treatment.
Treatment plan
The participants were randomly divided into experimental and control groups, they were treated with 2HREZ (S)/4HR and Silibinin capsules, and 2HREZ (S)/4HR only, respectively. The drugs were given as follows: isoniazid (H), 0.3 g/time, once a day, taken at a draught; rifampin, 600 mg/time for patients weighted ≥50 kg, or 450 mg/time for patients weighted <50 kg, once a day, taken at a draught before meal; pyrazinamide (Z), 0.5 g/time, three times a day, orally administered; ethambutol (E), 1.0 g/time for patients weighted ≥50 kg, or 0.75 g/time for patients weighted <50 kg, once a day, taken at a draught before meal; streptomycin (S), intramuscular injection of 0.75 g, once a day. Silibinin phospholipid complex capsules (35 mg/capsule, Tianjin Tasly Pharmaceutical Limited) were orally administered two capsules (70 mg) a time, with three times daily (210 mg/day) for 8 weeks. If patients had moderate or severe liver injury during the anti-TB treatment, the anti-TB drugs were discontinued or changed immediately, and other drugs were suggested to protect the liver. For patients with mild liver injury, investigators would decide whether to discontinue the drugs suspected of causing the liver injury based on the patient’s situation and the risk/benefit.
Evaluation
Liver function assessment
To assess DILI, serum blood tests were conducted for ALT, AST, AKP, TBiL and DBiL before and at 2, 4, 6, and 8 weeks of treatment. The severity of liver injury was classified according to Treatment Handbook on anti-TB Drug Adverse Reactions [4].
Liver injury symptom evaluation
Liver injury symptoms included fatigue, anorexia, nausea, vomiting and abdominal distension were scored as follows: 0, no symptoms; 1, mild symptoms that do not affect daily life and work; 2, moderated symptoms that slightly affect daily life and work; and 3, severe symptoms that significantly affect the daily life and work.
Evaluation of hepatoprotective effect
The hepatoprotective effect was assigned to one of the three results: (1) no abnormal liver function or liver injury symptom occurred after the completion of treatment; (2) transient abnormal liver function or liver injury symptom without interruption of the treatment during the treatment. In these patients, there were slight symptoms or signs, and abnormal ALT level but not worse enough to discontinue the treatment. After the completion of treatment plan, the symptoms or signs disappeared and liver functions returned to normal; and (3) patients had obvious liver function injury or symptoms, and the treatment was discontinued.
Evaluation of other clinical outcomes
Other clinical outcomes were assessed based on improvement of clinical symptoms, bacteriological results of sputum culture after 8 weeks of treatment and imaging analysis result. If ≥50% size of the original focus was absorbed; it was scored as significantly absorbed; if the reduction was <50%, it was scored as absorbed; if no obvious change was seen, it was scored as unchanged; and if the focus increased or diffused, it was scored as deteriorated.
Adverse events
Occurred adverse events were recorded during the treatment period, their relationship with drugs, severity, duration, measures taken and prognosis were analyzed.
Statistical analysis
The t-test and chi square test were used to analyze the measurement and count data, respectively. The Wilcoxon signed-rank test was used to assess nonparametric data. Analysis of variance or non-parametric analysis was used to compare data within the same curative effect indexes, and the CMH method was used to test the difference between groups. All statistical tests were two-sided and P<0.05 was considered as statistically significant. All statistical analyses were performed with the statistical software SAS9.13.
Results
Study subjects were recruited between November, 2012 and May, 2013, and the trial was completed in July, 2013. A total of 605 patients were screened and 37 were excluded due to missing age data (10), outside the inclusion ages (7), infection of hepatitis B (5), HIV (3), missing group information (1) and inappropriate liver functions (11). As a result, 568 patients were included with 277 in experiment group and 291 in control group, respectively.
The baseline characteristics of study subjects
Demographic analysis (Table 1) showed that there was no significant difference between the two groups in gender, age, height, weight, blood pressure, heart rate, past medical history, past medication history, concomitant diseases and concomitant medications (P>0.05).
Table 1.
General and demographic data in the study subjects
| Experimental group (%) | Control group (%) | P | |
|---|---|---|---|
| Gender (male) | 180 (64.98) | 194 (66.67) | 0.672 |
| Age (years) | 37.42±14.28 | 36.16±13.95 | 0.242 |
| Height (cm) | 1.68±0.07 | 1.68±0.07 | 0.426 |
| Weight (Kg) | 57.88±9.04 | 58.23±8.90 | 0.823 |
| Systolic blood pressure (mmHg) | 118.05±11.90 | 118.21±10.90 | 0.780 |
| Diastolic blood pressure (mmHg) | 77.71±8.37 | 74.36±7.59 | 0.822 |
| Heart rate (beats/min) | 83.38±9.37 | 82.39±10.12 | 0.165 |
| Past history | 35 (12.68) | 24 (8.25) | 0.084 |
| Past medical history | 34 (12.36) | 29 (10.07) | 0.388 |
| Concomitant diseases | 19 (6.88) | 24 (8.30) | 0.549 |
| Combination therapy | 58 (28.29) | 79 (30.62) | 0.586 |
Therapeutic outcomes
Liver function
As shown in Table 2, there was no difference in ALT, TBIL, DBIL, TP, and ALB between the two groups before or 2, 4 and 8 weeks after treatment (P>0.05, Figure 1). However, AST was significantly lower in experimental than in control groups at 2 and 4 weeks of treatment (P<0.05); and AKP was significantly lower in experimental than in control groups at 8 weeks of treatment (P<0.05). Over the treatment period, the average ALT and AST increased with slight difference between the groups; the average TBIL and DBIL decreased first and then increased without visible difference between the groups; the average AKP decreased in the experimental group, while increased in control group, where it was greater than in experimental group. The average TP and ALB increased over the treatment period the two groups (Figure 1).
Table 2.
Liver functions in the study subjects
| Liver function | Before therapy | P | Therapy for 2 W | P | Therapy for 4 W | P | Therapy for 8 W | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Experimental group | Control group | Experimental group | Control group | Experimental group | Control group | Experimental group | Control group | |||||
| ALT (U/L) | 17.14±10.07 | 17.23±9.91 | 0.690 | 25.72±23.11 | 29.59±36.13 | 0.070 | 25.57±18.57 | 32.81±43.85 | 0.053 | 26.79±24.70 | 30.95±60.82 | 0.583 |
| AST (U/L) | 19.20±7.32 | 19.42±9.17 | 0.678 | 26.44±30.33 | 27.96±29.42 | 0.018 | 24.69±11.23 | 32.04±45.33 | 0.030 | 25.21±15.74 | 30.41±38.74 | 0.064 |
| TBIL (μmol/L) | 10.64±5.48 | 10.83±6.14 | 0.811 | 8.88±4.97 | 9.18±4.74 | 0.287 | 9.58±4.51 | 10.15±5.93 | 0.715 | 10.25±4.47 | 10.42±6.23) | 0.515 |
| DBIL (μmol/L) | 4.20±3.03 | 4.01±2.45 | 0.334 | 3.69±3.31 | 5.90±25.02 | 0.551 | 3.69±2.05 | 4.03±2.78 | 0.466 | 3.86±1.90 | 5.40±19.12 | 0.593 |
| AKP (U/L) | 75.48±26.75 | 77.27±25.73 | 0.263 | 79.05±27.66 | 84.66±54.69 | 0.116 | 77.42±24.36 | 83.21±37.18 | 0.094 | 76.49±25.82 | 85.99±32.01 | 0.001 |
| TP (g/L) | 70.44±9.46 | 69.91±7.59 | 0.983 | 71.31±10.74 | 70.89±9.14 | 0.241 | ||||||
| ALB (g/L) | 40.88±5.87 | 41.32±6.14 | 0.533 | 42.89±5.60 | 43.14±6.01 | 0.754 | ||||||
Figure 1.
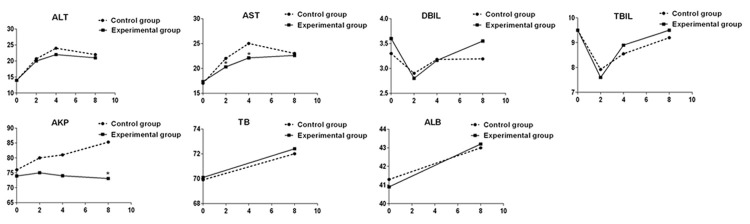
Comparison of assessment results of ALT, AST, AKB, TBIL, DBIL, TP, and ALB. *, P<0.05, compared with control group.
Liver protection
During the course of the study, 34.30% and 27.49% of the patients in experimental and control groups had transient abnormal liver function or symptoms, respectively; 3.25% and 6.19% of the patients in these two groups had liver function injury and symptoms that resulted in discontinuation of the treatment, respectively. However, these differences were not statistically significant (Table 3, P>0.05).
Table 3.
Analysis of liver therapy efficacy
| Result | Experimental group (%) | Control group (%) | Total | Statistics | P |
|---|---|---|---|---|---|
| No liver function abnormalities or symptoms | 173 (62.45) | 193 (66.32) | 366 | ||
| Transient liver function abnormalities or symptoms | 95 (34.30) | 80 (27.49) | 175 | -0.649 | 0.516 |
| Liver injury and symptoms with interrupted anti-TB treatment | 9 (3.25) | 18 (6.19) | 27 | ||
| Total | 277 | 291 | 568 |
Note: The groups were compared using the Wilcoxon test.
The incidence of liver injury
At 2, 4 and 8 weeks of treatment, the incidences of liver injury in experimental group were 3.97%, 1.44% and 2.17%, and in control group 4.12%, 4.12% and 2.41%. Statistical analysis showed that there was no difference in the incidences between the two groups at different treatment periods (P>0.05). Within the two months course of treatment, there were 18 cases (7.22%) of DILI in experimental group and 27 cases (9.28%) in control group. The difference between the two groups was not significant (P>0.05, Table 4).
Table 4.
Liver injury following anti-TB treatment in the study subjects
| Therapy for 2 W | Therapy for 4 W | Therapy for 8 W | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Result | Experimental group (%) | Control group (%) | Experimental group (%) | Control group (%) | Experimental group (%) | Control group (%) |
| No function abnormalities | 230 (83.03) | 233 (80.07) | 229 (82.67) | 214 (73.54) | 226 (81.59) | 215 (73.88) |
| Abnormal function | 27 (9.75) | 33 (11.34) | 26 (9.39) | 40 (13.75) | 21 (7.58) | 33 (11.34) |
| Mild injury | 6 (2.17) | 7 (2.41) | 3 (1.08) | 6 (2.06) | 1 (0.36) | 0 (0.00) |
| Moderate injury | 5 (1.81) | 2 (0.69) | 1 (0.36) | 3 (1.03) | 5 (1.81) | 6 (2.06) |
| Severe injury | 0 (0.00) | 3 (1.03) | 0 (0.00) | 3 (1.03) | 0 (0.00) | 1 (0.34) |
| Liver failure | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Undetermined | 9 (3.25) | 13 (4.47) | 18 (6.50) | 25 (8.59) | 24 (8.66) | 36 (12.37) |
| The overall incidence of liver injury | 11 (3.97) | 12 (4.12) | 4 (1.44) | 12 (4.12) | 6 (2.17) | 7 (2.41) |
| Statistics | 4.801 | 8.386 | 5.024 | |||
| P | 0.308 | 0.078 | 0.285 | |||
Note: The groups were compared using the Wilcoxon test.
Gastrointestinal symptoms
The records showed that experimental group had significantly less anorexic patients than control group at 4 weeks of treatment (2.26% vs 8.46%, P<0.01) and at 8 weeks of treatment (0.78% vs 3.28%, P<0.05). The incidence of nausea was significantly lower in experimental group than in control group at 4 weeks of treatment (1.14% vs 5.90%, P<0.05) and 8 weeks of treatment (0.39% vs 4.99%, P<0.01). For other symptoms such as vomiting, abdominal distension, fever, jaundice, and nervous system symptoms, the incidences varied between 0% and 0.39% at different treatment periods and were not statistically different between the groups. No diarrhea, edema, ascites and gastrointestinal bleeding were noticed.
Anti-tuberculosis effect
Sputum culture: the negative samples in sputum smear culture from patients in experimental group were significantly more than from control group at 8 weeks of treatment (98.30% vs 92.98%, P<0.05, Table 5). However, the difference was not significant in sputum culture between the two groups (P>0.05, Table 5).
Table 5.
Results of sputum culture at the end of the therapy
| Results | Experimental group (%) | Control group (%) | Statistics | P |
|---|---|---|---|---|
| Sputum smear culture | ||||
| Negative | 231 (98.30) | 225 (92.98) | -2.585 | 0.010 |
| Positive | 4 (1.70) | 17 (7.02) | ||
| Determined | 235 | 242 | ||
| Undetermined | 42 | 49 | ||
| Sputum culture | ||||
| Negative | 156 (95.71) | 153 (93.29) | -0.955 | 0.340 |
| Positive | 7 (4.29) | 11 (6.71) | ||
| Determined | 163 | 164 | ||
| Undetermined | 114 | 127 |
Note: the chi square test was used.
Imaging analysis: the focus absorption and cavity closing were similar between the two groups at 8 weeks of treatment (P>0.05, Table 6). Clinical symptoms: significant improvements in clinical symptoms such as cough, expectoration, fever, hemoptysis, chest tightness, chest pain, night sweat and fatigue were observed after treatment as compared to before treatment. However, there was no significant difference between the two groups (P>0.05).
Table 6.
Imaging analysis at the end of therapy
| Results | Experimental group (%) | Control group (%) | Statistics | P |
|---|---|---|---|---|
| Focus | ||||
| Significantly absorbed | 46 (18.25) | 50 (19.38) | -0.038 | 0.970 |
| Absorbed | 197 (78.17) | 195 (75.58) | ||
| Unchanged | 9 (3.57) | 11 (4.26) | ||
| Deteriorated | 0 (0.00) | 2 (0.78) | ||
| Determined | 252 | 258 | ||
| Undetermined | 25 | 33 | ||
| Cavity | ||||
| Closed | 50 (39.27) | 52 (40.63) | 0.501 | 0.616 |
| Unchanged | 6 (4.72) | 11 (8.59) | ||
| Reduced | 71 (55.91) | 64 (50.00) | ||
| Enlarged | 0 (0.00) | 1 (0.78) | ||
| Determined | 127 | 128 | ||
| Undetermined | 150 | 163 |
Note: The groups were compared using the Wilcoxon test.
Safety analysis
In addition to liver dysfunction and injury, there were other adverse events such as gastrointestinal tract reaction, drug fever, drug rash, leukopenia and hyperuricemia in both groups. The numbers of adverse events were similar between experimental and control groups (5 (1.81%) vs 3 (1.03%), P>0.05).
Discussion
The most common side effects of anti-TB drugs are DILI, which has been reported worldwide. Chinese herbal medicines and anti-TB drugs are the most common caused factors for DILI [5]. Mechanisms of DILI include mainly (1) direct toxic effects of drugs and their intermediate metabolites on the liver, whose effect is dose-dependent and predictable; (2) special drug reactions, such as allergic (immune idiosyncrasy) and metabolic (metabolic idiosyncrasy) reactions. DILI of this type is dose- and course-independent, and this type DILI occurs only in a small proportion of population, but not in the majority of the population, it is often unpredictable, and most of DILI cases observed clinically, the detail mechanisms and factors affecting the DILI are unclear in fact [6].
In recent years, in order to reduce the incidence of liver injury and improve the compliance and therapeutic effect of TB patients, some of Chinese specialist favor to use hepatoprotective drugs during anti-TB therapy, for the following reasons: (1) China has higher HBV infection rate with hepatitis B surface antigen carrying rate of 7.18% [7]. Higher HBV infection often results in higher incidence of severe liver disease and subsequent higher susceptibility to tuberculosis. Clinically, there is high concurrence of the two diseases. (2) Based on the mechanism underlying DILI, blocking the drug hepatotoxicity may reduce the liver injury of anti-TB drugs. For example, study with mouse model showed that isoniazid can inhibit the activity of thiol associated with free radical ligand glutathione, antioxidant glutathione peroxidase and catalase. In addition, N- acetyl cysteine, the substrate for glutathione synthesis, can inhibit the liver injury induced by isoniazid, and thus prevent mice from liver injury [8]. However, the use of hepatoprotective drugs in most of the patients has been questioned as a safe strategy for small proportion of patients to avoid DILI. The opponents believe that due to complexity of DILI, it would not be effective to use hepatoprotective drugs; and that hepatoprotective drugs may also have adverse effect, also unreasonable use of hepatoprotective drugs would increase the burden of TB patients [9]. In order to solve these questions and assess the effect of prophylactic liver treatment in the general population, we designed and conducted this study. Considering that DILI usually occurred at 2 to 8 weeks after anti-TB treatment, we set up our observation points at before treatment, 2, 4 and 8 weeks during the treatment. Our results showed that AST was significantly lower in experimental group than control group at 2 and 4 weeks of treatment; AKP was significantly lower in experimental group than control group at 8 weeks of treatment. However, the averages were all in normal ranges in both groups. Furthermore, other indexes such as ALT, TBIL, DBIL, TP and ALB were not statistically different between the two groups. At 2, 4 and 8 weeks of treatment, the incidences of liver injury were similar between groups (3.97%, 1.44% and 2.17% in experimental group vs 4.12%, 4.12% and 2.41% in control group). The total numbers of patients with liver injury within 2 months were also identical (18 (7.22%) in experimental group vs 27 (9.28%) in control group). These findings suggest that the overall incidence of liver injury does not change significantly in the preventive hepatoprotective therapy as designed in the trial.
Our results showed that there were higher incidences of transient abnormal liver function or symptoms during the anti-TB therapy (34.30% in experimental group vs 27.49% in control group). However, only a few had resulted in discontinuation of the therapy (3.25% in experimental group and 6.19% in control group). This might be an adaptive response of the liver triggered by anti-TB drugs, which lead to activation of genes or cellular pathways regulating antioxidation, anti-inflammation and anti-apoptosis, the proliferation of hepatocytes and generation of protective adaptation reaction. As a result, transaminase was transiently elevated [10]. A double-blind, prospective clinical study showed that in 173 hospitalized patients receiving 1 year preventive treatment with isoniazid, as high as 13.3% participants had increased ALT. However, the ALT level usually returned to normal as the medication continued. Generally, adaptive DILI often happens in asymptomatic patients with elevated serum enzymes, rarely accompanied with elevated jaundice. However, in this trial, many liver function indexes were measured, more patients were identified. If patients have liver function not suggestive of mild DILI, and are asymptomatic, they might be in process of liver adaptive reaction and should not discontinue the anti-TB drugs immediately. Instead, they should be followed-up closely for liver function evaluation.
The ultimate aim of preventive hepatoprotective treatment is to ensure curative effect of anti-TB therapy for better cure rate. In this study, no differences between the two groups were observed in improving TB symptoms, focus absorption and cavity closing. However, the negative rate of sputum smear culture was higher in experimental group than in control group at 8 weeks of treatment (98.30% vs 92.98%), and this might be resulted from lower discontinuation rate of anti-TB drugs in experimental group. This may help to reduce the occurrence of drug-tolerant bacteria. Our investigation showed that there were fewer patients with anorexia and nausea symptoms in experimental group than in control group, and the differences were significant at 4 and 8 weeks of treatment, suggesting that Silibinin may be beneficial to improve the gastrointestinal symptoms. Furthermore, the drug is relative safe with very low adverse event rate (1.81%), not significantly different from control.
There are some limitations in the trial, such as exclusion of some patients with high risk of DILI. Due to higher age, HIV infection, alcohol addiction, liver disease and malnutrition [1,11-15], therefore, the data presented here might have underestimated DILI in the overall population where the incidence of DILI may be higher. However, due to ethical considerations, it is hard to include these patients in the prospective study. In addition, the sample size in the trial was relatively small and some of the differences would become statistically significant with large sample size.
Disclosure of conflict of interest
None.
References
- 1.Baghaei P, Tabarsi P, Chitsaz E, Saleh M, Marjani M, Shemirani S, Pooramiri MV, Kazempour M, Farnia P, Fahimi F, Mansouri D, Masjedi M. Incidence, clinical and epidemiological risk factors, and outcome of drug-induced hepatitis due to antituberculous agents in new tuberculosis cases. Am J Ther. 2010;17:17–22. doi: 10.1097/MJT.0b013e31818f9eae. [DOI] [PubMed] [Google Scholar]
- 2.Liu Q, Garner P, Wang Y, Huang B, Smith H. Drugs and herbs given to prevent hepatotoxicity of tuberculosis therapy: systematic review of ingredients and evaluation studies. BMC Public Health. 2008;8:365. doi: 10.1186/1471-2458-8-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cha D, Cheng D, Liu M, Zeng Z, Hu X, Guan W. Analysis of fatty acids in sputum from patients with pulmonary tuberculosis using gas chromatography-mass spectrometry preceded by solid-phase microextraction and post-derivatization on the fiber. J Chromatogr A. 2009;1216:1450–1457. doi: 10.1016/j.chroma.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 4.Xiao T. Handbook for diagnosis and treatment of anti-TB drug-induced adverse reactions. People’s Health Publishing House; 2009. [Google Scholar]
- 5.Xu X, Wong X, Liu F, Zhang X, Peng W. The clinical investigation of drug induced liver injury and its influencing factors analysis. Chinese Journal of clinical pharmacology. 2014;30:216–218. [Google Scholar]
- 6.Grant LM, Rockey DC. Drug-induced liver injury. Curr Opin Gastroenterol. 2012;28:198–202. doi: 10.1097/MOG.0b013e3283528b5d. [DOI] [PubMed] [Google Scholar]
- 7.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, Wang F, Zheng H, Wang F, Guo J, Jia Z, Ma J, Wang H, Luo H, Li L, Jin S, Hadler SC, Wang Y. Reprint of: Epidemiological serosurvey of Hepatitis B in China--declining HBV prevalence due to Hepatitis B vaccination. Vaccine. 2013;31(Suppl 9):J21–28. doi: 10.1016/j.vaccine.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Saad EI, El-Gowilly SM, Sherhaa MO, Bistawroos AE. Role of oxidative stress and nitric oxide in the protective effects of alpha-lipoic acid and aminoguanidine against isoniazid-rifampicin-induced hepatotoxicity in rats. Food Chem Toxicol. 2010;48:1869–1875. doi: 10.1016/j.fct.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Xiao H. Controversial over concomitant use of hepatoprotective drugs in anti TB treatment. Chinese Journal of Tuberculosis and Respiratory Diseases. 2013;3:722–723. [Google Scholar]
- 10.Padmapriyadarsini C, Bhavani PK, Tang A, Kumar H, Ponnuraja C, Narendran G, Hannah E, Ramesh C, Chandrasekar C, Wanke C, Swaminathan S. Early changes in hepatic function among HIV-tuberculosis patients treated with nevirapine or efavirenz along with rifampin-based anti-tuberculosis therapy. Int J Infect Dis. 2013;17:e1154–1159. doi: 10.1016/j.ijid.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stirnimann G, Kessebohm K, Lauterburg B. Liver injury caused by drugs: an update. Swiss Med Wkly. 2010;140:w13080. doi: 10.4414/smw.2010.13080. [DOI] [PubMed] [Google Scholar]
- 12.Nanashima K, Mawatari T, Tahara N, Higuchi N, Nakaura A, Inamine T, Kondo S, Yanagihara K, Fukushima K, Suyama N, Kohno S, Tsukamoto K. Genetic variants in antioxidant pathway: risk factors for hepatotoxicity in tuberculosis patients. Tuberculosis (Edinb) 2012;92:253–259. doi: 10.1016/j.tube.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Sarda P, Sharma SK, Mohan A. Role of acute viral hepatitis as a confounding factor in antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2009;129:64–67. [PubMed] [Google Scholar]
- 14.Singla R, Sharma SK, Mohan A. Evaluation of risk factors for antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2010;132:81–87. [PubMed] [Google Scholar]
- 15.Babalik A, Arda H, Bakirci N, Agca S, Oruc K, Kiziltas S, Cetintas G, Calisir HC. Management of and risk factors related to hepatotoxicity during tuberculosis treatment. Tuberk Toraks. 2012;60:136–144. [PubMed] [Google Scholar]


