Abstract
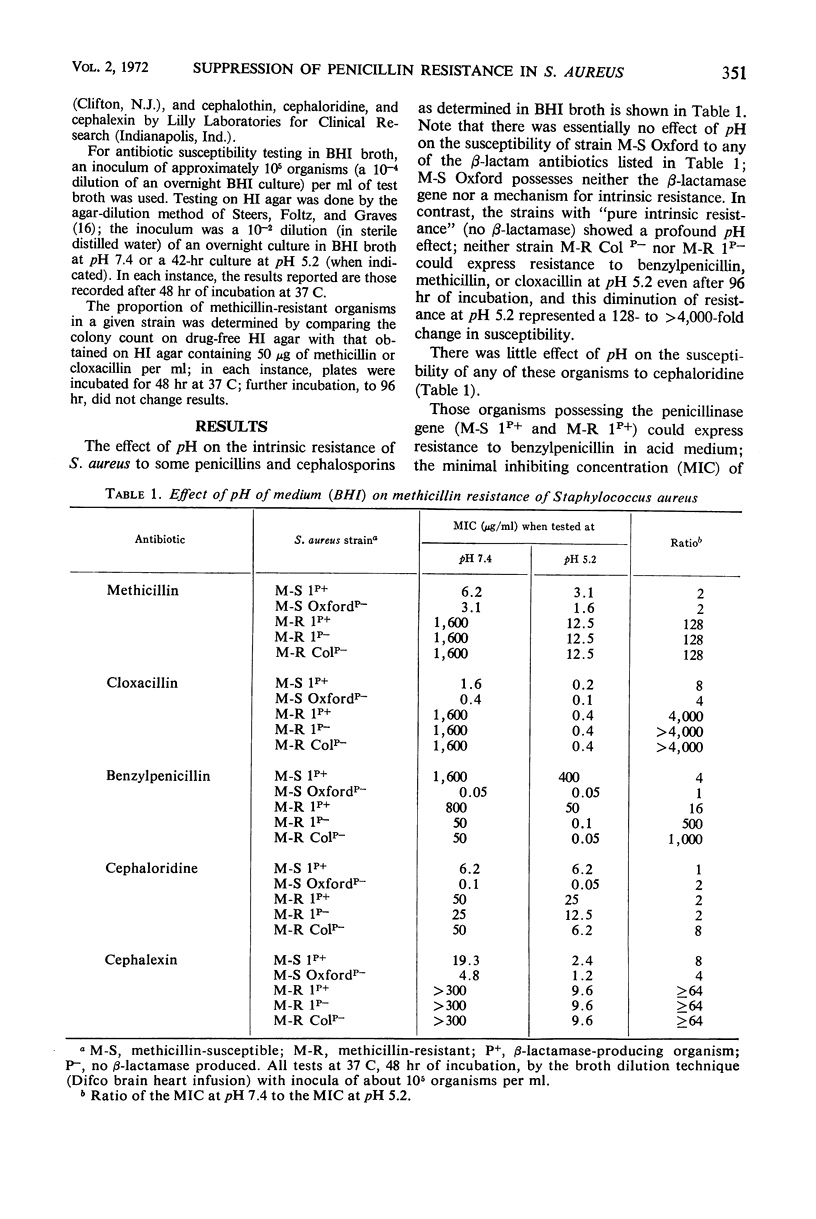
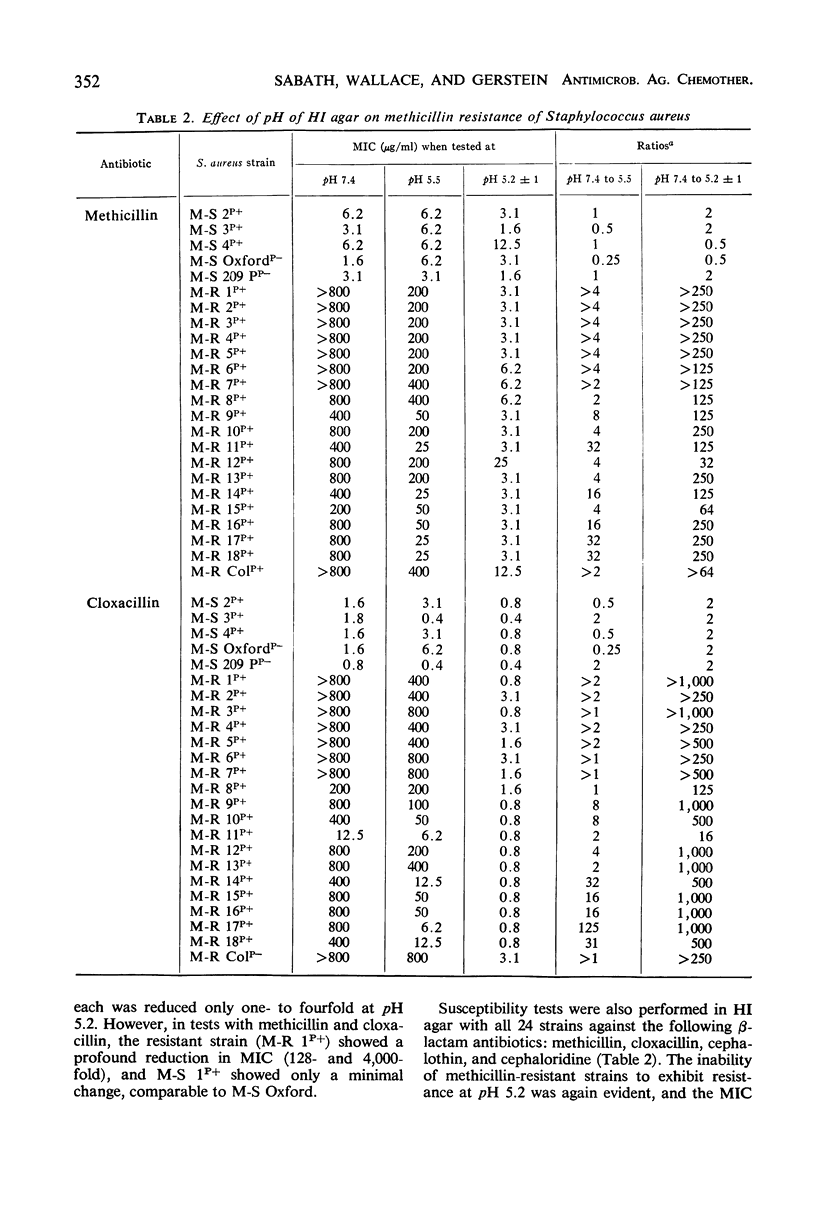
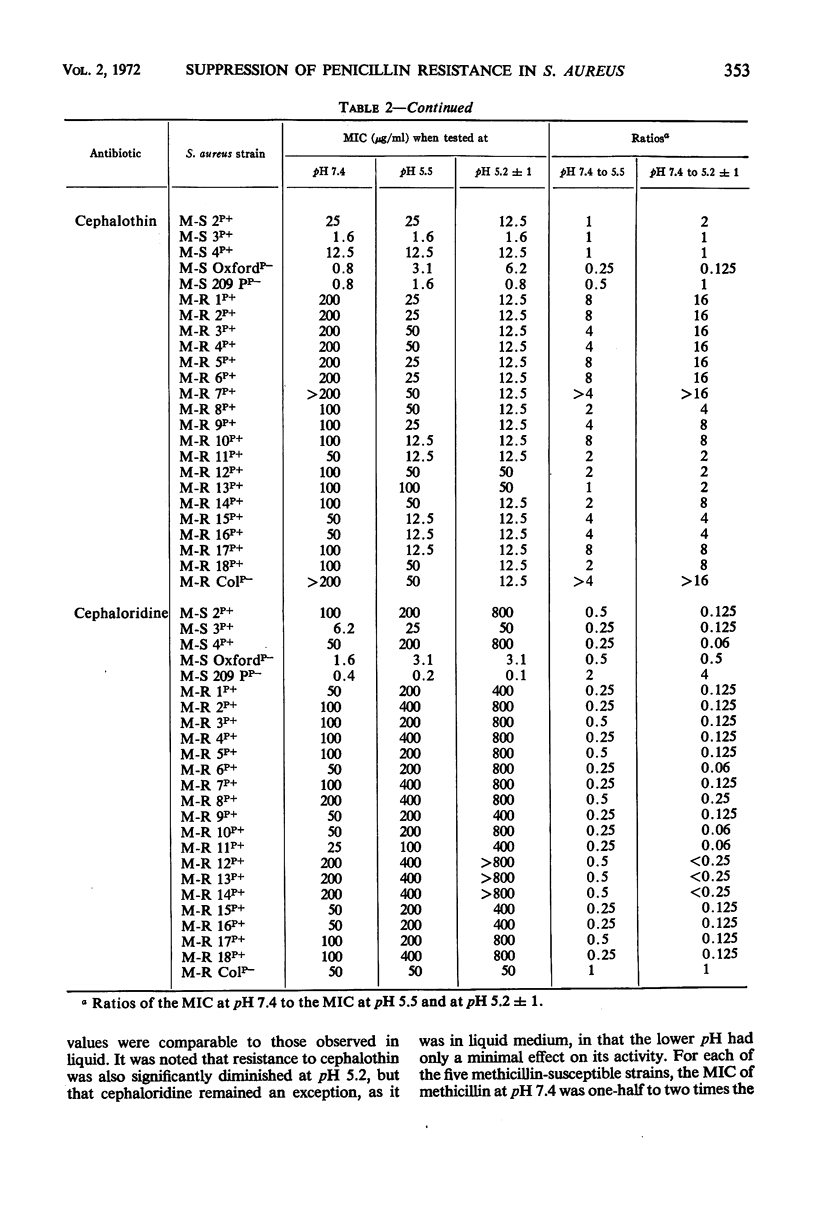
The pH of the medium in which staphylococcal susceptibility to penicillins was determined was found to make a profound difference (128- to 8,000-fold) in the expression of “intrinsic” resistance, whereas β-lactamase-mediated resistance was only slightly affected by pH; methicillin-resistant staphylococci that are β-lactamase-negative are models of pure intrinsic resistance, and the common β-lactamase-producing organisms (methicillin-susceptible) are examples of pure β-lactamase-mediated resistance. Methicillin-resistant staphylococci were unable to express their resistance at pH 5.2. However, growth of methicillin-resistant organisms in acid (pH 5.2) medium, followed by susceptibility testing at pH 7.4, showed no elimination of the genotype for intrinsic resistance, indicating that the pH effect was due to suppression, rather than to elimination of the gene determining the intrinsic resistance. These pH changes had little effect on the susceptibility of staphylococci that possessed neither intrinsic resistance nor β-lactamase-mediated resistance. Thus, the suppression of “intrinsic” resistance was highly specific, and probably not the result of a change in ionization of the antibiotic, which would have been expected to affect all cells essentially equally. It is unlikely that foci of inflammation in man become sufficiently acid to suppress methicillin resistance of the staphylococci causing infection and inflammation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annear D. I. The effect of temperature on resistance of Staphylococcus aureus to methicillin and some other antibioics. Med J Aust. 1968 Mar 16;1(11):444–446. [PubMed] [Google Scholar]
- Dyke K. G., Jevons M. P., Parker M. T. Penicillinase production and intrinsic resistance to penicillins in Staphylococcus aures. Lancet. 1966 Apr 16;1(7442):835–838. doi: 10.1016/s0140-6736(66)90182-6. [DOI] [PubMed] [Google Scholar]
- GRAVENKEMPER C. F., BRODIE J. L., KIRBY W. M. RESISTANCE OF COAGULASE-POSITIVE STAPHYLOCOCCI TO METHICILLIN AND OXACILLIN. J Bacteriol. 1965 Apr;89:1005–1010. doi: 10.1128/jb.89.4.1005-1010.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAIGHT T. H., FINLAND M. Modified Gots test for penicillinase production. Am J Clin Pathol. 1952 Aug;22(8):806–808. doi: 10.1093/ajcp/22.8_ts.806. [DOI] [PubMed] [Google Scholar]
- Houtsmuller U. M., van Deenen L. L. On the amino acid esters of phosphatidyl glycerol from bacteria. Biochim Biophys Acta. 1965 Dec 2;106(3):564–576. doi: 10.1016/0005-2760(65)90072-x. [DOI] [PubMed] [Google Scholar]
- Parker M. T., Hewitt J. H. Methicillin resistance in Staphylococcus aureus. Lancet. 1970 Apr 18;1(7651):800–804. doi: 10.1016/s0140-6736(70)92408-6. [DOI] [PubMed] [Google Scholar]
- SABATH L. D., POSTIC B., FINLAND M. Laboratory studies on methicillin. With special reference to patients treated for staphylococcal infections. Am J Med Sci. 1962 Oct;244:484–500. [PubMed] [Google Scholar]
- Sabath L. D., Gerstein D. A., Loder P. B., Finland M. Independent segregation of chloramphenicol resistance in Staphylococcus aureus. Antimicrob Agents Chemother (Bethesda) 1967;7:264–270. [PubMed] [Google Scholar]
- Sabath L. D., Leaf C. D., Gerstein D. A., Finland M. Altered cell walls of Staphylococcus aureus resistant to methicillin. Nature. 1970 Mar 14;225(5237):1074–1074. doi: 10.1038/2251074a0. [DOI] [PubMed] [Google Scholar]
- Sabath L. D. Synergy of antibacterial substances by apparently known mechanisms. Antimicrob Agents Chemother (Bethesda) 1967;7:210–217. [PubMed] [Google Scholar]
- Seligman S. J. Penicillinase-negative variants of methicillin-resistant Staphylococcus aureus. Nature. 1966 Mar 5;209(5027):994–996. doi: 10.1038/209994a0. [DOI] [PubMed] [Google Scholar]



