Abstract
To investigate the expression of H2 Relaxin (H2RLX) in chronic heart failure (CHF) patients and determine whether H2RLX level can predict cardiovascular events in CHF patients within 180 days after discharge. One hundred forty-six patients were selected for examination from July 2012 to January 2014. The CHF group included a total of 115 patients, while the control group comprised a total of 31 patients without CHF. In the early morning on the first day after admission, patients’ blood samples were obtained for measuring levels of brain natriuretic peptide (BNP), LDL-cholesterol, haemoglobin, fasting blood glucose, thyroid stimulating hormone, and creatinine clearance rate (Ccr). Enzyme-linked immunosorbent assay was performed to analyse the plasma concentration of H2RLX, collagen I, and collagen III. Echocardiography was used to estimate left ventricular ejection fraction. We followed patients for 6 months to record cardiovascular events (asymptomatic, symptomatic, re-hospitalisation for heart failure, and cardiac death). Plasma H2RLX in CHF patients was significantly higher than that in the control group (0.593 [0.542-0.644] vs. 0.390 [0.355-0.425] pg/mL; P < 0.01). With elevated cardiac dysfunction, plasma concentrations of both collagen I and H2RLX increased in all patients. Moreover, there was a significant correlation between H2RLX and collagen I (r = 0.890, P < 0.001). The area under the receiver operating characteristic (ROC) curve for prognosis was 0.816 (P < 0.01), suggesting that plasma H2RLX level predicts severe cardiovascular events (re-hospitalisation and cardiac death) within 180 days after discharge. Elevated H2RLX levels in CHF patients may be associated with disease severity, and H2RLX level may predict cardiovascular events in CHF patients within 180 days after discharge.
Keywords: Relaxin, chronic heart failure, cardiovascular events
Introduction
Relaxin (RLX) is a peptide hormone of the human reproductive system, secreted primarily by the corpus luteum and prostate, classified into H1, H2, and H3. H2RLX is the main hormone stored in human tissue and is present in the circulation system [1]. Previous studies found that RLX may prevent and reverse the formation of cardiac fibrosis [2]. The RELAX-AHF study showed that the clinical application of H2RLX in acute heart failure could improve the heart, kidney, and liver function of patients and reduce 180-day mortality [3,4]. However, its role in chronic heart failure (CHF) patients requires further study. Therefore, in the present study, H2RLX plasma levels were determined in CHF patients. In addition, the predictive role of H2RLX for the severe cardiovascular events of CHF patients was investigated.
Materials and methods
Subjects
One hundred forty-six patients in the Fifth People’s Hospital of Shanghai from July 2012 to January 2014 were selected and divided into the CHF group and control group. The CHF group included 115 patients (81 males and 34 females) and the control group comprised 31 cases (20 males and 11 females). The inclusion criteria for CHF patients was based on the European Society of Cardiology guidelines for the diagnosis and treatment of acute and CHF 2012 [5]: (1) typical symptoms (i.e. dyspnoea, ankle oedema, and fatigue); (2) typical signs (i.e. elevated jugular venous pressure, pulmonary rales, gallop, and displacement of the apical impulse); and (3) reduced left ventricular ejection fraction (LVEF) < 50%, or normal LVEF, left ventricular (LV) not dilated and relevant structural heart disease (LV hypertrophy/left atrial (LA) enlargement), and/or diastolic dysfunction. Basic diseases were hypertension (53 cases), coronary heart disease (36 cases), dilated cardiomyopathy (22 cases), hypertrophic cardiomyopathy (2 case), and alcoholic cardiomyopathy (2 case). Exclusion criteria were: (1) congenital heart disease; (2) pulmonary heart disease; (3) valvular heart disease; (4) pericardial disease, myocarditis, or acute myocardial infarction within one month; (5) pregnancy; and (6) combined with other severe organ dysfunction or tumour. CHF patients were classified according to NYHA class: 6 cases of class NYHA I, 21 of class II, 61 of class III, and 27 of class IV.
Inclusion criteria for the control group were as follows: patients in the hospital with no previous history of heart disease and no signs and symptoms of heart failure. All patients agreed to participate in the study and signed informed consent forms.
Measurements of H2RLX and collagen
In the early morning on the first day after admission, 5 mL of venous blood was drawn from each patient in both groups while they were in supine and resting status to detect brain natriuretic peptide (BNP), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), haemoglobin, fasting blood glucose, D-dimer, thyroid stimulating hormone (TSH), and creatinine clearance rate (Ccr).
An extra 5 mL blood was collected to detect H2RLX, collagen I, and collagen III by ELISA (minimum detectable concentration was 8 pg/L, 10 ng/mL, and 1 ng/mL, respectively).
Echocardiography
Echocardiography was performed after patients were admitted to the hospital. LVEF and left ventricular end-diastolic diameter (LVEDD) were examined.
Follow-up
A clinical or telephone follow-up was performed to record the occurrence of cardiovascular events (asymptomatic, symptomatic, re-hospitalisation for heart failure, and cardiac death) for 6 months after discharge. Severe cardiovascular events were defined as re-hospitalisation for heart failure and/or cardiac death.
Statistical analysis
SPSS 18.0 statistical software was used (SPSS Inc., Chicago, IL). Normally distributed data were expressed as mean ± standard deviation and were compared using t-tests between two independent samples. Non-normally distributed data were expressed as medians with interquartile range (Median [P25-P75]) and were compared using rank sum tests between two independent samples. Count data were compared using the chi-square test. Multiple groups were compared using generalized linear models. Linear correlation analysis was conducted to manage the indicators using the Spearman correlation. Prognosis was analysed with a receiver operating characteristic (ROC) curve. Significance was set at P < 0.05.
Results
Comparison of clinical parameters between the CHF and control groups
As shown in Table 1, BNP levels in chronic heart failure patients were significantly higher than in the control group (P < 0.01). LVEF and Ccr in the CHF group were significantly decreased compared with that in the control group (P < 0.05). However, no significant differences were found between the two groups for age, LDL-C, HDL-C, TSH, fasting glucose, D-dimer, and haemoglobin levels (P > 0.05).
Table 1.
Comparison of clinical characteristics in the CHF and control group
| CHF group (n = 115) | Control group (n = 31) | P | |
|---|---|---|---|
| Age (y) | 70.75 (68.54-72.96) | 67.52 (63.70-71.35) | 0.08 |
| Gender (M/F) | 81/34 | 20/11 | 0.528 |
| Fasting blood glucose (mmol/L) | 5.4 (4.6-7.0) | 5.9 (5.03-6.60) | 0.724 |
| Haemoglobin (g/L) | 123 ± 22 | 126 ± 19 | 0.607 |
| D-dimer (μg/L) | 1.08 (0.733-1.540) | 1.18 (1.69-2.73) | 0.769 |
| TSH (mIU/L) | 1.920 (1.105-2.415) | 2.09 (1.69-2.73) | 0.321 |
| LDL-C (mg/dL) | 2.311 ± 0.891 | 2.266 ± 0.93 | 0.865 |
| HDL-C (mg/dL) | 0.990 (0.790-1.230) | 1.135 (0.970-1.330) | 0.103 |
| Ccr | 0.64 (0.44-0.86) | 0.97 (0.65-1.13) | 0.011 |
| BNP (pg/mL) | 737 (357-1498) | 80 (30-200) | 0.000 |
| LVEF (%) | 41 (35-51) | 64 (56-71) | 0.000 |
P < 0.05 was considered statistically significant. Normally distributed data are expressed as mean ± standard deviation. Non-normally distributed data are expressed as median (interquartile range). TSH, thyroid-stimulating hormone; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Ccr, creatinine clearance rate; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction.
Comparison of the plasma H2RLX between the CHF and control groups
Plasma H2RLX in CHF patients was significantly higher than that in the control group (0.593 [0.542-0.644] vs. 0.390 [0.355, 0.425] pg/mL; P < 0.01).
Comparison of plasma H2RLX and collagen I when patients were grouped by NYHA class
As seen in Tables 2 and 3, H2RLX levels in the CHF group increased with higher NYHA class (P < 0.05) and compared with control group, it was much higher in patients with NYHA classes III and IV (P < 0.05). Although it was elevated in patients with NYHA class II compared to the control group, the difference was not significant (P > 0.05). H2RLX levels in patients with NYHA class I also were not significantly different from those in the control group (P > 0.05).
Table 2.
Comparison of plasma H2RLX when patients were grouped by NYHA class (pg/mL)
| NYHA class | Cases | H2RLX | F | P |
|---|---|---|---|---|
| Control group | 31 | 0.390 (0.309-0.471) | Ref | |
| YHA I | 6 | 0.393 (0.209-0.577) | 0.001 | 0.997 |
| NYHA II | 21 | 0.472 (0.374-0.570) | 1.604 | 0.205 |
| NYHA III | 61 | 0.588 (0.531-0.646) | 15.346 | 0.001 |
| NYHA IV | 27 | 0.743 (0.656-0.830) | 34.056 | 0.001 |
Ref: Reference; P < 0.05 was considered statistically significant. Data are expressed as median (interquartile range). NYHA, New York Heart Association.
Table 3.
Comparison of collagen I when patients grouped as NYHA class (ng/mL)
| NYHA class | Cases | Collagen I | F | P |
|---|---|---|---|---|
| Control group | 31 | 0.54 ± 0.09 | Ref | |
| NYHA I | 6 | 0.53 ± 0.20 | 0. 000 | 0.988 |
| NYHA II | 21 | 0.62 ± 0.11 | 0. 309 | 0.579 |
| NYHA III | 61 | 0.87 ± 0.07 | 9.345 | 0.002 |
| NYHA IV | 27 | 1.07 ± 0.10 | 16.691 | 0.001 |
Ref, Reference; P < 0.05 was considered statistically significant. Data are expressed as mean ± standard deviation.
Collagen I level was elevated with each NYHA class (P < 0.05), and compared with the control group, collagen I was much higher in patients of NYHA class IV (P < 0.05). Although it was elevated in NYHA class II and III patients, the difference was not significant (P > 0.05). Collagen I levels in patients of class NYHA I were not significantly different from those in controls (P > 0.05).
Spearman correlation analysis
A Spearman correlation analysis was performed for H2RLX level collagen, BNP, LVEF and Ccr. H2RLX had a significant linear correlation with collagen I (r = 0.89, P < 0.001) and BNP (r = 0.33, P < 0.05), and a significant negative linear correlation with Ccr (r = -0.31, P < 0.01).
H2RLX predicts severe cardiovascular events in CHF patients within 180 days after discharge
The area under the ROC curve was 0.816 (95% confidence interval (CI), 0.724-0.909, P < 0.01) (Figure 1), suggesting H2RLX could predict severe cardiovascular events in CHF patients within 180 days after discharge.
Figure 1.
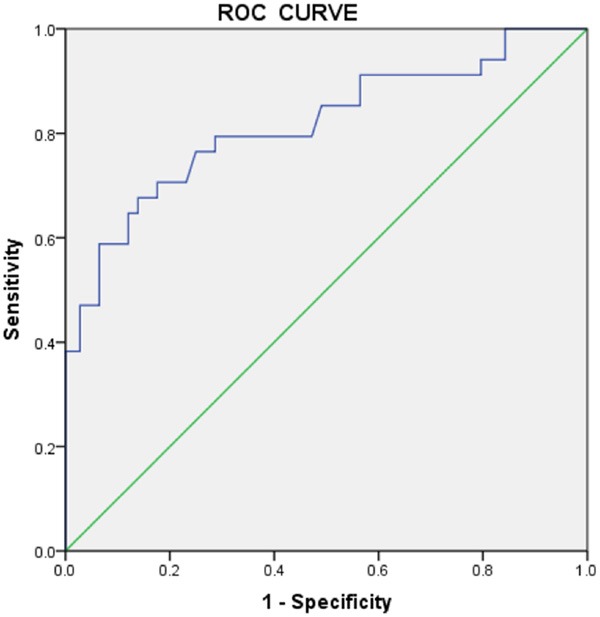
The receiver operating characteristic curve for H2RLX levels predicts severe cardiovascular events within 180 days after hospital discharge in patients with chronic heart failure.
Discussion
RLX was initially considered a pregnancy-related hormone. It was discovered in recent years that RLX also plays a role in non-reproductive systems, such as the heart and kidneys [2]. RLX exerts a protective effect on the heart by antagonizing myocardial fibrosis, improving ventricular remodelling and haemodynamics, reducing cardiac load, and inhibiting the inflammatory response [2]. However, its role in CHF patients’ needs further study.
This study showed that plasma H2RLX levels in CHF patients were much higher than those in the control group. H2RLX increased based on NYHA class, so it seems that plasma H2RLX may play a role in the preliminary assessment of CHF severity. Similar to our study, Dschiezig et al. [6] showed that plasma concentrations of RLX in patients with heart failure was higher than in the control group, while RLX plasma levels and RLX mRNA expression were proportional to the severity of heart failure. Therefore, H2RLX is expected to be used for assessing CHF severity.
Myocardial fibrosis is primarily due to fibroblast cell proliferation and activation, increased collagen synthesis and deposition, and reduced collagen decomposition. Collagen is mainly composed of type I and III. Collagen I is mainly crude fibre, with higher stiffness determining ventricular strength. Therefore, change of the concentration of collagen I and the proportion of collagen can lead to myocardial fibrosis [7-12]. Studies in vivo indicate that cardiac collagen and pro-collagen levels expressed in RLX gene knock-out mice were significantly higher than in mice whose RLX genes were not knocked out [13,14], showing the inhibitory effects of RLX on collagen synthesis. In our study, we found that collagen I levels in CHF patients increased with NYHA class, especially in patients of NYHA class III and IV. H2RLX and collagen I were positively correlated (r = 0.89, P < 0.001). Therefore, we hypothesized that in CHF, over-expression of collagen I can cause a compensatory rise of H2RLX.
H2RLX is released and secreted by the heart, and our study showed that CHF patients had higher levels of plasma H2RLX. Therefore, we assume that H2RLX can predict the prognosis of patients with chronic heart failure. In this study, patients were followed for cardiovascular events (cardiovascular death or re-hospitalisation for heart failure) for 180 days, and then the ROC curve was determined (Figure 1). The area under the ROC curve was 0.816, with a 95% CI of 0.724-0.909 (P < 0.01). In severe heart failure, RLX showed a close inverse correlation with plasma endothelin-1, the most potent vasoconstrictor in heart failure and a powerful mediator of sodium retention and myocardial remodelling [6]. In heart failure, pathologically elevated levels of endothelin contribute to symptoms and are associated with adverse outcomes [2]. Therefore, it is predicted that RLX may prove valuable in assessing the prognosis of heart failure and represents a potential target for future therapeutic strategies [6]. Our study suggests that H2RLX levels can help to identify high-risk patients, direct clinical treatment, and reduce the incidence of cardiovascular events.
In short, H2RLX plasma levels may contribute to the assessment of CHF severity and can predict severe cardiovascular events occurring within 180 days of hospital discharge. There are some limitations of our study because of the modest sample size of our study, so further study in a larger population is necessary.
Disclosure of conflict of interest
None.
References
- 1.Bathgate RA, Samuel CS, Burazin TC, Layfield S, Claasz AA, Reytomas IG, Dawson NF, Zhao C, Bond C, Summers RJ, Parry LJ, Wade JD, Tregear GW. Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. Novel members of the relaxin peptide family. J Biol Chem. 2002;277:1148–1157. doi: 10.1074/jbc.M107882200. [DOI] [PubMed] [Google Scholar]
- 2.Teichman SL, Unemori E, Teerlink JR, Cotter G, Metra M. Relaxin: review of biology and potential role in treating heart failure. Curr Heart Fail Rep. 2010;7:75–82. doi: 10.1007/s11897-010-0010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld L. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld LR. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 6.Dschietzig T, Richter C, Bartsch C, Laule M, Armbruster FP, Baumann G, Stangl K. The pregnancy hormone relaxin is a player in human heart failure. FASEB J. 2001;15:2187–2195. doi: 10.1096/fj.01-0070com. [DOI] [PubMed] [Google Scholar]
- 7.Doering CW, Jalil JE, Janicki JS, Pick R, Aghili S, Abrahams C, Weber KT. Collagen network remodelling and diastolic stiffness of the rat left ventricle with pressure overload hypertrophy. Cardiovasc Res. 1988;22:686–695. doi: 10.1093/cvr/22.10.686. [DOI] [PubMed] [Google Scholar]
- 8.Eghbali M, Weber KT. Collagen and the myocardium: fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Mol Cell Biochem. 1990;96:1–14. doi: 10.1007/BF00228448. [DOI] [PubMed] [Google Scholar]
- 9.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5:15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laviades C, Mayor G, Díez J. Treatment with lisinopril normalizes serum concentrations of procollagen type III amino-terminal peptide in patients with essential hypertension. Am J Hypertens. 1994;7:52–58. doi: 10.1093/ajh/7.1.52. [DOI] [PubMed] [Google Scholar]
- 11.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 12.Marijianowski MM, Teeling P, Mann J, Becker AE. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J Am Coll Cardiol. 1995;25:1263–1272. doi: 10.1016/0735-1097(94)00557-7. [DOI] [PubMed] [Google Scholar]
- 13.Du XJ, Samuel CS, Gao XM, Zhao L, Parry LJ, Tregear GW. Increased myocardial collagen and ventricular diastolic dysfunction in relaxin deficient mice: a gender-specific phenotype. Cardiovasc Res. 2003;57:395–404. doi: 10.1016/s0008-6363(02)00663-6. [DOI] [PubMed] [Google Scholar]
- 14.Samuel CS, Unemori EN, Mookerjee I, Bathgate RA, Layfield SL, Mak J, Tregear GW, Du XJ. Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo. Endocrinology. 2004;145:4125–4133. doi: 10.1210/en.2004-0209. [DOI] [PubMed] [Google Scholar]


