Abstract
Objectives: To evaluate the expression and significance of CK19, TPO, and HBME-1 in the differential diagnosis of papillary thyroid carcinoma (PTC) and nonmalignant nodules. Methods: Tissue samples were obtained from 257 patients with PTC and 149 patients with nonmalignant thyroid specimens, and immunohistochemical staining for CK-19, TPO, and HBME-1 was performed. Results: The expression of CK-19, TPO, and HBME-1 was 96.3%, 12.0%, and 85.3%, respectively, for the PTC group. For nonmalignant thyroid lesions group, the expression of these markers was 40.4%, 86.2%, and 37.2%, respectively. Further, the expression of CK-19 and HBME-1 in PTCs was much higher than that in the benign thyroid lesions (P < 0.05). However, the positive expression of TPO in PTC specimens was much lower than that in the nonmalignant specimens (P < 0.05). CK-1 had the highest sensitivity (96.30%) for PTCs. The combination of the positive expression of CK-19 and negative expression of TPO had the highest sensitivity (98.50%), while that of the positive expression of HBME-1 and negative expression of TPO had the highest specificity (92.90%). Conclusions: The combination of positive expression of CK-19 or HBME-1 or negative expression of TPO can improve the specificity of the diagnosis of PTC.
Keywords: Papillary thyroid carcinoma, cytokeratin-19, thyroid peroxidase, human bone marrow endothelial cell-1
Introduction
Papillary thyroid carcinoma (PTC) is the most common malignant thyroid neoplasm and accounts for 80% of all thyroid cancers [1,2]. Both cytological and histological examinations of the specimens for pathological diagnosis of PTC are based on the typical nuclear morphology; however, it is often difficult to differentiate PTC from benign papillary hyperplasia when the diagnostic nuclear features are insufficient to make a clear diagnosis. Immunohistochemical markers such as calcitonin, BRAF, CK19, and galectin-3 may help distinguish PTCs from nonmalignant thyroid lesions [3-5].
Although immunohistochemical staining for various protein markers has been used in the diagnosis of PTC in recent years, the use of combinations of several proteins to increase the accuracy of PTC diagnosis is still controversial [6,7].
Therefore, in this study, we aimed at evaluating the diagnostic performances of 3 immunohistochemical markers-CK19, TPO, and HBME-1-for PTC and determining the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of these 3 proteins individually and in combination with one another to distinguish PTC from other nonmalignant thyroid lesions.
Patients and methods
Patient population
We retrospectively identified patients who had histologically confirmed papillary thyroid carcinoma and were treated between December 2011 and January 2014 at the Department of Breast and Thyroid Surgery, Union Hospital Wu Han, China. This study was approved by the ethics committees of the Union Hospital.
Tissue samples and immunohistochemistry (IHC)
Samples used for this study were obtained from the patients mentioned above at the Department of Breast and Thyroid Surgery, Union Hospital. The tissues were embedded in paraffin and continuously sliced into 3-4 μm sections. For IHC analysis, tissue sections were routinely treated as follows: The tissue was deparaffinized in xylene and rehydrated in descending alcohol dilutions. Subsequently, it was heated in a boiling 0.01 mol/L citrate buffer (PH 6.0) for 20 min for antigen retrieval. After cooling it to room temperature, sections were treated with 3% hydrogen peroxide for 10 min to block endogenous peroxidase and then blocked with goat serum (1: 10 dilution in 0.1 M phosphate-buffered saline [PBS; pH 7.4]) for 20 min at room temperature. Thereafter, the sections were incubated with the following primary antibodies overnight at 4°C: CK-19 (Product ID: MAB-0056, clone: A53-B/A2.26), thyroid peroxidase (TPO) (Product ID: MAB-0630, clone: AC25), and HBME-1 (Product ID: MAB-0130, clone: HBME-1).
PBS-incubated slides were used as a negative control. The slides were washed 3 times in 0.1 M PBS (pH 7.4) for 3 min and then incubated with a biotinylated horseradish-peroxidase goat anti-mouse/rabbit secondary antibody for 20 min at room temperature. Thereafter, the sections were visualized with 0.05% diaminobenzidine in 0.01 M PBS (pH 7.4) for 5 min at room temperature. Hematoxylin was used for counterstaining. After dehydrating the sections in descending alcohol dilutions, they were sealed and covered with glass coverslips. All slides were processed by the same pathologist.
The sliced sections were stained with hematoxylin and eosin for histological examination. Two independent pathologists reviewed all histological sections and evaluated the IHC staining results according to the criteria of the World Health Organization. In case of disagreement, the results were reanalyzed until a consensus was reached.
Statistical analysis
EpiData software (version 3.1; EpiData Association, Odense, Denmark) was used for entry of initial clinical and pathological data. Statistical analyses were performed using SPSS software (version 13.0; IBM SPSS, Inc., Chicago, IL, USA). Fisher’s exact test was used to determine the significance of differences between the PTC group and benign group. P values < 0.05 were considered statistically significant.
Results
Tissue samples were obtained from 257 patients with PTC (56 men and 201 women; age range, 19-79 years) and 149 patients with nonmalignant thyroid nodules (34 men and 115 women; age range, 22-80 years). No statistically significant difference was noted in the sex and age distribution between patients with PTC and those with benign thyroid nodules.
Immunohistochemical staining for CK-19, TPO, and HBME-1 was conducted for all specimens (Table 1; Figure 1). Patients with PTC more frequently expressed positive CK-19 and HBME-1 and negative TPO compared to patients with benign thyroid nodules (all P < 0.001). When analyzed individually, positive expression of CK-19 had the highest sensitivity (96.3%) for PTCs; the sensitivity of negative TPO expression and positive expression of HBME-1 were 88.0% and 85.3%, respectively, in PTCs.
Table 1.
Immunohistochemical scores for the staining with the 3 protein markers in surgical specimens of thyroid cancer from patients with papillary thyroid carcinoma and samples from patients with thyroid nodules
| Protein staining | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Immunohistochemical score | Positive (%) | Statistical significance (P value) | ||||
| Protein markers | Group | n | - | + to +++ | ||
| Ck-19 | PTC | 240 | 9 | 231 | 96.3 | < 0.001 |
| Benign | 146 | 87 | 59 | 40.4 | ||
| TPO | PTC | 234 | 206 | 28 | 12.0 | < 0.001 |
| Benign | 145 | 20 | 125 | 86.2 | ||
| HBME-1 | PTC | 136 | 20 | 116 | 85.3 | < 0.001 |
| Benign | 86 | 54 | 32 | 37.2 | ||
Corresponding immunohistochemical score: -, negative; + to +++, weakly positive to strongly positive. CK-19, cytokeratin-19; TPO, thyroid peroxidase; HBME-1, human bone marrow endothelial cell-1.
Figure 1.
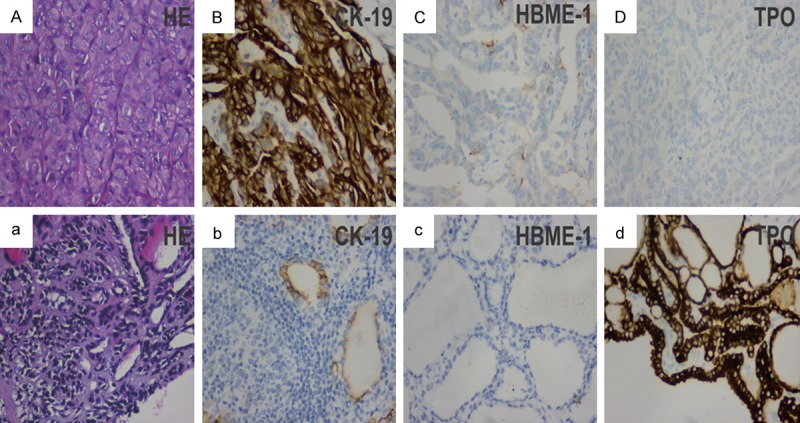
Immunoreactivity of CK19, TPO, and HBME-1 in PTCs and nonmalignant thyroid lesions. A-D: HE, CK19, TPO, and HBME-1 staining in PTCs; a-d: HE, CK19, TPO, and HBME-1 staining in nonmalignant thyroid lesions.
The performance of positive expression of CK-19 (CK19+) in the diagnosis for PTC was as follows: sensitivity, 96.30%; specificity, 40.40%; positive predictive value (PPV), 72.60%; negative predictive value (NPV), 86.80%; and accuracy, 75.10%. The performance of negative expression of TPO (TPO-) for the diagnosis of PTC was as follows: sensitivity, 88.00%; specificity, 86.20%; PPV, 91.20%; NPV, 81.70%; and accuracy, 87.30%. Further, the performance for positive expression of HBME-1 (HBME-1+) for the diagnosis of PTC was as follows: sensitivity, 85.30%; specificity, 62.80%; PPV, 85.10%; NPV, 73.00%; and accuracy, 76.60% (Table 2).
Table 2.
Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the 3 protein markers in the diagnosis of papillary thyroid carcinoma
| Protein markers | Sensitivity | specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| CK-19+ | 96.30% | 40.40% | 72.60% | 86.80% | 75.10% |
| TPO- | 88.00% | 86.20% | 91.20% | 81.70% | 87.30% |
| HBME+ | 85.30% | 62.80% | 85.10% | 73.00% | 76.60% |
CK-19, cytokeratin-19; TPO, thyroid peroxidase; HBME-1, human bone marrow endothelial cell-1.
Regarding the combinations of these protein markers, we found that the combination of CK19+ and TPO- had the highest sensitivity (98.50%), followed by the combination of CK19+, TPO-, and HBME-1+ (98.00%). Thus, although the combined use of the 3 markers did not further improve the diagnostic sensitivity, it showed good diagnostic performance. The combination of HBME-1+ and TPO- had the highest specificity (92.90%). Again, the combined use of the 3 markers did not further improve the diagnostic specificity (89.30%); however, it did improve the diagnostic accuracy more than the combination of any 2 proteins (Table 3).
Table 3.
Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of different combinations of the 3 protein markers in the diagnosis of papillary thyroid carcinoma
| Protein markers combination | Sensitivity | specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| CK19 + and TPO- | 98.50% | 77.90% | 93.10% | 94.60% | 93.40% |
| CK19 + and HBME-1+ | 97.40% | 51.00% | 82.60% | 89.30% | 83.70% |
| HBME-1+ and TPO- | 94.30% | 92.90% | 96.10% | 89.70% | 93.80% |
| CK19 +, TPO- and HBME-1+ | 98.00% | 89.30% | 97.00% | 92.60% | 96.10% |
CK-19, cytokeratin-19; TPO, thyroid peroxidase; HBME-1, human bone marrow endothelial cell-1.
Discussions
Thyroid cancer is the most common malignant tumor of the endocrine system, and its incidence has increased dramatically [8-13]. The most frequent type of thyroid malignancy is PTC, which comprises 90% of all thyroid cancers [14-16]. Thus, it is critical to accurately diagnose PTC.
Immunohistochemistry techniques facilitate diagnosis of PTC when it is difficult to distinguish using histological criteria. According to the World Health Organization classification, PTC may be classified into 15 variants based on histological structure, tumor size, cell shape, cell type, and extracellular matrix. However, when there is a paucity of diagnostic nuclear features, it is often difficult to distinguish PTC from benign papillary hyperplasia. In this condition, many molecular alterations in thyroid cancer, such as CK19, TPO, and HBME-1, may help to differentiate malignant from benign thyroid lesions [5].
CK-19 (keratin 19), a member of the keratin family, plays an important role in the structural integrity of epithelial cells. The role of CK-19 in the diagnosis of thyroid carcinoma is still controversial [3-5,17-19]. Zhu et al. suggested that CK-19 was not a specific marker of PTC [18].
In our study, we observed CK-19 expression in some cases of benign thyroid lesions (59/146), but CK-19 was a highly sensitive (96.30%) marker in the diagnosis of PTC. In addition, when assessing CK-19 as a single protein marker, there was a significant difference between PTC specimens and benign thyroid nodules (P < 0.01).
HBME-1 is a component of the microvilli on the surface of mesothelial cells and has been used for the diagnosis of tumors that originate from mesothelial cells [20,21]. Previous studies demonstrated that a high rate of HBME-1-positivity, by immunohistochemistry, was observed in malignant thyroid tissues, and HBME-1 was a sensitive marker for PTCs [22,23]. In our study, when assessing the diagnostic performance of HBME-1 as a single protein marker, the sensitivity was 85.3%, similar to that in previous studies.
Griffith et al. had found that TPO was also a practical marker in terms of diagnostic utility in distinguishing nonmalignant thyroid nodules from thyroid cancer tissue [24,25]. Negative staining for TPO was a powerful identifier of differentiated thyroid carcinomas with an established cut-off value, and in addition, lower TPO mRNA expression was associated with a higher thyroid cancer stage [6,26,27]. Assessing the combination of TPO and HBME-1 resulted in the best accuracy for diagnosis of thyroid carcinoma, similar to our results (93.80%).
Morphological diagnosis methods such as immunohistochemistry and cytology of fine-needle aspiration biopsy play an important role in providing a definitive diagnosis and therapeutic guidance. Testing for expression of CK-19 and HBME-1 -has been shown to improve the diagnostic accuracy for thyroid malignant nodules [28-32]. The combination of CK-19, HBME-1, and TPO may contribute to an accurate diagnosis of thyroid papillary carcinoma by fine-needle aspiration biopsy.
Our current study demonstrated that the combination of 2 or 3 protein markers, namely CK-19, HBME-1, and TPO, provided greater diagnostic accuracy than a single protein marker. Therefore, we suggest that immunohistochemical analysis of 2 or 3 protein markers is necessary for the diagnosis of PTC, especially because of the paucity of significant diagnostic morphological features. This would also help avoid technical or operational bias. However, our study still have some limitations such as data from single center, the number of patients with benign thyroid nodules is relatively small, loss more protein markers for evaluation et. At.
In conclusion, we have verified that CK19, TPO, and HBME-1 may provide significant contributions in distinguishing PTC from benign thyroid lesions. The combination of these markers may improve the diagnostic accuracy of PTCs.
Disclosure of conflict of interest
None.
References
- 1.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z, Wang L, Yi P, Wang CY, Huang T. Risk factors for central lymph node metastasis of patients with papillary thyroid microcarcinoma: a meta-analysis. Int J Clin Exp Pathol. 2014;7:932–937. [PMC free article] [PubMed] [Google Scholar]
- 3.Song Q, Wang D, Lou Y, Li C, Fang C, He X, Li J. Diagnostic significance of CK19, TG, Ki67 and galectin-3 expression for papillary thyroid carcinoma in the northeastern region of China. Diagn Pathol. 2011;6:126. doi: 10.1186/1746-1596-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung SY, Park ES, Park SY, Song JY, Ryu HS. CXC motif ligand 12 as a novel diagnostic marker for papillary thyroid carcinoma. Head Neck. 2014;36:1005–1012. doi: 10.1002/hed.23404. [DOI] [PubMed] [Google Scholar]
- 5.Wu G, Wang J, Zhou Z, Li T, Tang F. Combined staining for immunohistochemical markers in the diagnosis of papillary thyroid carcinoma: improvement in the sensitivity or specificity? J Int Med Res. 2013;41:975–983. doi: 10.1177/0300060513490617. [DOI] [PubMed] [Google Scholar]
- 6.Paunovic I, Isic T, Havelka M, Tatic S, Cvejic D, Savin S. Combined immunohistochemistry for thyroid peroxidase, galectin-3, CK19 and HBME-1 in differential diagnosis of thyroid tumors. APMIS. 2012;120:368–379. doi: 10.1111/j.1600-0463.2011.02842.x. [DOI] [PubMed] [Google Scholar]
- 7.Gabryel B, Brominski G, Owecki M, Michalak M, Ruchala M. The prevalence of thyroid nodular disease in patients with increased titers of anti-thyroidal peroxidase antibodies. Neuro Endocrinol Lett. 2012;33:442–445. [PubMed] [Google Scholar]
- 8.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 9.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N, Zhang Y. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang C, Yang L, Wang N, Li L, Xu M, Chen GG, Liu ZM. High expression of GPER1, EGFR and CXCR1 is associated with lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2014;7:3213–3223. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang N, Dong CR, Jiang R, Tang C, Yang L, Jiang QF, Chen GG, Liu ZM. Overexpression of HIF-1alpha, metallothionein and SLUG is associated with high TNM stage and lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2014;7:322–330. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Q, Ming J, Liu C, Shi L, Xu X, Nie X, Huang T. Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Ann Surg Oncol. 2013;20:746–752. doi: 10.1245/s10434-012-2654-2. [DOI] [PubMed] [Google Scholar]
- 13.Hambleton C, Kandil E. Appropriate and accurate diagnosis of thyroid nodules: a review of thyroid fine-needle aspiration. Int J Clin Exp Med. 2013;6:413–422. [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Xun X, Wang Y, Mei L, He L, Zeng W, Wang CY, Tao H. MRI and ultrasonography detection of cervical lymph node metastases in differentiated thyroid carcinoma before reoperation. Am J Transl Res. 2014;6:147–154. [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Abdel-Mageed AB, Mondal D, Kandil E. MicroRNA expression profiles in differentiated thyroid cancer, a review. Int J Clin Exp Med. 2013;6:74–80. [PMC free article] [PubMed] [Google Scholar]
- 16.Huang DP, Ye XH, Xiang YQ, Zhang XH. Thymectomy in central lymph node dissection for papillary thyroid cancer. Int J Clin Exp Med. 2014;7:1135–1139. [PMC free article] [PubMed] [Google Scholar]
- 17.Erkilic S, Aydin A, Kocer NE. Diagnostic utility of cytokeratin 19 expression in multinodular goiter with papillary areas and papillary carcinoma of thyroid. Endocr Pathol. 2002;13:207–211. doi: 10.1385/ep:13:3:207. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X, Sun T, Lu H, Zhou X, Lu Y, Cai X, Zhu X. Diagnostic significance of CK19, RET, galectin-3 and HBME-1 expression for papillary thyroid carcinoma. J Clin Pathol. 2010;63:786–789. doi: 10.1136/jcp.2010.076901. [DOI] [PubMed] [Google Scholar]
- 19.Scognamiglio T, Hyjek E, Kao J, Chen YT. Diagnostic usefulness of HBME1, galectin-3, CK19, and CITED1 and evaluation of their expression in encapsulated lesions with questionable features of papillary thyroid carcinoma. Am J Clin Pathol. 2006;126:700–708. doi: 10.1309/044V-86JN-2W3C-N5YB. [DOI] [PubMed] [Google Scholar]
- 20.Saleh HA, Feng J, Tabassum F, Al-Zohaili O, Husain M, Giorgadze T. Differential expression of galectin-3, CK19, HBME1, and Ret oncoprotein in the diagnosis of thyroid neoplasms by fine needle aspiration biopsy. Cytojournal. 2009;6:18. doi: 10.4103/1742-6413.55894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Lois C, Ballestin C, Sotelo MT, Lopez-Rios F, Garcia-Prats MD, Villena V. Combined use of novel epithelial (MOC-31) and mesothelial (HBME-1) immunohistochemical markers for optimal first line diagnostic distinction between mesothelioma and metastatic carcinoma in pleura. Histopathology. 2001;38:528–534. doi: 10.1046/j.1365-2559.2001.01157.x. [DOI] [PubMed] [Google Scholar]
- 22.Nga ME, Lim GS, Soh CH, Kumarasinghe MP. HBME-1 and CK19 are highly discriminatory in the cytological diagnosis of papillary thyroid carcinoma. Diagn Cytopathol. 2008;36:550–556. doi: 10.1002/dc.20841. [DOI] [PubMed] [Google Scholar]
- 23.Mase T, Funahashi H, Koshikawa T, Imai T, Nara Y, Tanaka Y, Nakao A. HBME-1 immunostaining in thyroid tumors especially in follicular neoplasm. Endocr J. 2003;50:173–177. doi: 10.1507/endocrj.50.173. [DOI] [PubMed] [Google Scholar]
- 24.Griffith OL, Melck A, Jones SJ, Wiseman SM. Meta-analysis and meta-review of thyroid cancer gene expression profiling studies identifies important diagnostic biomarkers. J. Clin. Oncol. 2006;24:5043–5051. doi: 10.1200/JCO.2006.06.7330. [DOI] [PubMed] [Google Scholar]
- 25.Prasad ML, Pellegata NS, Huang Y, Nagaraja HN, de la Chapelle A, Kloos RT. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol. 2005;18:48–57. doi: 10.1038/modpathol.3800235. [DOI] [PubMed] [Google Scholar]
- 26.Segev DL, Clark DP, Zeiger MA, Umbricht C. Beyond the suspicious thyroid fine needle aspirate. A review. Acta Cytol. 2003;47:709–722. doi: 10.1159/000326594. [DOI] [PubMed] [Google Scholar]
- 27.Lazar V, Bidart JM, Caillou B, Mahe C, Lacroix L, Filetti S, Schlumberger M. Expression of the Na+/I- symporter gene in human thyroid tumors: a comparison study with other thyroid-specific genes. J Clin Endocrinol Metab. 1999;84:3228–3234. doi: 10.1210/jcem.84.9.5996. [DOI] [PubMed] [Google Scholar]
- 28.Yip L, Ferris RL. Clinical Application of Molecular Testing of Fine-needle Aspiration Specimens in Thyroid Nodules. Otolaryngol Clin North Am. 2014;47:557–571. doi: 10.1016/j.otc.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitt AC, Cohen C, Siddiqui MT. Paired box gene 8, HBME-1, and cytokeratin 19 expression in preoperative fine-needle aspiration of papillary thyroid carcinoma: diagnostic utility. Cancer Cytopathol. 2010;118:196–202. doi: 10.1002/cncy.20082. [DOI] [PubMed] [Google Scholar]
- 30.Bonzanini M, Amadori PL, Sagramoso C, Dalla Palma P. Expression of cytokeratin 19 and protein p63 in fine needle aspiration biopsy of papillary thyroid carcinoma. Acta Cytol. 2008;52:541–548. doi: 10.1159/000325595. [DOI] [PubMed] [Google Scholar]
- 31.Raggio E, Camandona M, Solerio D, Martino P, Franchello A, Orlandi F, Gasparri G. The diagnostic accuracy of the immunocytochemical markers in the pre-operative evaluation of follicular thyroid lesions. J Endocrinol Invest. 2010;33:378–381. doi: 10.1007/BF03346607. [DOI] [PubMed] [Google Scholar]
- 32.Flanagan JN, Pineda P, Knapp PE, De Las Morenas A, Lee SL, Braverman LE. Expression of cytokeratin 19 in the diagnosis of thyroid papillary carcinoma by quantitative polymerase chain reaction. Endocr Pract. 2008;14:168–174. doi: 10.4158/EP.14.2.168. [DOI] [PubMed] [Google Scholar]


