Abstract
Objective: To evaluate the correlation of epicardial adipose tissue volume (EATV) with the coronary artery lesion and its severity. Methods: Inpatients with suspicious stable angina of coronary heart lesion were recruited. For patients with coronary artery lesions in CTA, further coronary angiography (CAG) was performed to evaluate the coronary artery lesion. Gensini scoring system was employed to assess the severity of coronary artery lesions. Results: Patients were classified as coronary heart disease (CHD) group (n = 160). Results showed the mean EATV was 192.57 ± 30.32 cm3 in CHD group, which was significantly larger than that in control group (138.56 ± 23.18 cm3; P < 0.01). The coronary artery stenosis was classified as mild, moderate and severe stenosis according to the extent of coronary artery lesions, and results showed marked difference in the EATV among patients with different severities of coronary artery stenosis (P < 0.005). The Gensini score was positively related to EATV (r = 0.285, P = 0.000). The EATV increased with the increase in the number of affected coronary arteries. Multivariate Logistic regression analysis showed EATV was an independent risk factor of CHD after adjusting other confounding factors (OR = 1.023, P = 0.013). Conclusion: EATV is closely related to the severity of coronary artery lesions: the larger the EATV, the more severe the coronary artery lesions. Moreover, EATV is an independent risk factor of CHD.
Keywords: Volume of epicardial adipose tissue, coronary artery lesion, coronary arteriography, computer-assisted tomography
Introduction
The epicardial adipose tissues (EAT) refer to the fat tissues depositing outside of pericardial sac. Recent studies showed that the volume of EAT (EATV) was related to the occurrence of coronary heart diseases (CHD). Several studies proposed that EATV was an independent risk factor of CHD as other traditional risk factors of CHD such as hypertension, diabetes, smoking and abdominal obesity [1-3]. However, few studies have been conducted to investigate the relationship between EATV and CHD due to the limitation of detection methods and small sample sizes. In the present study, 256-slice spiral CT coronary angiography (CTA) was performed to measure the EATV in patients with suspicious stable angina, and the severity of coronary artery lesions was determined by coronary angiography (CAG). The correlation of EATV with coronary artery lesions and their severity was further evaluated.
Materials and methods
Subjects
From April 2013 to August 2014, patients with suspicious stable angina received CTA or CAG to confirm the diagnosis in our hospital. Following conditions were excluded: acute myocardial infarction; irregular heart rhythm (such as frequent premature beats, atrial fibrillation and other malignant arrhythmias); untreated congestive heart failure; severe liver or kidney dysfunction; concomitant fever or acute infection; severe electrolyte disturbance; sensitivity to iodine contrast agent and unavailable informed consent. A total of 208 patients were recruited into the present study, and there were 133 males and 75 females with a mean age of 58.5 ± 9.52 years old.
Collection of clinical information
Following clinical information was collected: age and past medical history (CHD, and risk factors of CHD such as hypertension, diabetes, hyperlipidemia and smoking). According to the WHO criteria, the body mass index (BMI) was calculated as follow: BMI = body weight/height2 (kg/m2). The waist circumference (WC) was also measured: patients stood with both foot separating 25-0 cm, and the WC was measured at the middle point of the line between anterior superior iliac spine and lower edge of 12th rib.
Laboratory detections
Fasting blood was collected in the morning, and automatic biochemical analyzer was used to detect triglycerides, total cholesterol, high density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDLC), fasting blood glucose (FBG), creatinine, blood urea nitrogen, high-sensitivity C-reactive protein (Hs-CRP), white blood cells and platelets.
256-slice spiral CT coronary angiography
All the patients routinely received 256-slice spiral CT coronary angiography (CTA; Philips). Patients held the breath for 10 s before scanning. Then, patients lied in a supine position and connected to an electrocardiogram monitor. Following inspiration, the patients held the breath, followed by scanning. Before scanning, 20 ml of contrast agent was injected via the cubital vein. Scanning was performed at the plane of ascending aortic root (1 frame/s), and the peak concentration of intravascular contrast (CT value) was measured to determine the scan trigger threshold. Then, 65 ml of contrast was injected into one tube and 40 ml of normal saline into the other tube of binocular high-pressure syringe. After bolus injection of 65 ml of contrast (5-5.5 ml/s), normal saline was injected immediately. When the CT value of the ascending aorta was higher than the pre-designed threshold, the scanning was triggered. Scanning of the whole heart was performed during the breath-holding period (10 s).
The parameters for scanning varied among patients: voltage: 120-140 kV; effective current: 600-800 mA; width of collimation: 256 × 0.6 mm; pitch: 0.2; time of rotating scanning: 0.35. Scanning was done within 3-5 s from the tracheal bifurcation to the cardiac base. Following scanning, R-R phases of 35%, 45% and 75% was constructed. The image post-processing workstation was employed to post-process the original figures. Construction was done by multiplanar reconstruction (MPR), curved planar reformation (CPR), volume reconstruction (VR) and maximum intensity projection. At the same time, intelligent vessel analysis software was employed to analyze the severity of coronary artery stenosis and the morphology of atherosclerotic plaques. Above methods of construction were flexibly used on the basis of specific condition to assure the best image quality and data measurement which was independently done by two experienced radiologists. Disagreements were resolved by discussion.
Measurement of EATV: Volume measurement software of CTA was employed to detect EATV. The slice thickness was 2.5 mm; voltage was 12.-140 kV, current 300-320 mA, field 250 mm, matrix 512 × 512, heart scanning mode was used, retrospective ECG-gated scanning was performed from the aortic arch to the cardiac base (including the whole heart). During the scanning, patients held the breath, to minimize the respiratory motion artifacts. The pericardium was manually outlined, and the heart image was obtained. The fat tissues were outlined with the width of the window at -250~-30 HU, followed by measurement of EATV.
The findings from CTA were classified according to the criteria developed by the American Heart Association. The coronary artery was divided into 15 segments: Left main artery and left anterior descending artery include 5-10 segments: left main artery (LMA), proximal left anterior descending artery (LAD-P), middle left anterior descending artery (LAD-M) and distal left anterior descending artery (LAD-D), the first diagonal branch (D1), the second diagonal branch (D2); Left circumflex artery includes 11-15 segments: proximal left circumflex artery (LCX-P), Obtuse marginal branch (OM), distal left circumflex artery (LCX-D), posterior lateral branch (PL), posterior descending branch (PD); Right coronary artery includes 1-4 segments: proximal right coronary artery (RCA-P), middle right coronary artery (RCA-M), distal right coronary artery (RCA-D), posterior descending artery/acute marginal branch/branch of sinuatrial node (PDA/AM/SN). The most stenotic segment of the artery was evaluated. Severe stenosis was defined when one or more main segments had > 75% stenosis; moderate stenosis was defined when one or more main segments had 50-75% stenosis; mild stenosis was defined when one or more main segments had < 50% stenosis. Patients without coronary arterial stenosis at CTA served as controls.
CAG
For patients with coronary stenosis at CTA, further CAG was performed with Artis zee Ceiling angiography machine. Puncture was done at the radial artery or femoral artery, and 3000 IU of heparin was injected. Multi-position projection was employed as usually, to completely expose different segments of coronary artery. If necessary, nitroglycerin was injected into the coronary artery to relieve coronary spasm. The images with the most severe stenosis were captured and the severity of coronary stenosis was evaluated by two experienced radiologists independently by using the method of quantitative coronary angiography.
Images from CAG were also used to evaluate the coronary artery lesions and their severity as above mentioned. When there was discrepancy in the severity of coronary steonsis evaluated with CTA and CAG, the results from CAG were used; when coronary artery lesions were present at ATC and coronary artery stenosis was not evident at CAG, non-CHD was defined. Gensini scoring system was employed to evaluate the coronary arterial stenosis of each segment: scoring was done according to the severity of stenosis and its significance. The most stenotic segment was used to determine the severity of stenosis: score 1, 1-25% stenosis; 2, 26%-50%; 4, 51%-75%; 8, 76%-90%; 16, 91%-99%; 32, 100%. The above score was multiplied by different coefficients depending on the site of stenosis: left main artery: 5; proximal left anterior descending artery and proximal Left circumflex artery: 2.5; middle left anterior descending artery: 1.5; proximal, middle, and distal right anterior descending artery, posterior right anterior descending artery, distal left anterior descending artery, the first diagonal branch, obtuse marginal branch, distal left circumflex artery and posterior descending artery: 1; remaining segments: 0.5. The total score was used as the final score of a specific patient. The sites of lesions included left main artery, anterior descending artery, circumflex artery and right coronary artery. The lesions at branches were classified as main artery lesions and the number of branches involved was determined: single branch, two-branch and three-branch lesions.
Statistical analysis
Statistical analysis was performed with SPSS version 13.0. Quantitative data are expressed as mean ± standard deviation, and qualitative data as percentages. Comparisons of quantitative data were done with independent t test between two groups. Multivariate logistic regression analysis was adopted to evaluate the risk factors of CHD. The correlation of EATV with Gensini score was evaluated with Pearson correlation analysis. A value of P < 0.05 was considered statistically significant.
Results
Of 208 patients receiving CTA, coronary stenosis was found in 164 patients, and there was no evident coronary stenosis in remaining 44 patients who then served as controls. Of 164 patients, further CAG was performed, and coronary stenosis was identified in 160 patients who were included into CHD group, and remaining 4 patients were included into control group. Thus, a total of 48 patients were included in control group.
There were no significant differences in the age, gender, hypertension, diabetes, smoking, height, WC, body weight, BMI, FBG, TG, TC, HDL-C, LDL-C, creatinine, uric acid, white blood cells, platelets and hs-CRP between CHD patients and controls (P > 0.05). The EATV was 192.57 ± 30.32 cm3 in CHD patients, which was significantly larger than that in control group (138.56 ± 23.18 cm3; P < 0.01; Table 1). Linear regression analysis showed significant correlations of EAVT with numbers and locations of coronary artery lesions (P < 0.01, Figure 1).
Table 1.
General information and EATV in CHD group and control group
| Control group n = 48 | CHD group n = 160 | t/x2 | P | |
|---|---|---|---|---|
| Age (yr) | 56.21 ± 8.91 | 60.75 ± 9.51 | -1.335 | 0.138 |
| Male (%) | 20 (41.67%) | 113 (70.63%) | 0.024 | 0.911 |
| Hypertension (%) | 19 (39.58%) | 110 (68.75%) | 1.560 | 0.324 |
| Diabetes (%) | 10 (20.83%) | 102 (63.75%) | 0.802 | 0.424 |
| Smoking (%) | 13 (20.83%) | 96 (60.0%) | 2.011 | 0.217 |
| Family history of CHD | 10 (20.83%) | 92 (57.5%) | 0.221 | 0.602 |
| Height (cm) | 169.4 ± 4.02 | 170.5 ± 7.06 | -1.072 | 0.264 |
| Body weight (Kg) | 69.81 ± 8.90 | 72.82 ± 7.55 | -1.031 | 0.232 |
| WC (cm) | 97.23 ± 8.10 | 99.82 ± 9.04 | -1.222 | 0.048 |
| BMI/Kg/m2 | 25.52 ± 28.76 | 27.41 ± 3.86 | -0.620 | 0.576 |
| FBG (mmol/l) | 5.11 ± 1.02 | 6.58 ± 4.46 | -1.670 | 0.076 |
| TG (mmol/l) | 2.02 ± 1.32 | 2.15 ± 0.95 | -1.611 | 0.070 |
| TC (mmol/l) | 4.70 ± 0.82 | 4.76 ± 1.37 | -1.120 | 0.245 |
| HDL-C (mmol/l) | 1.37 ± 0.30 | 1.30 ± 0.36 | -0.810 | 0.314 |
| LDL-C (mmol/l) | 2.56 ± 0.72 | 2.63 ± 0.66 | -0.371 | 0.712 |
| Creatinine (umol/l) | 81.63 ± 18.88 | 76.21 ± 21.28 | 0.875 | 0.368 |
| Uric acid (umol/l) | 307.14 ± 78.52 | 306.70 ± 67.87 | -0.315 | 0.715 |
| WBC | 6.48 ± 1.70 | 6.57 ± 1.45 | -0.017 | 0.960 |
| PLT | 187.1 ± 12.2 | 187.6 ± 11.4 | 0.740 | 0.481 |
| Hs-CRP (mg/dl) | 4.1 ± 3.7 | 6.0 ± 4.74 | -1.021 | 0.22 |
| EATV (cm3) | 138.56 ± 23.18 | 192.57 ± 30.32 | -3.127 | 0.001* |
P < 0.01, CHD group compare with control group.
Figure 1.
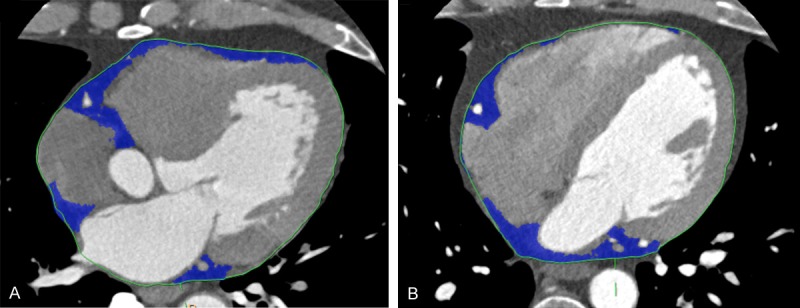
Imaging of epicardial fat tissue from both groups. A. Epicardial tissue (blue) in coronary heart disease group; B. Epicardial tissue (blue) in control group.
Univariate logistic regression analysis was employed to evaluate the correlation of EATV with other risk factors. Results showed EATV and WC were risk factors of CHD; HDL-C and LDL-C were protective factors of CHD. Multivariate logistic regression analysis showed EATV was still a risk factor of CHD after adjusting other confounding factors (OR = 1.023, P = 0.013) (Table 2).
Table 2.
Logistic regression analysis of risk factors of CHD
| Risk factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | P | OR (95% CI) | P | |
| Age/(yr) | 1.02 (0.972~1.231) | 0.132 | 1.023 (0.885~1.012) | 0.079 |
| Male | 2.18 (0.757~6.020) | 0.123 | 1.116 (0.231~4.805) | 0.734 |
| Hypertension | 1.23 (0.715~2.245) | 0.214 | 1.078 (0.451~2.561) | 0.615 |
| Diabetes | 1.64 (0.723~3.235) | 0.202 | 1.601 (0.620~3.250) | 0.252 |
| Smoking | 1.36 (0.626~2.420) | 0.348 | 1.457 (0.630~3.241) | 0.320 |
| Family history of CHD | 0.85 (0.311~1.852) | 0.610 | 0.584 (0.251~2.020) | 0.511 |
| BMI/Kg/m2 | 0.84 (0.321~1.870) | 0.103 | 0.722 (0.594~1.003) | 0.063 |
| WC (cm3) | 1.075 (1.021~1.151) | 0.006 | 1.081 (0.984~1.122) | 0.067 |
| HDL-C (mmol/l) | 0.13 (0.030~0.721) | 0.051 | 0.112 (0.020~1.021) | 0.053 |
| LDL-C (mmol/l) | 0.85 (0.520~1.567) | 0.855 | 0.782 (0.279~1.620) | 0.526 |
| EATV (cm3) | 1.02 (1.002~1.010) | 0.001 | 1.023 (1..002~1.021) | 0.013 |
In CHD group, results showed 68 patients had mild stenosis, of whom there were 305 lesions and the Gensini score was 9.6 ± 5.9; 47 patients had moderate stenosis of whom there were 192 lesions and the Gensini score was 28.9 ± 8.1; 45 patients had severe stenosis, of whom there were 136 lesions and the Gensini score was 51.3 ± 15.4. Analysis showed the Gensini score was positively related to EATV (r = 0.285, P = 0.000) (Table 3).
Table 3.
Relationship between EATV and Gensini score
| Severity of stenosis | n | EATV (cm3) | Gensini score |
|---|---|---|---|
| Mild | 68 | 145.26 ± 32.8 | 9.6 ± 5.9 |
| Moderate | 47 | 171.56 ± 47.30 | 28.9 ± 8.1 |
| Severe | 45 | 195.58 ± 37.45 | 51.3 ± 15.4 |
In addition, 63 patients had lesions at a single branch, 56 patients at two branches and 21 patients at three branches. The EATV increased with the increase in the number of branches involved. The EATV was the largest in patients with lesions at three branches and was significantly different from that in patients with lesions at single branch or two branches (two vs single: P < 0.001; three vs single: P < 0.001). However, there was no marked difference in the EATV between patients with lesions at two and three branches (P = 0.305; Table 4).
Table 4.
Correlation of EATV with the number and sites of coronary lesions
| Branch of coronary lesions | n | X ± S (cm3) |
|---|---|---|
| Single branch | ||
| LAD | 30 | 165 ± 39.2 |
| LCX | 11 | 152 ± 34.9 |
| RCA | 22 | 167 ± 27.9 |
| Two-branch | ||
| LAD + LCX | 22 | 178 ± 35.5 |
| LAD + RCA | 23 | 184 ± 39.2 |
| LCX + RCA | 11 | 186 ± 34.5 |
| Three branch | 21 | 195 ± 43.7 |
Discussion
It is well known that obesity is an independent risk factor of CHD and studies have confirmed that the localized visceral fat play more important roles in the progression of cardiovascular lesions and diseases as compared to systemic fat. In recent year, increasing evidence shows that EATV is important for the occurrence and development of coronary atherosclerosis. Epicardium is a visceral membrane covering the heart. EAT refers to the fat tissues depositing around the heart, especially at the sites around the coronary artery. EAT covers more than 80% of the heart and accounts for 1% of systemic fat, but EATV accounts for 15-20% of the heart volume [4]. Generally, the differentiated white adipose tissues completely cover the atrioventricular groove and interatrial groove and extend to the apex of the heart. Adipose tissues mainly localize in the right ventricle. When the adipose tissues increase, they may fill the gap between the ventricles and cover the whole epicardium. Some adipose tissues may extend along the branches of coronary artery into the myocardium. Anatomically and functionally, the adipose tissues are closely related to the myocardium, and supplied by the coronary artery. Thus, the EAT is crucial for the pathophysiology of myocardium and coronary artery.
MRI has been used as a golden standard for the measurement of EAT [2]. However, MRI is time consuming and costly. With the introduction of multiple slice CT (MSCT), “green” scanning with low radiation dose has been developed and used in clinical practice and has a short time of scanning. Thus, MSCT is an economic, affordable, accurate, reliable, repeatable and safe tool for the measurement of adipose tissues [5] and currently used in the measurement of EAT. The thickness of adipose tissues around the coronary artery and whole EATV can be measured, both of which are more accurate when compared with those determined by heart ultrasonography [6]. Thus, in the present study, 256-splice spiral CT was performed to measure EATV, aiming to investigate the correlation of EATV with coronary lesions and their severity.
Chaowalit et al [7] investigated 139 patients with CHD. Results showed EATV was closely related to the CHD and its severity. Other studies also confirmed that EATV in non-CHD patients is significantly smaller than that in CHD patients, but it serves as an independent risk factor of CHD [8]. Oyama et al investigated 957 females. Results showed, when compared with females without a history of CHD, females with a history of CHD had larger EATV. Mahabadi et al [3] employed MSCT to measure the EATV, visceral fat volume (VEV) and intrathoracic fat volume. Their results showed, after adjusting gender, age and BMI, EATV was still an independent risk factor of CHD. Cheng et al followed up 232 patients without symptoms of CHD for 4 years, and results indicated that EATV in patients with cardiovascular events was larger than that in patients without these events, and to measure EATV was helpful to predict cardiovascular events [1]. In addition, studies also confirm that EATV is a risk factor of CHD and may serve as a non-invasive parameter to predict CHD [9,10]. Among numerous clinical indicators, EATV has significant influence on the Gensini score, suggesting EATV is positively related to the severity of coronary stenosis. In the present study, EATV was measured in 208 patients and results confirmed the extent of adipose tissues covering on the heart. When compare with controls, patients with CHD had a significantly larger EATV (P < 0.05), suggesting EATV was related to CHD. In addition, EATV in patients with severe stenosis was also markedly larger than that in patients with mild or moderate stenosis. Further quantification revealed that the larger the EATV, the more severe the Gensini score was, suggesting that quantification of EATV is important for the determination of CHD severity. Moreover, results showed there was no marked correlation between EATV and single-branch lesions; EATV increased with the increase in the number of branches involved; patients with three-branch lesions had the largest EATV.
There is evidence showing that EAT is significantly related to risk factors of CHD [11]. The EAT increases over age, and is related parameters of obesity, fasting insulin and arterial blood pressure. Some investigators have shown that EAT is closely related to risk factors of CHD such as blood pressure, blood glucose, TC, TG, LDL-C, HDL-C, impaired insulin sensitivity and fasting blood glucose [12]. Greif et al [13] found EATV increased with the increase in the number of risk factors of cardiovascular diseases and significantly associated with hypertension, diabetes, age and hyperlipidemia. In the present study, univariate logistic regression analysis showed EATV and WC were independent risk factors of CHD. Multivariate logistic regression analysis showed EATV was still a risk factor of CHD after adjusting other confounding factors.
However, the mechanism underlying the relationship between EAT and CHD is still unclear. Studies have revealed that cytokines secreted by adipose tissues may act on adjacent coronary artery and myocardium via paracrine or supporting vessels, which may be involved in the occurrence and development of cardiovascular disease, especially the CHD [14]. There are a large amount of inflammatory mediators in the visceral adipose tissues such as tumor necrosis factor, monocyte chemotactic factor-1, interleukin-1b, interleukin-6, soluble interleukin-6 receptor, and resistin. These cytokines in the adipose tissues may serve as key inflammatory mediators to induce atherosclerosis and increase the risk for CHD [15]. EAT around the coronary artery may focally intensify the systemic pro-atherogenic effect via outside-in signal transduction to promote the occurrence and development of coronary atherosclerosis.
Taken together, 256-splice spiral CTA is able to accurately and conveniently evaluate the thickness and volume of epicardial adipose tissues. EATV is an independent risk factor of CHD and may serve as a non-invasive indicator used to predict CHD. EATV is closely related to the severity of coronary lesions, and the larger the EATV, the more severe the coronary lesion is.
Disclosure of conflict of interest
None.
References
- 1.Iacobellis G, Willens HJ, Barbaro G, Sharma AM. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity (Silver Spring) 2008;16:887–892. doi: 10.1038/oby.2008.6. [DOI] [PubMed] [Google Scholar]
- 2.Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK, Rhee SJ, Lee EM, Lee J, Yoo NJ, Kim NH, Park JC. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007;71:536–539. doi: 10.1253/circj.71.536. [DOI] [PubMed] [Google Scholar]
- 3.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O’Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010;17:115–130. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- 5.Sarin S, Wenger C, Marwaha A, Qureshi A, Go BD, Woomert CA, Clark K, Nassef LA, Shirani J. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am J Cardiol. 2008;102:767–771. doi: 10.1016/j.amjcard.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 6.Gorter PM, van Lindert AS, de Vos AM, Meijs MF, van der Graaf Y, Doevendans PA, Prokop M, Visseren FL. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197:896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Chaowalit N, Somers VK, Pellikka PA, Rihal CS, Lopez-Jimenez F. Subepicardial adipose tissue and the presence and severity of coronary artery disease. Atherosclerosis. 2006;186:354–359. doi: 10.1016/j.atherosclerosis.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Djaberi R, Schuijf JD, van Werkhoven JM, Nucifora G, Jukema JW, Bax JJ. Relation of epicardial adipose tissue to coronary atherosclerosis. Am J Cardiol. 2008;102:1602–1607. doi: 10.1016/j.amjcard.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R, Berman DS, Lahiri A. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012;220:223–230. doi: 10.1016/j.atherosclerosis.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 10.Sengul C, Cevik C, Ozveren O, Oduncu V, Sunbul A, Akgun T, Can MM, Semiz E, Dindar I. Echocardiographic epicardial fat thickness is associated with carotid intima-media thickness in patients with metabolic syndrome. Echocardiography. 2011;28:853–858. doi: 10.1111/j.1540-8175.2011.01471.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 12.Wang CP, Hsu HL, Hung WC, Yu TH, Chen YH, Chiu CA, Lu LF, Chung FM, Shin SJ, Lee YJ. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol (Oxf) 2009;70:876–882. doi: 10.1111/j.1365-2265.2008.03411.x. [DOI] [PubMed] [Google Scholar]
- 13.Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, Tittus J, Parhofer K, Becker C, Reiser M, Knez A, Leber AW. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:781–786. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 14.Langheim S, Dreas L, Veschini L, Maisano F, Foglieni C, Ferrarello S, Sinagra G, Zingone B, Alfieri O, Ferrero E, Maseri A, Ruotolo G. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol. 2010;298:H746–753. doi: 10.1152/ajpheart.00617.2009. [DOI] [PubMed] [Google Scholar]
- 15.Sade LE, Eroglu S, Bozbas H, Ozbicer S, Hayran M, Haberal A, Muderrisoglu H. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2009;204:580–585. doi: 10.1016/j.atherosclerosis.2008.09.038. [DOI] [PubMed] [Google Scholar]


