Abstract
The present paper reports the effects of Jinlida (JLD), a traditional Chinese medicine which has been given as a treatment for high-fat-diet (HFD)-induced insulin resistance. A randomized controlled experiment was conducted to provide evidence in support of the affects of JLD on insulin resistance induced by HFD. The affect of JLD on blood glucose, lipid, insulin, adiponectin, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL) in serum and lipid content in skeletal muscle was measured. Genes and proteins of the AMPK signaling pathway were analyzed by real time RT-PCR and Western blot. Adiponectin receptor 1 and 2 (ADIPOR1, ADIPOR2) and other genes involved in mitochondrial function and fat oxidation were analyzed by real time RT-PCR. Histological staining was also performed. JLD or pioglitazone administration ameliorated fasting plasma levels of glucose, insulin, triglyceride (TG), total cholesterol (TC), ALT, AST and non-esterified fatty acid (NEFA) (P < 0.05). Treatment with JLD or pioglitazone significantly reverted muscle lipid content (P < 0.05). JLD (1.5 g/kg) significantly increased plasma adiponectin concentration by 60.17% and increased AMPK and acetyl-CoA carboxylase (ACC) phosphorylation in skeletal muscle (P < 0.05). JLD administration increased levels of ADIPOR1 and ADIPOR2 by 1.48 and 1.29 respectively. Levels of genes involved in mitochondrial function and fat oxidation were increased. This study provides the molecular mechanism by which JLD ameliorates HFD-induced insulin resistance in rats.
Keywords: Jinlida (JLD), insulin resistance, skeletal muscle, lipid accumulation, AMP-activated protein kinase, mitochondria
Introduction
The incidence of type 2 diabetes mellitus (T2DM) is increasing worldwide. Recently, a survey evaluates that more than 439 million people will suffer from diabetes in nearly all countries by the year 2030 [1]. Insulin resistance is an important risk factor in the development of T2DM. Currently, agents used to treat T2DM are synthetic drugs and insulin. Such agents have considerable side effects [2]. Consequently, there is an increasing requirement for anti-diabetic agents from natural resources with fewer side effects. Jinlida (JLD) superfine powder (also known as JLD Recipe) is a Chinese herbal compound that has been widely used in the treatment of insulin resistance and T2DM in China [3]. The underlying mechanisms of JLD are poorly understood. As JLD can lower circulating serum lipid levels in clinical settings, it was hypothesized that the mechanisms by which JLD ameliorates insulin resistance may be related to reduction of lipid content in the plasma, or also in the muscle.
The major peripheral organ systems involved in insulin resistance include skeletal muscle, adipose tissue and the liver [4]. Skeletal muscle, the major tissue contributing to whole-body energy metabolism, is the main site for insulin-stimulated glucose uptake. Therefore, responsiveness to insulin in skeletal muscle greatly influences whole-body glucose homeostasis [5]. In addition, several studies have reported that insulin resistance is characterized by excessive lipid accumulation in skeletal muscle due to a reduction in fatty acid oxidation [6].
The mechanisms involved in lipid accumulation in skeletal muscle are complex. The adenosine monophosphate-(AMP-) activated protein kinase (AMPK), is a metabolic sensor that plays an important role in regulating lipid metabolism, as well as glucose homeostasis. AMPK can be activated by conditions that deplete energy and by adiponectin [7]. Several studies have shown that activation of AMPK can inactivate Acetyl-CoA carboxylase (ACC) and reduce the concentration of malonyl coenzyme A, an inhibitor of carnitine palmitoyl transferase (CPT)-1 in muscle [8]. In addition, activation of AMPK up-regulates the expression of CPT1, drives the entrance of fatty acids into the mitochondria for β-oxidation and decreases circulating lipids and ectopic fat deposition in skeletal muscle. In contrast, it has been suggested that impairment of mitochondrial function is a crucial factor in the pathogenesis of insulin resistance due to reduced fatty acid oxidation in skeletal muscle [9]. The expression of peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha), and other genes involved in mitochondrial function, have been reported to be reduced in subjects with insulin resistance [10].
Thiazolidinediones (TZDs), a peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist, has been used as an insulin sensitizer in patients with T2DM [2]. Pioglitazone increases plasma adiponectin levels, thus activating the AMPK pathway in skeletal muscle, increasing nonesterified fatty acid (NEFA) oxidation and mitochondrial function in human skeletal muscle [11]. Consequently, in the present study, pioglitazone was chosen as a positive control. This study aimed to investigate the mechanism of JLD in insulin-sensitivity in insulin resistant rats on a high-fat-diet (HFD). Lipid content in skeletal muscle, expression of proteins involved in the AMPK signaling pathway and genes involved in mitochondrial function were detected, thus providing support for the traditional Chinese use of JLD in the management of insulin resistance and T2DM.
Methods
Preparation of JLD
Some Chinese herbs have demonstrated safety and efficacy in the management of T2DM in either animal models or humans [12-14]. JLD are a specific TCM extract obtained from 17 types of herbs (Table 1). JLD were approved by China Food and Drug Administration for the treatment of T2DM in 2005 (Approval Number of JLD is Zhunzi Z20050845).
Table 1.
Complex compounds contained in JLD
| Material | Species | Combination Principle | Origin | Medicinal Parts | Concocted |
|---|---|---|---|---|---|
| Ginseng Radix et Rhizoma | Panax ginseng C. A. Mey | King | Jilin | Roots and Rhizomes | Drying |
| Polygonatirhizoma | Polygonatum kingianum Coll. Et Hemsl | Minister | Jilin | Rhizomes | Drying |
| Atractylodisrhizoma | Atractylodes lancea (Thunb.) DC | Minister | Jiangsu | Rhizomes | Drying |
| Sophoraeflavescentisradix | Sophora flavescens Ait | Minister | Neimenggu | Roots | Drying |
| Ophiopogonisradix | Ophiopogon japonicas (L.f) Ker-Gawl | Assistant | Zhejiang | Tubers | Drying |
| Rehmanniaeradix | Rehmanniag lutinosa Libosch | Assistant | Henan | Tubers | Drying |
| Polygonimuui | Polygonum multiflorum Thunb | Assistant | Guangdong | Tubers | Boil, Steam, and Drying |
| Cornifructus | Cornus officinalis Sieb. et Zucc | Assistant | Henan | Flesh | Drying |
| Poria | Poriacocos (Schw.) Wolf | Assistant | Anhui | Sclerotia | Drying |
| Eupatoriiherba | Eupatorium fortune Turcz | Assistant | Jiangsu | Aboveground parts | Drying |
| Coptidisrhizoma | Coptis chinensis Franch. | Assistant | Sichuan | Rhizomes | Drying |
| Anemarrhenaerhizoma | Anemarrhena asphodgfoides Bge | Assistant | Hebei | Rhizomes | Drying |
| Epimediifolium | Epimedium brevicornu Maxim | Assistant | Guizhou | Leaves | Drying |
| Salviaemiltiorrhizaeradixetrhizoma | Salvia miltiorrhiza Bge. | Assistant | Jiangsu | Roots and Rhizomes | Drying |
| Lyciicortex | Lycium chinense Mill. | Assistant | Hebei | Velamina | Drying |
| Puerariaelobataeradix | Pueraria lobata (Willd.) Ohwi | Ambassador | Anhui | Roots | Drying |
| Litchisemen | Litchi chinensis Sonn | Ambassador | Shangdong | Seeds | Drying |
Animals
Thirty-six male Sprague Dawley (SD) rats aged 6 weeks (130±10 g), were supplied by the Institute for Laboratory Animal Resources of National Institutes for food and drug Control (NIFDC), Beijing, China, (Certificate No. scxk (Jing) 2009-0017) and housed at a controlled temperature (22±2°C), humidity-controlled (30-70%) and with a standard 12 h light/dark cycle. The animals were provided with food and water ad libitum. Animal studies and relative protocols were approved by the Animal Care and Use Committee at the Hebei Medical University.
Animal grouping and treatment
After 1 week of acclimatization, rats were randomly divided into two groups consisting of 6 rats receiving a normal diet of rodent chow (ND group) and a second group of 30 rats receiving a HFD. The normal rodent chow diet was obtained from Hebei medical university animal laboratory, China, containing 10.3% fat, 24.2% protein and 65.5% carbohydrate (kcal). The HFD contained flour, bean flour, bean pulp, fish meal, bone meal, bran and lard oil and consisted of 59.8% fat, 20.1% protein and 20.1% carbohydrate (kcal). Rats of each group were subjected to a euglycemic-hyperinsulinemic clamp after 6 weeks of diet to confirm the onset of insulin resistance in the HFD group. The 30 insulin resistant rats on a HFD were subdivided into five subgroups: HFD group, HFD with pioglitazone and HFD with 3 different doses of JLD (n=6 per group).
The pioglitazone group was gavaged with 4.5 mg/kg pioglitazone (Takeda Pharmaceutical Company Limited, Japan). The low, middle and high dose JLD groups were gavaged with 0.75 g/kg, 1.5 g/kg or 3 g/kg JLD after 6 weeks of HFD. Pioglitazone and JLD superfine powder were suspended in 0.5% carboxymethyl cellulose sodium and administered daily between 9-10 am for 8 weeks by oral gavage. Correspondingly, mice of HFD and ND groups were administered with 0.5% carboxymethyl cellulose sodium. The rats were allowed to continue to feed on their respective diets until the end of the study. Body weight and food intake were monitored weekly. Blood glucose levels were measured by Accu-chek Active Meter (ACCU-CHEK® Active; Roche, Germany). Animals were sacrificed at week 14 after 12 h overnight fasting. Blood samples were collected from the abdominal aorta. Skeletal muscle samples of rats were collected immediately and kept at -80°C after being quickly frozen in liquid nitrogen.
Glucose tolerance test
The intraperitoneal glucose tolerance test was performed at 14 weeks of the experiment. Following overnight fasting, glucose level of blood obtained from the tail vein was measured at the initial time. Rats were then injected intraperitoneally with 50% glucose 2 g/kg of body weight. Blood glucose levels were monitored at 0, 5, 10, 30, 60 and 120 min after glucose injection. The area under the curve (AUC) was calculated based on the blood glucose curves [15].
Euglycemic hyperinsulinemic clamp
Hyperinsulinemic clamp studies were performed as previously described [16]. Rats were given general anesthesia (3% pelltobarbitalum natricum, 40 mg/kg) intraperitoneally, and two catheters inserted into the right jugular vein and carotid artery of rats respectively and exteriorized from the back of the neck subcutaneously. The catheters were flushed with isotonic saline containing heparin (50 U/mL). Rats were allowed to rest for a minimum of 3 d completely, and only rats that had lost less than 5% of their preoperative weights were used. Insulin was infused at 4 mU/min through the jugular vein catheter for 90 min. Glucose concentrations were clamped at euglycemic levels by a changeable rate infusion of 30% glucose. Blood glucose levels were monitored during the process. A stable glucose infusion rate (GIR) was achieved within approximately 60 min insulin infusion. Euglycemic hyperinsulinemic clamp tests were operated on fasted, awake and unrestrained animals.
Measurement of biochemical indices in serum
Serum was separated and stored at -80°C. The concentration of insulin, nonesterified fatty acid (NEFA) and adiponectin in serum were measured using a rat enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturers’ instructions (Jian Cheng Biological Engineering Institute, Nanjing, China). Triglyceride (TG), total cholesterol (TC), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL) concentrations in serum were assayed on Automated Clinical System (HITACHI, model: 7600-110, Japan).
Tissue lipid analysis
For TG and NEFA assays, muscle samples were weighed and homogenized with anhydrous ethanol (1 g: 9 mL) and centrifuged at 2500 rpm/min for 10 min. The supernatant was collected to determine the total amount of tissue lipids using the manufacturers’ protocol (Jian Cheng Biological Engineering Institute, Nanjing, China). The long-chain fatty acyl-CoA (LCACoA) was determined by ELISA assay (Jian Cheng Biological Engineering Institute, Nanjing, China).
Oil red O staining
Pathological changes in skeletal muscle were monitored by oil red O staining using the Oil red O kit (Jian Cheng Biological Engineering Institute, Nanjing, China). Frozen sections were placed on a staining rack at room temperature (RT) for 10 min, then immersed into a staining jar containing dye liquor A for 15 min and washed with distilled water at approximately 37°C for 20 s. Frozen sections were stained by dye liquor B for 5 min, washed for 60 seconds and fixed with neutral balsam mounting agent. The selected specimens were viewed and photographed with an electron microscope (HITACHI H7500, Japan).
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from skeletal muscle tissue according to the TRIzol protocol (Invitrogen, United States). Total RNA was quantified by measuring absorbance at 260 and 280 nm with a ratio ≥ 1.8. Integrity of the RNA was determined by visual inspection of the two ribosomal RNAs, 18S and 28S, on an agarose gel. Reverse transcription was performed using the First-Strand cDNA Synthesis system (Promega, United States), and quantitative real-time RT-PCR performed using an ABI PRISM 7300 PCR System (Applied Biosystems, United States) using Syber Green I GoTaq® qPCR Master Mix (Promega, United States). The PCR primer sequences are shown in Table 4. PCR was performed as follows: one cycle at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 58°C for 20s and 72°C for 30 s. Analysis of the melting curve of the PCR products was performed to confirm amplification specificity. The data obtained was analyzed by comparative cycle threshold method, normalized to the β-actin expression value and expressed as fold change. Relative expression levels were calculated by the formula 2-ΔΔCt comparing HFD, JLD-treated, pioglitazone-treated and normal samples.
Table 4.
Primer sequences for quantitative polymerase chain reaction
| Gene | Forward primer (5’-3’) bp | Reverse primer (5’-3’) bp | Amplified fragment length |
|---|---|---|---|
| ADIPOR1 | CCGCATCCACACAGAAACT | ACATCCCGAAGACCACCTT | 139 |
| ADIPOR2 | TGTAAGGTGTGGGAAGGTCG | GGAAAGAAGGCATAGGAGGC | 111 |
| PPAR γ | CCACCAACTTCGGAATCAG | GATGTCAAAGGAATGGGAGTG | 68 |
| PPARα | GGTCCGATTCTTCCACTGCT | GGTAACCTGGTCATTCAAGTCC | 115 |
| ACADM | TGACGGAGCAGCAGAAAGAG | TTGATGAGAGGGAACGGGT | 115 |
| NRF1 | AGACACGGTTGCTTCGGAA | CGCACCACATTCTCCAAAG | 148 |
| COX IV | TCGCTGAGATGAACAAGGG | AGTGAAGCCGATGAAGAACA | 74 |
| PGC1α | AAGACCAGGAAATCCGAGC | TTGCCATCCCGTAGTTCAC | 103 |
| CPT1 | CCAAACATCACTGCCCAA | GGAAATAGGCTTCGTCATCC | 100 |
| GLUT4 | ATACTCATTCTCGGACGGTTC | CCCACATACATAGGCACCAA | 74 |
| ACCβ | TGCCCACTTTCTTCTATCACG | ACATAAACCTCCAGGGACGC | 61 |
| AMPKα1 | AATCCAAACACCAAGGCG | TGCTCTACACACTTCTGCCAT | 95 |
| β-actin | TGTGATGGTGGGTATGGGT | AGGATGCCTCTCTTGCTCTG | 69 |
ADIPOR1: Adiponectin receptor 1; ADIPOR2: Adiponectin receptor 2; PPARγ: Peroxisome proliferator-activated receptor gamma; PPARα: Peroxisome proliferator -activated receptor α; ACADM: acyl-CoA dehydrogenase; NRF1: nuclear respiratory factor 1; COX IV: cytochrome c oxidase subunit IV; PGC1α: Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha; CPT1: Carnitine palmitoyltransferase 1; ACC: Acetyl-CoA carboxylase; AMPKα1: Adenosine 5’-monophosphate-activated protein kinase α1; GLUT4: Glucose transporter type 4.
Western blot analysis
Protein samples were prepared using RIPA buffer containing a cocktail of protease inhibitors. Protein concentration was estimated by BCA protein assay kit (Merck Chemicals, Darmstadt, Germany) with BSA as a standard. A total of 60 μg of each sample was separated by 10% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis), then transferred to PVDF membranes. Membranes were blocked with 5% fat-free milk powder with 1% Triton X-100 in Tris-buffered saline (TBS-T) (20 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.4) for 2 h at RT. Membranes were incubated with the appropriate diluted primary antibodies for GLUT4 (Signalway Biotechnology, Pearland, USA), AMPK α-1 (Abcam, Cambridge, UK), phospho-AMPKα Thr172 (P-AMPK), Acetyl-CoA carboxylase (ACC), phospho-acetyl-CoA carboxylase Ser79 (P-ACC) (Cell Signaling, Boston, USA), CPT1, PGC-1α and β-actin (Santa cruz, Texas, USA) respectively overnight at 4°C. The primary antibody was removed by washing the membranes three times in TBS-T, 5 min each. Membranes were then incubated with the respective secondary antibody (Santa cruz, Texas, USA) for 2 h at RT. Following three washes in TBS-T, protein bands were detected on an X-ray film using Pierce ECL Western Blotting Substrate (Santa cruz, Texas, USA). The internal loading control was β-actin. The expression of proteins was calculated as [(OD target protein/OD β-actin) experiment group]/[(OD target protein/OD β-actin) control group]. The standard deviation (SD) in the ND group was set at 1. The experiments were replicated three times.
Statistical analysis
All results are expressed as mean ± SD. One-way analysis of variance (ANOVA) was used to determine statistically significant differences among groups followed by a Dunnett post-hoc test using PASW Statistics 18 (IBM, USA). A P value of < 0.05 was considered statistically significant.
Results
Body weight development and food intake
Treatment with different concentrations of JLD (0.75 g/kg, 1.5 g/kg, 3 g/kg) for 8 weeks resulted in less weight gain compared to the HFD group (9.5%, 16.2%, 18.4% respectively) (P < 0.05). JLD or pioglitazone administration did not affect food intake, indicating that the decreased weight gain, was not due to altered food intake (Table 2).
Table 2.
Body weight, energy intake, lipid profile and liver function of the rats
| ND | HFD | Pioglitazone | JLD (0.75 g/Kg) | JLD (1.5 g/Kg) | JLD (3 g/Kg) | |
|---|---|---|---|---|---|---|
| Body weight (g) | 471.50±4.53* | 644.83±15.77 | 539.00±10.55*,# | 583.5±17.41*,# | 540.67±17.43*,# | 526.50±18.08*,# |
| Energy intake (kcal/day) | 87.32±6.01* | 117.06±6.25 | 110.34±7.92 | 109.83±6.04 | 101.59±5.05 | 103.26±7.33 |
| APN (ng/mL) | 17.43±0.93* | 12.68±0.93 | 22.17±1.25*,# | 18.28±1.01* | 20.31±1.14* | 17.14±0.82* |
| Lipid profile | ||||||
| TG (mmol/L) | 0.37±0.03* | 0.52±0.03 | 0.23±0.03*,# | 0.26±0.02*,# | 0.15±0.01*,# | 0.16±0.01*,# |
| NEFA (mmol/L) | 0.64±0.05* | 1.40±0.08 | 0.76±0.05* | 0.88±0.06* | 0.76±0.06* | 0.77±0.08* |
| TC (mmol/L) | 1.04±0.07 | 1.15 ±0.05 | 1.01±0.06 | 0.76±0.08* | 0.75±0.05* | 0.84±0.07* |
| Liver function | ||||||
| ALT (IU/L) | 42.25±3.47* | 116.98±4.90 | 46.52±2.70* | 84.77±3.29#,* | 66.25±5.32#,* | 88.48±2.30#,* |
| AST (IU/L) | 119.38±4.44* | 218.67±5.12 | 141.63±4.44#,* | 161.18±3.94#,* | 132.98±3.44#,* | 169.18±5.74#,* |
| TBIL (μmol/L) | 0.56±0.05 | 0.81±0.08 | 0.70±0.06 | 0.74±0.06 | 0.71±0.06 | 0.69±0.07 |
Data are means ± SD. n = 6 per group.
P < 0.05 compared with the HFD group;
P < 0.05 compared with the ND group.
APN: adiponectin; TG: triglyceride; NEFA: nonesterified fatty acid; TC: total cholesterol; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TBIL: total bilirubin. 12. 68±0.93.
Affects of JLD on insulin resistance
JLD (0.75 g/kg, 1.5 g/kg, 3 g/kg) or pioglitazone administration significantly ameliorated fasting plasma levels of glucose (14.2%, 21.2%, 21.9%, 32.9% respectively) and insulin (37.2%, 43.5%, 41.4%, 39.8% respectively) (P < 0.05). Glucose infusion rate (GIR) was also calculated. JLD administration (0.75 g/kg, 1.5 g/kg, 3 g/kg) up-regulated GIR by 84.1%, 99.6% and 85.5% respectively. Pioglitazone administration up-regulated GIR by 88.8% (Figure 1A-C) (P < 0.05). Plasma glucose levels during IPGTT were significantly increased in HFD compared to ND. Treatment with JLD or pioglitazone attenuated plasma glucose levels in mice fed a HFD (Figure 1D) (P < 0.05). The AUC values of plasma glucose levels during IPGTT were significantly increased in the HFD group and this increase was attenuated by 28.2%, 23.3% and 22.9% at the doses of 0.75, 1.5, 3 g/kg of JLD, and by 22.6% in the pioglitazone group (Figure 1E) (P < 0.05). In conjunction, the results indicate that JLD effectively attenuates insulin resistance.
Figure 1.
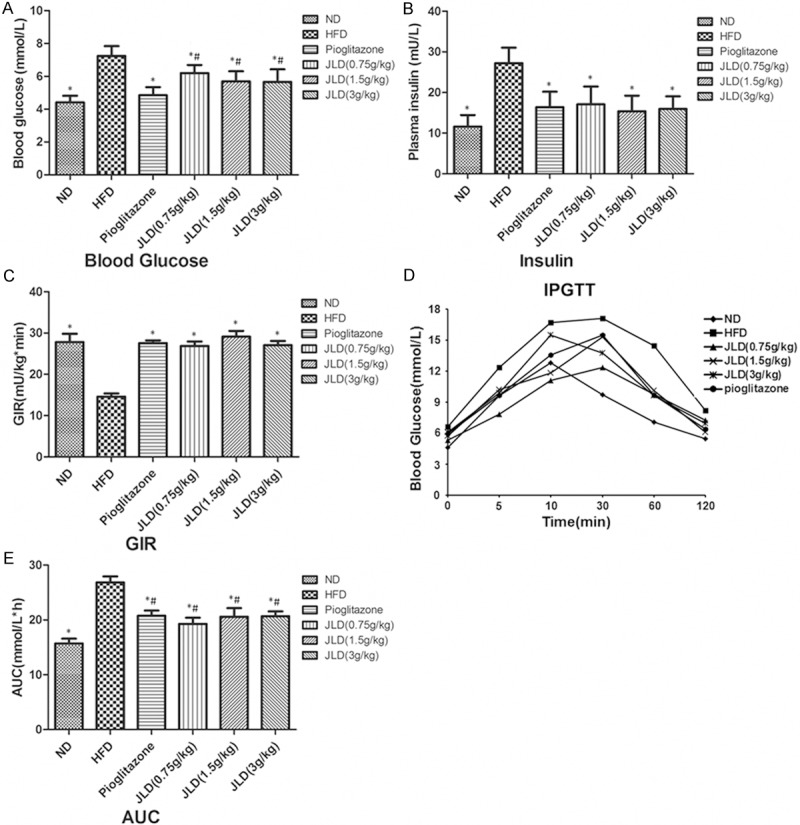
The affect of Jinlida on insulin resistance. The fasting plasma levels of glucose (A), insulin (B) and GIR (C) were used to assess insulin sensitivity of the rats. The intraperitoneal glucose tolerance test (IPGTT) was performed. Area under the curve (AUC) was calculated based on the blood glucose curves (D, E). n = 6 per group; *P < 0.05 compared with the high-fat-diet group (HFD); #P < 0.05 compared with the normal diet group (ND).
Affects of JLD on plasma and skeletal muscle lipid parameters
Both TG and NEFA in serum were significantly increased by HFD, but were ameliorated by JLD or pioglitazone administration (P < 0.05). The increase of TC in serum was ameliorated by JLD (P < 0.05), not by pioglitazone (Table 2). The TG, NEFA and LCACoA content in skeletal muscle were also increased in HFD rats compared with the control rats, suggesting the development of lipid accumulation (Table 3) (P < 0.05). Treatment with JLD or pioglitazone significantly reverted the fat content of muscle tissue to that of normal muscle (Table 3) (P < 0.05).
Table 3.
TG, NEFA and LCACoA in skeletal muscle
| ND | HFD | Pioglitazone | JLD (0.75 g/Kg) | JLD (1.5 g/Kg) | JLD (3 g/Kg) | |
|---|---|---|---|---|---|---|
| TG (mmol/g) | 0.07±0.01* | 0.26±0.01 | 0.07±0.01* | 0.19±0.02*,# | 0.07±0.01* | 0.11±0.01*,# |
| NEFA (mmol/g) | 77.20±6.41* | 150.06±6.36 | 64.80±6.16* | 110.00±6.20*,# | 79.59±6.62* | 92.30±6.22*,# |
| LCACoA (nmol/g) | 3.82±0.26* | 7.23±0.34 | 4.68±0.21*,# | 5.48±0.22*,# | 5.00±0.13*,# | 6.21±0.37*,# |
Data are means ± SD. n = 6 per group.
P < 0.05 compared with the HFD group;
P < 0.05 compared with the ND group.
TG: triglyceride; NEFA: nonesterified fatty acid; LCACoA: long-chain fatty acyl-CoA.
JLD alleviated skeletal muscle steatosis
The Oil red-O technique is a common method used to stain for neutral lipids. Oil-red-O is fat-soluble dyes which stain the fat droplets red orange. The tissue sections in the HFD group exhibited diffuse skeletal muscle steatosis under a light microscope, while JLD and pioglitazone alleviated skeletal muscle steatosis with less red fat droplets. These results indicated that the accumulation of excess lipid in the skeletal muscle was ameliorated by JLD or pioglitazone treatment (Figure 2).
Figure 2.
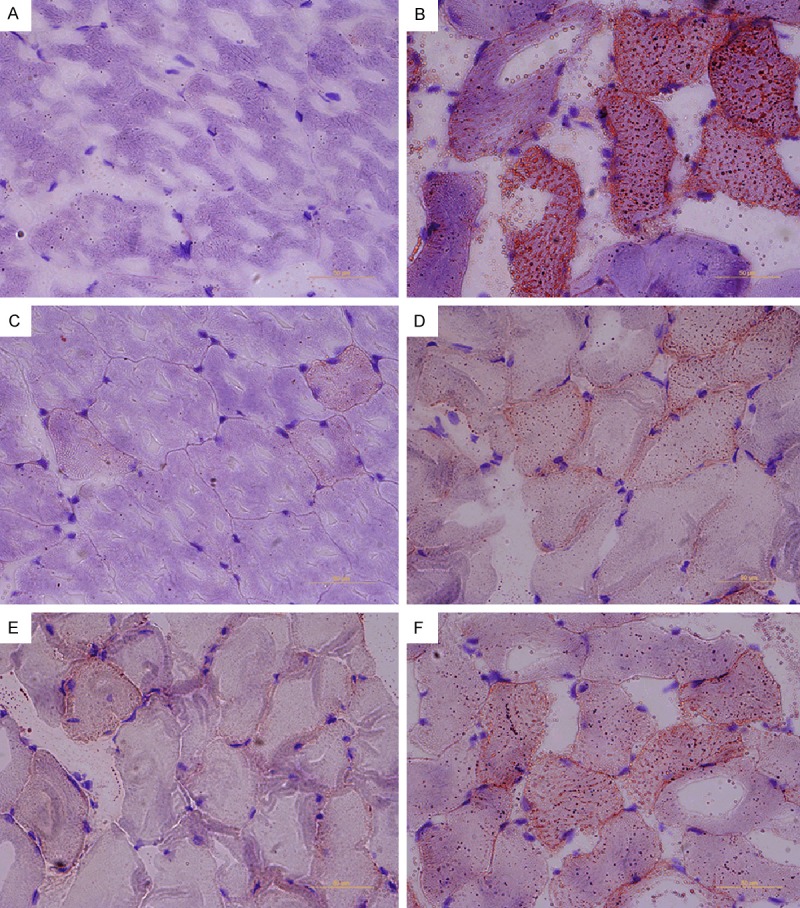
Jinlida alleviated skeletal muscle steatosis. Oil red O staining of skeletal muscle sections from ND group (A), HFD group (B), pioglitazone treatment group (C) and the Jinlida (0.75 g/Kg, 1.5 g/Kg, 3 g/Kg) treatment group (D-F) (×400).
Effect of JLD on AMPK signaling and adiponectin receptor gene expression
To investigate the mechanism by which JLD reversed lipid accumulation in skeletal muscle in insulin-resistant rats, the expression of proteins involved in the AMPK signaling pathway were investigated, as AMPK plays an important role in lipid metabolism [17]. In the current study, both JLD (0.75 g/kg, 1.5 g/kg) and pioglitazone treatment significantly increased AMPK and ACC phosphorylation in skeletal muscle (P < 0.05). Administration of JLD (3 g/kg) also increased AMPK and ACC phosphorylation, however, the difference was not significant (Figure 3F-K). Consistent with mRNA levels, there was no difference in expression of AMPK or ACC protein between the 6 groups, suggesting that treatment with JLD or pioglitazone had no effect on AMPK or ACC protein (Figure 3). The enzyme that catalyses the transport of long-chain fatty acids into the mitochondria for β-oxidation, CPT1, was significantly increased after treatment with JLD (0.75 g/kg, 1.5 g/kg) or pioglitazone (Figure 5) (P < 0.05). Several studies have demonstrated that phosphorylation of AMPK can activate the expression of GLUT4. Consistent with this theory, both JLD and pioglitazone treatment significantly increased GLUT4 protein expression in the present study and this was consistent with GLUT4 mRNA levels (Figure 3) (P < 0.05). To investigate the mechanism involved in the stimulatory affect of JLD on AMPK activity, plasma adiponectin concentration was examined. Adiponectin concentration in the HFD group was 12.68±0.93 ng/mL. This was lower than the HFD group treated with JLD at a dose of 0.75, 1.5 or 3 g/kg (18.28±1.01 ng/mL, 20.31±1.14 ng/mL, 17.14±0.82 ng/mL respectively) (Table 2) (P < 0.05). The increase in adiponectin concentration in the HFD group treated with JLD was accompanied by elevated expression of the ADIPOR1 and ADIPOR2 genes (Figure 3A-E) (P < 0.05).
Figure 3.
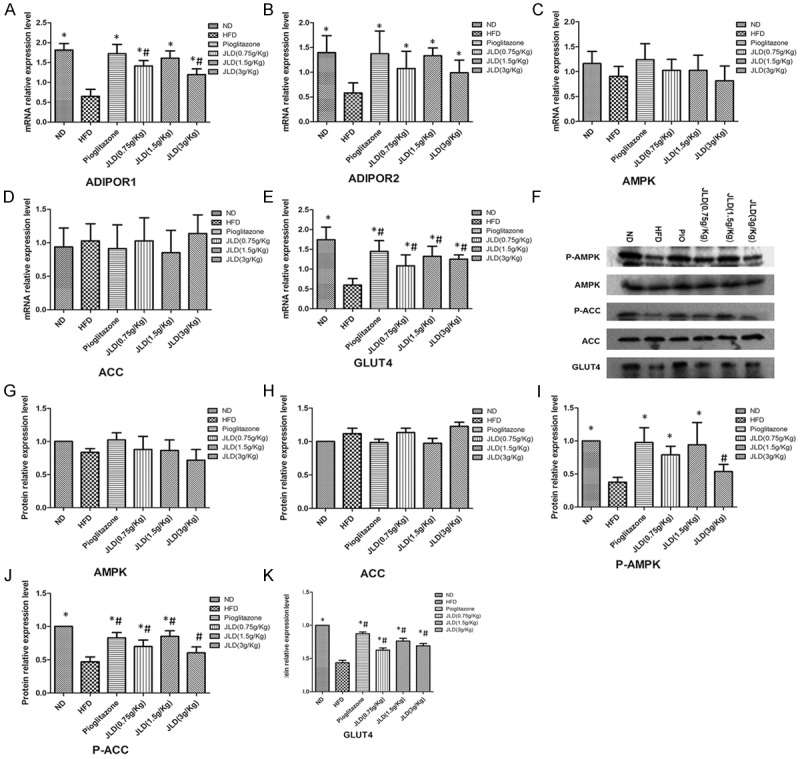
The effects of Jinlida on AMPK signaling and adiponectin receptor gene expression in skeletal muscle in rats. The mRNA expression levels of adiponectin receptor 1 (ADIPOR1), adiponectin receptor 2 (ADIPOR2), AMP-activated protein kinase (AMPK), Acetyl-CoA carboxylase (ACC) and glucose transporter type 4 (GLUT4) were examined by quantitative real-time-polymerase chain reaction (A-F). The corresponding bands of western blotting were showed (H).The protein levels of P-AMPK, P-ACC, AMPK, ACC and GLUT4 were detected by Western blotting (G-K). We calculated the expression of proteins as [(OD target protein/OD β-actin) experiment group]/[(OD target protein/OD β-actin) control group]. So the standard deviation in ND group was 1. n = 6 per group; *P < 0.05 compared with the HFD group; #P < 0.05 compared with the ND group.
Figure 5.
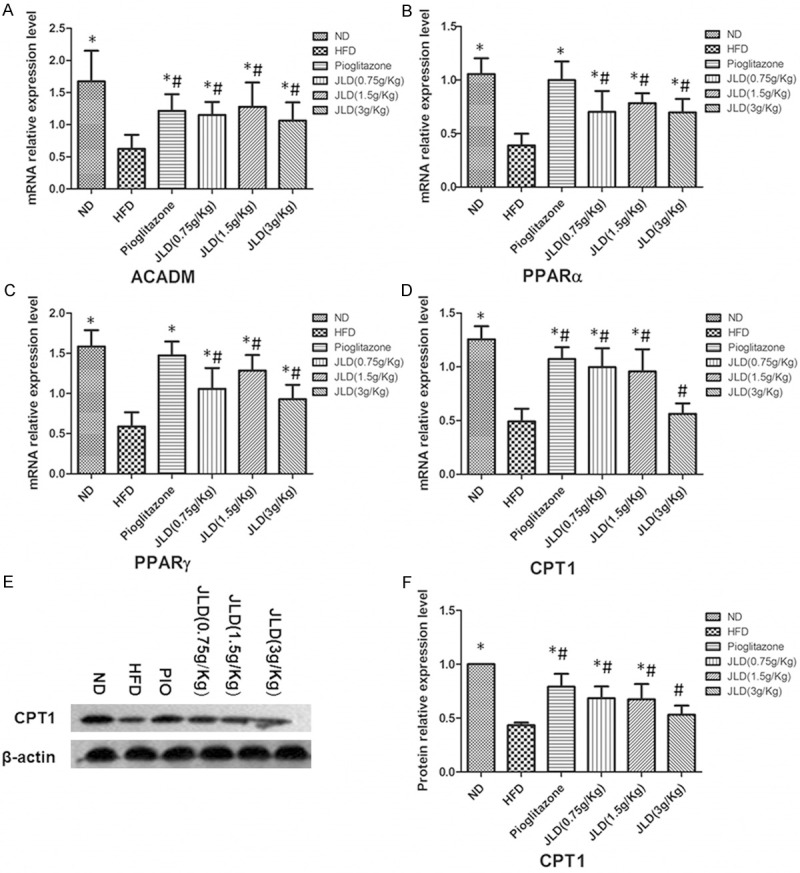
The affect of Jinlida on the expression of genes involved in NEFA oxidation in skeletal muscle in rats. The mRNA expression levels of acyl-CoA dehydrogenase (ACADM), peroxisome proliferator-activated receptor alpha (PPARα), PPARγ and carnitine palmitoyl transferase1 (CPT1) were examined by quantitative real-time-polymerase chain reaction (A-D). Protein level of CPT1 was detected by Western blot (E, F). n = 6 per group; *P < 0.05 compared with the HFD group; #P < 0.05 compared with the ND group.
Effect of JLD on PGC1α expression and genes involved in mitochondrial function and NEFA oxidation
To further investigate the mechanism by which JLD reversed lipid accumulation in skeletal muscle, we detected the expression of proteins and genes of mitochondrial. The mRNA and protein expression levels of PGC1αwere significantly increased following JLD (1.5 g/Kg) and pioglitazone treatment (Figure 4) (P < 0.05). The genes for nuclear respiratory factor 1 (NRF1) and cytochrome c oxidase subunit IV (COX IV) were not affected by pioglitazone, but the expression of COX IV was increased by the middle dose of JLD (Figure 4A-C) (P < 0.05).
Figure 4.
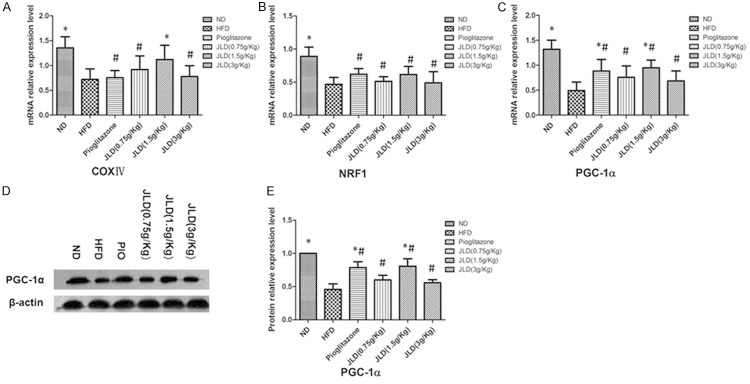
The affect of Jinlida on expression of PGC1α and genes involved in mitochondrial function in skeletal muscle in rats. The mRNA expression levels of peroxisome proliferator-activated receptor gamma, coactivator 1α (PGC1α), nuclear respiratory factor 1 (NRF1) and cytochrome c oxidase subunit IV (COX IV) were examined by quantitative real-time-polymerase chain reaction (A-C); The protein level of PGC1α was detected by Western blotting (D, E). n = 6 per group; *P < 0.05 compared with the HFD group; #P < 0.05 compared with the ND group.
The mRNA level of genes involved in NEFA oxidation in mitochondrial: CPT1 was significantly increased after JLD (0.75 g/kg, 1.5 g/kg) and pioglitazone treatment (Figure 5A-D) (P < 0.05), while there was no change in the high dose of JLD. Genes for acyl-CoA dehydrogenase (ACADM), peroxisome proliferator-activated receptor alpha (PPARα) and PPARγ were significantly increased after JLD and pioglitazone treatment (Figure 5A-D) (P < 0.05).
Effect of JLD on liver function
Changes in liver function were assessed by the levels of ALT, AST and TBIL (Table 2). The results show that the concentrations of ALT were 84.77±3.29 IU/L, 66.25±5.32 IU/L and 88.48±2.30 IU/L at doses of 0.75, 1.5 and 3 g/kg of JLD respectively. These concentrations were significantly lower than the HFD group (116.98±4.90 IU/L) (P < 0.05). Concentrations of AST in the HFD group treated with JLD were 161.18±3.94 IU/L, 132.98±3.44 IU/L and 169.18±5.74 IU/L respectively. Again, these concentrations were significantly lower than the HFD group (218.67±5.12 IU/L) (P < 0.05). TBIL in serum was unchanged. These results indicate that JLD treatment is beneficial for the liver.
Discussion
Recently available therapies for diabetes mellitus include insulin and many oral hypoglycemic agents, such as biguanides, thiazolidinediones and sulfonylureas. However, these drugs have a series of limitations, such as adverse effects and high rates of secondary failure [18]. The traditional Chinese medicine (TCM) is an excellent example in alternative and complementary medicine with a long history and unique theory system [19]. Considerable attention has been focused on the protective role of natural drugs in the prevention and treatment of T2DM in recent years. JLD, one traditional Chinese medicine, has exhibited the activity on lowering blood glucose, increasing insulin sensitivity and regulating lipid metabolism, etc [3]. In our study we find that JLD can ameliorate HFD-induced insulin resistance.
Pioglitazone, used as positive control drug, has been reported to ameliorate insulin resistance by activating AMPK and ACC in muscle, up-regulating the expression of CPT1, augmenting fatty acid entry into mitochondria for oxidation. Coletta et al [11] reported that chronic treatment with pioglitazone increased plasma adiponectin levels as well as AMPK and ACC phosphorylation expression in the muscle. Fur-thermore, expression of genes involved in mitochondrial function and fat oxidation were up-regulated. Treatment with pioglitazone improves plasma adiponectin, reduces fasting plasma insulin, in part, by a coordinated up-regulation of genes involved in mitochondrial oxidative phosphorylation and ribosomal protein biosynthesis in muscle in PCOS [20]. JLD can lower circulating serum lipid levels in patients with dyslipidemia in addition to improving insulin sensitivity. Consequently, it was hypothesized that the mechanism by which JLD ameliorates insulin resistance may be related to reduced lipid content in plasma, or also in the muscle. To test this hypothesis, pioglitazone was selected as a positive control.
In the present study, the insulin-sensitizing affects of JLD were investigated using the obese insulin resistant rat model fed a high-fat-diet that would closely mimic the natural history of the disease as well as the metabolic characteristics associated with human T2DM. Treatment with JLD for 8 weeks significantly improved glycaemic control (FPG, AUC and GIR) in HFD-induced insulin resistant rats. Insulin resistance induced by a high-fat-diet correlated with dyslipidaemia. Serum TG, TC and NEFA levels were reduced significantly following treatment with JLD for 8 weeks, suggesting that JLD improves metabolic homeostasis in T2DM. The decrease of plasma lipid levels was an indication of enhanced energy expenditure in the whole body. It was also found that HFD-induced insulin resistance correlated with lipid deposits in skeletal muscle, the precise mechanism responsible for this accumulation remained controversial. Elevated ectopic triglyceride deposition in muscle might be due to increased fatty acid uptake, decreased oxidation and increased esterification, or a combination of these. Otherwise, increasing fatty acid oxidation in muscle could improve insulin sensitivity. Treatment with JLD for 8 weeks alleviated the accumulation of TG, NEFA and LCACoA in muscle in insulin-resistant rats, as shown by Oil red O staining.
To investigate the mechanism by which JLD reversed the lipid accumulation in skeletal muscle in insulin-resistant rats, we detected the expression of proteins in AMPK signal pathway. In our study we found that JLD (1.5 g/kg) increased the phosphorylation of AMPK in skeletal muscle, which suggest that activation of AMPK pathway plays a role in the insulin-sensitizing effect of JLD. Many studies have indicated that activation of AMPK increased fatty acid oxidation by activating P-ACC and CPT1 in muscle [11]. ACC phosphorylation at Ser79 by AMPK reduces malonyl-CoA, an allosteric inhibitor of CPT1, which regulates the rate-limiting LCACoA entry into mitochondria for β-oxidation [21]. Consistent with this scenario, 8-week treatment with JLD at 1.5 g/kg increased ACC phosphorylation and expression of CPT1 and several genes involved in fatty acid oxidation. In addition, several studies have demonstrated that the phosphorylation of AMPK can activate the expression of GLUT4 [22]. Consistent with this theory, in the current study, AMPK phosphorylation was associated with up-regulation of the expression of GLUT4.
The increase in adiponectin concentrations in response to JLD treatment may, to some extent, explain the increase in phosphorylation of AMPK and ACC. In rodents, adiponectin may stimulate AMPK activity in the muscle, liver and fat, resulting in increased fatty acid oxidation and glucose transport in the muscle and liver [23,24]. As JLD can increase the concentration of adiponectin and the phosphorylation of AMPK, the in vivo effect of JLD on the level of genes involved in adiponectin signaling in skeletal muscle were also investigated. The level of both ADIPOR1 and ADIPOR2 were significantly decreased in the muscle of HFD mice. This was consistent with a study by Tsuchida [25]. As with pioglitazone, an increase in ADIPOR1 and ADIPOR2 mRNA following JLD treatment in insulin resistant rats was observed. Whilst the results of this study are in accordance with previous observations in rodents, the results do not allow definitive establishment of whether the stimulatory affect of JLD on AMPK activity is mediated via adiponectin, or if it the stimulation represents a direct affect of JLD on AMPK. Further research is required to conclusively determine the affect of JLD on AMPK signaling.
Mitochondrial dysfunction is now considered an important event in whole-body metabolic disturbance, particularly in T2DM. Impaired fatty acid oxidation may be a result of mitochondrial dysfunction [26-28]. Insulin-resistant patients have decreased expression of nuclear-encoded mitochondrial genes, along with decreased expression of PGC-1α, which is considered key regulators of mitochondrial biogenesis and function [10,29]. In accordance with previous studies [30], the current study shows that levels of PGC1α and several other genes involved in mitochondrial function in skeletal muscle, were decreased during insulin resistance. Treatment with JLD (1.5 g/Kg) increased the expression of PGC1α as well as its downstream targets in skeletal muscle including cytochrome c oxidase subunit IV (COX IV), Peroxisome proliferator-activated receptor gamma (PPARγ), Peroxisome proliferator-activated receptor alpha (PPARα), Carnitine palmitoyltransferase 1 (CPT1), acyl-CoA dehydrogenase (ACADM). However, unlike JLD, pioglitazone did not increase the expression of COXIV nor NRF-1. Similarly, Schrauwen-Hinderling et al [31] demonstrated that rosiglitazone treatment for 2 months did not change muscle mitochondrial function in patients with T2DM. In contrast, treatment with pioglitazone for 6 months significantly increased the level of genes involved in coding mitochondrial proteins in patients with T2DM [11]. The different treatment period may account for these two varying observations.
It is plausible that there may be a more complex mechanism by which JLD ameliorates HFD-induced insulin resistance as TCM often consists of many components. Therefore, further research is required to understand the molecular mechanisms of JLD. A cell culture study would be beneficial to further investigate the expression of proteins involved in the AMPK or other related pathways. In the present study, the middle dose of 1.5 g/kg of JLD was more effective than the low and high dose in insulin resistance conditions. Based on all above, the effects of JLD are non-dose-dependent, and we consider that the middle dose of JLD (1.5 g/Kg) is the best effective to ameliorate insulin resistance in our study.
In summary, the current study demonstrates that treatment with JLD up-regulates plasma adiponectin level and the expression of both ADIPOR1 and ADIPOR2 in skeletal muscle. Treatment with JLD activated the expression of proteins involved in the AMPK signaling pathway, increased the level of genes involved in mitochondrial function and fat oxidation, induced a decrease in toxic intracellular lipid accumulation (TG, NEFA and LCACoA) and augmented insulin sensitivity. The results provide support for the folkloric use of JLD in the management of insulin resistance and T2DM.
Acknowledgements
We wish to express our deepest thanks and appreciation to all who have graciously collaborated with us, we also thank the Department of Clinical Medical Research Center and Geriatric Key Laboratory of Hebei General Hospital for its support and of this research.
Disclosure of conflict of interest
None.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Aher SB, Auti PB, Washimkar MH. Thiazolidinediones : A class of pharmacologically important molecules. Mini Rev Med Chem. 2013 [Epub ahead of print] [PubMed] [Google Scholar]
- 3.Shi JL, Wu Y, Song YP, Han C, Liu ZM. Protective effect of Jinlida granules on islet β cells in diabetes mellitus rats. Academic Journal of Second Military Medical University. 2012;33:385–389. [Google Scholar]
- 4.Prentki M, Madiraju SR. Glycerolipid/free fatty acid cycle and islet beta-cell function in health, obesity and diabetes. Mol Cell Endocrinol. 2012;353:88–100. doi: 10.1016/j.mce.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boren J, Taskinen MR, Olofsson SO, Levin M. Ectopic lipid storage and insulin resistance: a harmful relationship. J Intern Med. 2013;274:25–40. doi: 10.1111/joim.12071. [DOI] [PubMed] [Google Scholar]
- 7.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean D, Daugaard JR, Young ME, Saha A, Vavvas D, Asp S, Kiens B, Kim KH, Witters L, Richter EA, Ruderman N. Exercise diminishes the activity of acetyl-CoA carboxylase in human muscle. Diabetes. 2000;49:1295–1300. doi: 10.2337/diabetes.49.8.1295. [DOI] [PubMed] [Google Scholar]
- 9.Holloway GP, Bonen A, Spriet LL. Regulation of skeletal muscle mitochondrial fatty acid metabolism in lean and obese individuals. Am J Clin Nutr. 2009;89:455S–462S. doi: 10.3945/ajcn.2008.26717B. [DOI] [PubMed] [Google Scholar]
- 10.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 11.Coletta DK, Sriwijitkamol A, Wajcberg E, Tantiwong P, Li M, Prentki M, Madiraju M, Jenkinson CP, Cersosimo E, Musi N, Defronzo RA. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia. 2009;52:723–732. doi: 10.1007/s00125-008-1256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie W, Zhao Y, Zhang Y. Traditional chinese medicines in treatment of patients with type 2 diabetes mellitus. Evid Based Complement Alternat Med. 2011;2011:726723. doi: 10.1155/2011/726723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang J, Wei H, Sun Y, Zhang X, Liu W, Chang Q, Wang R, Gong Y. Regulation of podocalyxin expression in the kidney of streptozotocin-induced diabetic rats with Chinese herbs (Yishen capsule) BMC Complement Altern Med. 2013;13:76. doi: 10.1186/1472-6882-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao HL, Sui Y, Qiao CF, Yip KY, Leung RK, Tsui SK, Lee HM, Wong HK, Zhu X, Siu JJ, He L, Guan J, Liu LZ, Xu HX, Tong PC, Chan JC. Sustained antidiabetic effects of a berberine-containing Chinese herbal medicine through regulation of hepatic gene expression. Diabetes. 2012;61:933–943. doi: 10.2337/db11-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higuchi N, Hira T, Yamada N, Hara H. Oral Administration of Corn Zein Hydrolysate Stimulates GLP-1 and GIP Secretion and Improves Glucose Tolerance in Male Normal Rats and Goto-Kakizaki Rats. Endocrinology. 2013;154:3089–3098. doi: 10.1210/en.2012-2275. [DOI] [PubMed] [Google Scholar]
- 16.Meirelles K, Ahmed T, Culnan DM, Lynch CJ, Lang CH, Cooney RN. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg. 2009;249:277–285. doi: 10.1097/SLA.0b013e3181904af0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Liu X, Han L, Gao X, Liu E, Wang T. Regulation of lipid and glucose homeostasis by mango tree leaf extract is mediated by AMPK and PI3K/AKT signaling pathways. Food Chem. 2013;141:2896–2905. doi: 10.1016/j.foodchem.2013.05.121. [DOI] [PubMed] [Google Scholar]
- 18.Tahrani AA, Piya MK, Kennedy A, Barnett AH. Glycaemic control in type 2 diabetes: targets and new therapies. Pharmacol Ther. 2010;125:328–361. doi: 10.1016/j.pharmthera.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Yin J, Zhang H, Ye J. Traditional chinese medicine in treatment of metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2008;8:99–111. doi: 10.2174/187153008784534330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skov V, Glintborg D, Knudsen S, Tan Q, Jensen T, Kruse TA, Beck-Nielsen H, Hojlund K. Pioglitazone enhances mitochondrial biogenesis and ribosomal protein biosynthesis in skeletal muscle in polycystic ovary syndrome. PLoS One. 2008;3:e2466. doi: 10.1371/journal.pone.0002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamontagne J, Pepin E, Peyot ML, Joly E, Ruderman NB, Poitout V, Madiraju SR, Nolan CJ, Prentki M. Pioglitazone acutely reduces insulin secretion and causes metabolic deceleration of the pancreatic beta-cell at submaximal glucose concentrations. Endocrinology. 2009;150:3465–3474. doi: 10.1210/en.2008-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song H, Guan Y, Zhang L, Li K, Dong C. SPARC interacts with AMPK and regulates GLUT4 expression. Biochem Biophys Res Commun. 2010;396:961–966. doi: 10.1016/j.bbrc.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, Kamon J, Kobayashi M, Suzuki R, Hara K, Kubota N, Terauchi Y, Froguel P, Nakae J, Kasuga M, Accili D, Tobe K, Ueki K, Nagai R, Kadowaki T. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817–30822. doi: 10.1074/jbc.M402367200. [DOI] [PubMed] [Google Scholar]
- 26.Qi Z, Ding S. Transcriptional Regulation by Nuclear Corepressors and PGC-1alpha: Implications for Mitochondrial Quality Control and Insulin Sensitivity. PPAR Res. 2012;2012:348245. doi: 10.1155/2012/348245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meugnier E, Faraj M, Rome S, Beauregard G, Michaut A, Pelloux V, Chiasson JL, Laville M, Clement K, Vidal H, Rabasa-Lhoret R. Acute hyperglycemia induces a global downregulation of gene expression in adipose tissue and skeletal muscle of healthy subjects. Diabetes. 2007;56:992–999. doi: 10.2337/db06-1242. [DOI] [PubMed] [Google Scholar]
- 28.Goodpaster BH. Mitochondrial deficiency is associated with insulin resistance. Diabetes. 2013;62:1032–1035. doi: 10.2337/db12-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attane C, Foussal C, Le Gonidec S, Benani A, Daviaud D, Wanecq E, Guzman-Ruiz R, Dray C, Bezaire V, Rancoule C, Kuba K, Ruiz-Gayo M, Levade T, Penninger J, Burcelin R, Penicaud L, Valet P, Castan-Laurell I. Apelin treatment increases complete Fatty Acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes. 2012;61:310–320. doi: 10.2337/db11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schrauwen-Hinderling VB, Mensink M, Hesselink MK, Sels JP, Kooi ME, Schrauwen P. The insulin-sensitizing effect of rosiglitazone in type 2 diabetes mellitus patients does not require improved in vivo muscle mitochondrial function. J Clin Endocrinol Metab. 2008;93:2917–2921. doi: 10.1210/jc.2008-0267. [DOI] [PubMed] [Google Scholar]


