Abstract
Aim: This study was designed to compare the effects and mechanisms of transcutaneous electroacupuncture (TEA) on rectal distention (RD)-induced intestinal dysmotility with EA. Methods: six female dogs chronically implanted with a duodenal fistula, a proximal colon fistula and intestinal serosal electrodes were studied. EA and TEA were performed via needles and cutaneous electrodes placed at bilateral ST-36 (Zusanli) acupoints respectively; their effects on postprandial intestinal dysmotility (slow waves, contractions and transit) induced by RD, and autonomic functions were compared. Results: RD at a volume of 140 ml suppressed intestinal contractions; the motility index was reduced with RD (P = 0.001). Both EA and TEA ameliorated the suppressed contractions (P = 0.003 and 0.001) and their effects were comparable. RD reduced the percentage of normal intestinal slow waves (P = 0.002) that was increased with both EA and TEA (P = 0.005 and 0.035). No significant difference was noted between EA and TEA. EA and TEA reduced small bowel transit time (P = 0.001 and 0.007); these prokinetic effects were blocked by atropine. Both EA and TEA increased vagal activity assessed by the spectral analysis of heart rate variability (both P = 0.03). Conclusion: RD inhibits postprandial intestinal motility. Both EA and TEA at ST-36 are able to improve the RD-induced impairment in intestinal contractions, transit and slow waves mediated via the vagal mechanism. Needleless TEA is as effective as EA in ameliorating the intestinal hypomotility.
Keywords: Heart rate variability, intestinal motility, intestinal slow waves, rectal distension, transcutaneous electroacupuncture
Introduction
Patients with chronic constipation often have gastrointestinal dysmotility. While numerous studies reported colonic and gastric dysmotility caused by constipation [1-6], much less is known on the effects of constipation on small bowel motility [7].
Rectal distension (RD) is known to inhibit jejunal and ileal motility in humans by an inhibitory reflex called “recto-enteric reflex” [8]. It’s proposed that RD mimicking fecal stasis in the colorectal region directly causes reflexive inhibition of proximal gastrointestinal motility [9-11]. Some studies have shown that RD inhibits intestinal transit and contractions [6,12]. However, little was known about the mechanism of this reflex. It’s reported that it may be mediated via the neural reflex and either the nociceptive or non-nociceptive afferent pathway is involved [13]. It is not clear whether sympathetic and vagal activities are altered with RD.
Few medications are available for treating small bowel dysmotility. The effective agents in the treatment of these disorders are prucalopride, neostigmine, bethanechol, metoclopramide, cisapride, and loperamide [14-17]. Due to moderate prokinetic effects, poor symptomatic responses and the presence of adverse effects, major prokinetic agents have limited uses in clinic [14]. Intestinal electrical stimulation has been reported to be a promising therapeutic option [18]. However, the requirement of surgical implantation of stimulation electrodes and a pulse generator limits its application in clinical practice.
Acupuncture is a Traditional Chinese Medicine therapy performed by inserting needles on specific acupoints through the skin. Electroacupuncture (EA) stimulates acupoints with electric current instead of manual manipulations and appears to be more reproducible in both clinical and animal research settings. Transcutaneous electroacupuncture (TEA) is new method of electrical stimulation via cutaneous electrodes placed at acupoints without needles. It’s a non-invasive method that can be easily accepted by patients and even self-administrated at home. If proven effective, this method would have clinical applications.
As we know, acupuncture has been shown effective in treating nausea and vomiting associated with postoperative and chemotherapy [19]. There have also been studies investigating the effects of acupuncture on constipation and irritable bowel syndrome [20-22]. Animal and human studies have explored the effects of EA on gastric motility and gastric myoelectrical activity [23-27]. However, little is known on the effects and mechanisms of EA on intestinal dysmotility. EA at ST36 was found to improve glucagon-induced small intestinal hypomotility and accelerate intestinal transit via the opioid and cholinergic pathway in dogs [28]. In a previous clinical study, we have shown that needless TEA is effective in improving dyspeptic symptoms in patients with functional dyspepsia [29]. It is unknown whether needless TEA is effective in treating RD-induced intestinal dysmotility, and whether it is as effective as EA.
The aims of this study were to explore the effects and mechanisms of EA and TEA on RD-induced impairment in small bowel motility and to compare the performance of needleless TEA with traditional EA in dogs.
Materials and methods
Animals
Six healthy female hound dogs (2-3 years old, 22-31 kg) fasted overnight underwent abdominal surgery under anesthesia using a previously established method [18,30]. Five mg/kg thiopental sodium (Abbott Laboratories, North Chicago, Ill, USA) was intravenously infused and maintained on 1.5% isoflurane inhalation in 1:1 oxygen-nitrous oxide carrier gases. One pair of 28-gauge cardiac pacing wires (A & E Medical, Farmingdale, NJ, USA) was implanted on the serosal surface of small bowel 35 cm beyond the pylorus. The two electrodes in the pair were 1 cm apart. The electrode wires were subcutaneously tunneled through the anterior abdominal wall along the right trunk and placed outside the skin around the right hypochondrium for intestinal slow wave recording. Two cannulas were implanted in each dog; one in the duodenum about 20 cm distal to the gastric pylorus and the other in the ascending colon about 5 cm distal to the cecum. The duodenal cannula was used for placement of a manometric catheter to the small bowel lumen for the recording of intestinal motor activity. It was also used for ingestion of phenol red for the measurement of intestinal transit. The colon cannula was used for the observation of outflow of phenol red from the small intestine.
The study was initiated after the dogs completely recovered from the surgery about 2 weeks later. All experiments were performed in the conscious state with the animal placed on an experimental table and slightly restrained. All dogs were free from any drugs within one week before the experiments and fasted overnight prior to each experiment. On the experiment day, one bottle of enema liquid (Fleet enema; C.B. Fleet Co. Inc., Lynchburg, VA, USA) was used for the clearance of the rectum before the experiment. The washout time between two sessions in each dog was at least one week. The protocol was approved by the Animal Use and Care Committee of the University of Texas Medical Branch at Galveston, Texas.
Experimental protocols
The study included two experiments, one to study effects and mechanisms of EA and TEA on postprandial small bowel transit with RD and the other to investigate the effects of EA and TEA on postprandial small bowel contractions and slow waves, and autonomic functions with RD.
Experiment 1: Effects and mechanisms of EA and TEA on small bowel transit with RD
The experiment was composed of six sessions: control (sham-EA), EA, TEA, atropine, EA + atropine, and TEA + atropine. Small bowel transit was measured as previously described [31,32]. The experiment was performed in the postprandial state immediately after the dog was fed with 237 ml liquid meal (Boost; Novartis Medical Health, Minneapolis, MN). In the sessions with atropine, atropine was intravenously administrated immediately after the meal at a dose (0.05 mg kg-1) used in previous studies [18]. Immediately after feeding, 15 mg of phenol red mixed with 30 ml of saline was injected into the small intestine via the duodenum cannula. The colon cannula was opened for the collection of intestinal contents every 3 min. The complete disappearance of phenol red (absence of phenol red in three consecutive collections) after its appearance was determined by the spectrophotometer method. The small intestinal transit time (SBTT) was defined as the time interval between the injection of phenol red and the complete disappearance of phenol red collected from the colon cannula.
Experiment 2: Effects of EA and TEA on small intestinal contractions and slow waves, and autonomic functions
The experiment consisted of three sessions: control (sham-EA), EA, and TEA. Each session was composed of 4 consecutive periods: 1) a 30 min postprandial baseline recording, 2) a 30 min recording with RD (140 ml), 3) a 30 min recording with RD and sham, EA or TEA, and 4) a 30 min recording of recovery without RD, sham, EA or TEA. The experiment was performed in the postprandial state after feeding of one can of 375 g chopped chicken (Pedigree; Mars Inc, Vernon California, USA). Through the experiment, intestinal manometry was recorded from a water-perfused catheter inserted into the small bowel via the duodenal cannula; the electrocardiography (ECG) was recorded from three cutaneous electrodes placed on the surface of the chest and the left leg; and intestinal myoelectrical activity was recorded from the implanted serosal electrodes.
Rectal distension
A small balloon was fixed to the tip of a catheter with thread. Polyoxygen was twined above the thread to strengthen the sealing. The sealing of balloon was checked by air inflation of the balloon in water. Before the experiment, the balloon was inserted into the rectum and positioned so that the caudal pole of the balloon lay 8 cm from the anal verge [33].
Preliminary testing was performed to determine appropriate distention volume as follows: the balloon was inflated with air at different volumes (110, 120, 130, 140, 150 mL) for a period of 30 min in a randomized order and the animal behaviors were closely monitored during the RD period. The distension volume of 140 ml was found to be the largest volume the dogs could tolerate without adverse behaviors suggestive of pain or discomfort. Consequently, this volume was used in all formal experiments.
Small bowel manometry
Small bowel manometry was performed as previously described [12]. The catheter connected to the pressure transducer was passed through the duodenal cannula towards the small bowel with a depth of 35 cm (distal direction). There were four sensors (side-hole) spaced at 5 cm intervals in the catheter. The recorded manometric signals were amplified by a multichannel manometric system (Ningbo MaiDa Medical Device Company, Ningbo, China). The recording was displayed on a monitor and saved on a PC.
The contractile activity was evaluated by using the mean area under the curve (AUC) per second, a parameter called Motility Index (MI). A high value in MI represented an increased contractile activity of the small bowel.
Recording and analysis of small bowel slow waves
Small bowel slow waves were recorded using a Biopac system (EOG 100A; Biopac Systems, Inc., Santa Barbara, Calif., USA) as described previously [32,34]. Dominant frequency (DF), Dominant power (DP), the percentage of normal intestinal slow waves (N%) and arrhythmia (A%) was analyzed by the adaptive spectral analysis software. DF refers to the mean frequency of intestinal slow waves. DP refers to the mean power of intestinal slow waves at the dominant frequency. N% is the percentage of time during which regular normal intestinal slow waves (18-22 cycles/min) are present over the given period. The establishment of normal slow wave frequency range of regular 18-22 cpm in dogs was based on our previous studies [13,35,36].
Assessment of autonomic function
The ECG was recorded from two body surface electrodes attached to the left and right supraclavicular fossa and a ground electrode attached to the left leg of the dog using a special amplifier (model 2283 Fti Universal Fetrode Amplifier, UFI, Morro Bay, CA) [37]. The heart rate variability (HRV) signal was derived from the original ECG recording by using a special validated program developed in our laboratory [38]. The program was used to identify R waves, calculate and interpolate R-R interval data at 100 Hz, and finally down-sample the interpolated data to a frequency of 1 Hz. The low frequency (LF) and high frequency (HF) components were extracted from the overall power spectrum of the heart rate variability signal. LF was defined as the AUC in the frequency range of 0.04 to 0.15 Hz, and reflects mainly sympathetic or adrenergic activity. HF was defined as the AUC in the frequency range of 0.15 to 0.50 Hz and reflects purely parasympathetic or vagal activity [37].
Electroacupuncture (EA) and transcutaneous electroacupuncture (TEA)
Both EA and TEA were performed via bilateral ST-36 (Zusanli, stomach-36). The selection of ST-36 acupoint was based on our previous studies in dogs [39]. The location of ST-36 is established at the proximal one-fifth of craniolateral surface of the rear leg distal to the head of the tibia in a depression between the muscles of the cranial tibia and long digital extensor.
For EA, a pair of acupuncture needles was inserted into bilateral ST-36 acupoints to a depth of 3-5 mm. The needles were connected to one electro-needling instrument (electrotherapeutic apparatus, Model D-860; Jinshan Electronic Device, Shanghai, China). The electrical stimuli were composed of pulse trains. The frequency of the pulse trains was 12 trains/min, the duration of each train was 2 s, the frequency of pulses in each train was 25 Hz and the pulse amplitude was 6 mA. These stimulation parameters were shown in a previous study to improve impaired intestinal motility attributed to glucagon in dogs [13].
For TEA, cutaneous ECG electrodes were placed on bilateral Zusanli (ST-36) and connected to the electrical stimulator. The stimulation parameters were the same as EA.
In the control (or called sham-EA) session, EA at acupoints located on the back (bladder-21) were used for sham EA. Bladder-21 (BL21) is located 1 cm lateral of the spinous process of the 12th thoracic vertebrae [23,28].
Statistical analysis
Data are reported as mean ± standard error. Student’s t-test was used to analyze the difference between two sessions. The ANOVA was used to analyze the differences among three or more sessions. If the ANOVA revealed a significant difference, the Dunnett’s test was used to study the differences between any two sessions among three or more sessions. P < 0.05 was considered as statistical difference.
Results
As shown in the preliminary testing, all dogs tolerated RD at a volume of 140 ml without adverse behaviors indicative of pain, gasping for breath or writhing.
Effects and mechanisms of RD, EA and TEA on small intestinal transit
Both EA and TEA accelerated small bowel transit (Figure 1). The SBTT was reduced from 190.8 ± 9.3 min in the control (sham) session to 100.8 ± 15.3 min in the EA session (P = 0.001) and 136.7 ± 8.0 min in the TEA session (P = 0.007). Although EA seemed more potent than TEA, no significant difference was noted in the accelerative effect between the two methods.
Figure 1.
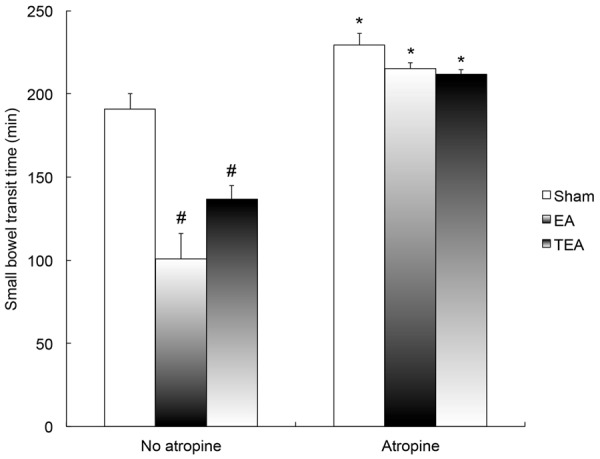
Effects of EA and TEA on small bowel transit time (SBTT). EA and TEA significantly reduced SBTT compared with sham (control) (P < 0.01). Atropine blocked the effect. After the administration of atropine, there is no difference among the three sessions (ANOVA P > 0.05). #P < 0.01 vs. Sham; *P < 0.001 vs. No atropine.
Atropine blocked the accelerative effects of EA and TEA on intestinal transit (Figure 1). At the presence of atropine, the SBTT became 229.3 ± 7.2 min in the control (sham) session, 215.2 ± 3.4 min in the EA session and 211.8 ± 2.7 min in the TEA session (ANOVA, P = 2.89).
Effect of RD, EA and TEA on small intestinal contractions in fed state
As expected, RD suppressed intestinal contractions. When RD was used, intestinal contractions were decreased immediately (Figures 2 and 3). In the control session, the mean MI was significantly reduced from 8.5 ± 0.3 at baseline to 6.2 ± 0.3 during distension (P = 0.001), and the MI recovered to 7.9 ± 0.6 (vs. 8.5 ± 0.3 at baseline, P = 0.13) after the distension was ceased.
Figure 2.
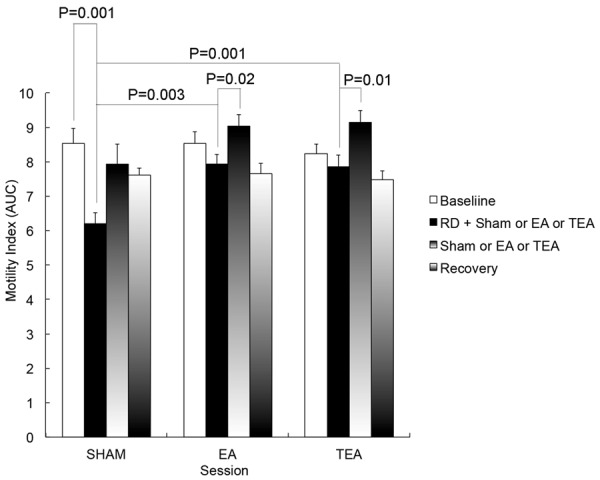
Effects of RD, EA and TEA on small bowel contractions. The motility index was reduced with RD in comparison with its baseline in the sham session (P = 0.001), and was increased with EA and TEA (P = 0.003, P = 0.001 vs. sham).
Figure 3.
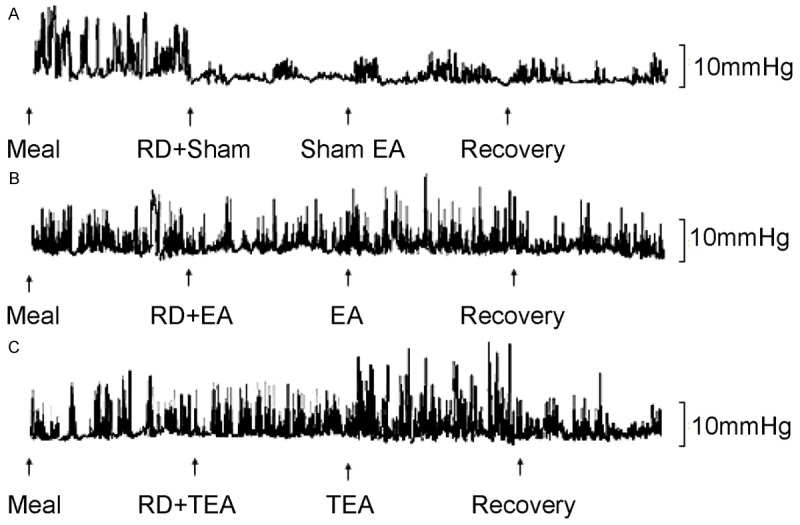
Typical tracings of intestinal contractions. A: Sham session; B: EA session; C: TEA session.
EA and TEA at ST36 ameliorated RD-induced impairment in intestinal motility (Figure 2). The MI was 7.9 ± 0.3 during RD + EA and 7.8 + 0.2 during RD + TEA, which were significantly higher than during RD with sham (6.2 ± 0.3, P = 0.003 and 0.001) and comparable to the baseline without RD (P = 0.17 for the EA session and P = 0.29 for the TEA session). Interestingly, the prokinetic effects were also observed when RD was stopped; as shown in Figure 2, during the third period without RD, the MI was increased to 9.0 ± 0.3 (P = 0.02) with EA and to 9.1 ± 0.4 (P = 0.01) with TEA; these were even higher than that at baseline without RD (7.9 ± 0.6, P = 0.04 and 0.03 for both EA and TEA). In the recovery period, they rapidly returned to 7.7 ± 0.3 (P = 0.002) in EA session and 7.5 ± 0.2 (P = 0.001) in TEA session.
No differences were found in the prokinetic effects between the EA session and the TEA session (P = 2.64) (See Figure 2).
Effects of RD, EA and TEA on small intestinal slow waves
Similarly, RD also impaired small intestinal slow waves (Figure 4). In control (sham) session, the percentage of normal slow waves was 92.0 ± 4.0% at baseline, significantly decreased to 51.1 ± 3.1% during RD (P = 0.002) and recovered to 77.0 ± 7.0% (P = 0.06 vs. baseline) after RD was stopped. However, RD showed no effects on dominant frequency or power of the intestinal slow waves.
Figure 4.
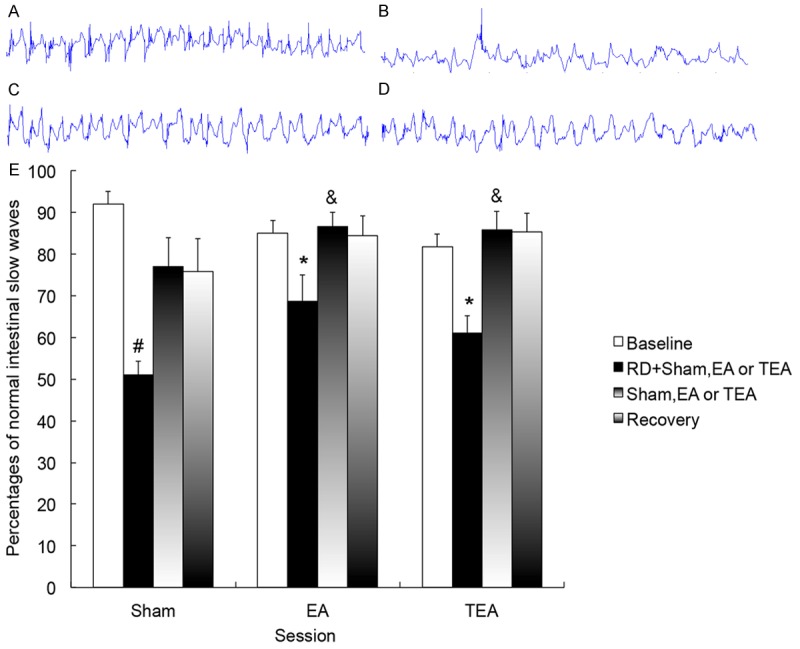
Effects of RD, EA and TEA on intestinal slow waves in dogs. A-D. Tracing of small intestinal slow waves (1min): A. Control: Regular, 18-22 cpm; B. RD: Irregular, < 18 cpm; C. RD + EA: Regular, 18 cpm; D. RD + TEA: Regular, 19 cpm. E. The percentages of normal slow waves was reduced with RD (P < 0.01), and increased with EA and TEA (P < 0.05). #P < 0.01 vs. Baseline; *P < 0.05 vs. Sham; &P < 0.05 vs. RD + Sham.
EA and TEA improved RD-induced impairment in intestinal slow waves. As shown in Figure 4, the percentage of normal slow waves was increased from 51.1 ± 3.1% during RD + sham to 68.7 ± 6.2 % (P = 0.005) during RD + EA and 61.1 ± 4.1% (P= 0.035) during RD + TEA. When RD was terminated, the percentage of normal slow waves was increased to 86.6 ± 3.5 % with EA and to 85.8 ± 4.6% with TEA; these were higher than that in the corresponding period in the control session (77.0 ± 7.0 %, P = 0.02 vs. both EA and TEA). In the recovery period, the percentage of normal slow waves in the EA and TEA sessions remained higher than that in the control session (both P = 0.04).
No difference was noted in the ameliorating effect on intestinal slow waves between EA and TEA (See Figure 4). Neither EA nor TEA showed any significant effect on dominant frequency or power of the intestinal slow waves.
Effects of RD, EA and TEA on vagal activity
The sympathetic activity (LF) assessed by the spectral analysis of the HRV showed no differences during the same periods among the three sessions (ANOVA, P = 1.58).
However, the vagal activity (HF) was altered by RD, EA and TEA (Figure 5). In the control (sham) session, RD decreased the vagal activity (HF) from 1.8 ± 0.4 at baseline to 0.5 ± 0.1 with RD (P = 0.001). EA increased HF during RD to 0.9 ± 0.2 (P = 0.03 vs. control) and so did TEA (0.9 ± 0.1, P = 0.03 vs. control). When RD was terminated, the HF with EA was higher than that with sham (2.5 ± 0.2 vs. 1.2 ± 0.4, P = 0.03), and similarly the HF with TEA was also higher than that with sham (2.1 ± 0.5 vs. 1.2 ± 0.4, P = 0.04). In the recovery period, no difference was noted in HF among three session of sham, EA and TEA (ANOVA P = 2.35). No difference was found in vagal activity between the EA session and the TEA session (see Figure 5).
Figure 5.
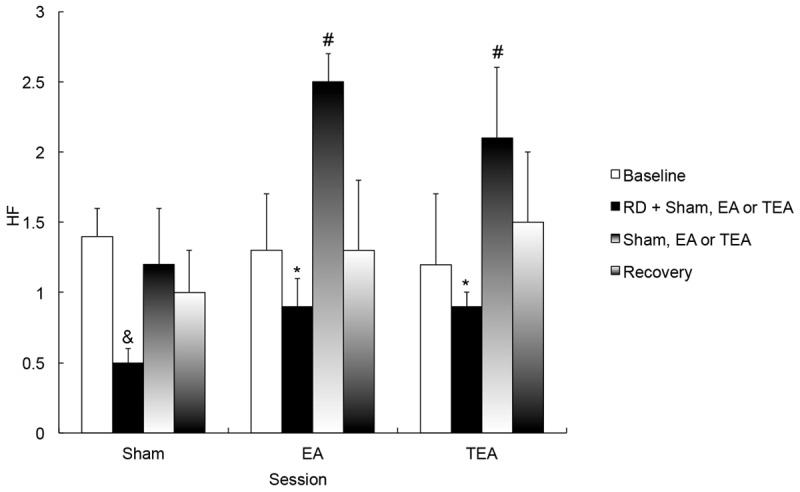
Effects of RD, EA and TEA on the high-frequency (HF) component representing vagal activity. *P < 0.0 vs. RD + Sham, #P < 0.05 vs. Sham; &P < 0.01 vs. Baseline.
Discussion
In this study, we investigated the effects and mechanisms of EA and TEA at ST-36 on small intestinal slow waves, contractions and transit, and vagal activity. We found that both EA and TEA at ST-36 improved small intestinal dysrhythmia, enhanced vagal activity and increased intestinal contractions and transit in dogs with RD, and their effects were comparable. Atropine blocked the accelerative effect of EA and TEA on small bowel transit, suggesting a cholinergic mechanism.
RD has been frequently used in studying visceral sensation [40], colorectal reflex [41], rectoanal inhibitory reflex (RAIR) [13,42], anal sphincter function [43] and gastrointestinal motility [12,23]. RD is a commonly used model to induce both upper and lower gastrointestinal motility disorders. In this study we used RD to impair intestinal motility and then study the effects of EA and TEA on intestinal dysmotility. Our previous studies in animals have revealed that RD was able to impair gastric tone, accommodation and antral contractions, and the RD-induced inhibition on gastric motility [23,44]. One clinical study has suggested that RD induces upper or lower abdominal symptoms and impairs gastric slow waves in healthy volunteers possibly by inhibiting vagal activity [45]. However, little was known on the effect of RD on intestinal motility. Our previous study revealed that RD inhibited postprandial small intestinal motor activity in a distension volume-dependent manners in dogs, and this inhibitory effect was at least partially mediated via the alpha and beta adrenergic pathways [12]. Shafik, et al reported that rectal balloon distension inhibited jejunal and ileal motility possibly mediated by the recto-enteric reflex in healthy volunteers [7]. Some studies in clinic also showed that patients with constipation exhibited intestinal hypomotility and dysmotility [6,7,46,47]. In the present study, we found that RD could slow small bowel transit, inhibit small bowel contractions and impair intestinal slow waves; meanwhile it reduced vagal activity. These findings demonstrated that RD was an appropriate model of intestinal dysmotility for studying the prokinetic effects of EA and TEA.
In clinic, no effective drugs are available for treating small bowel hypomotility. EA was reported to improve intestinal motility in canine model [18]. While a number of studies reported improved gastric emptying in animals and humans with EA and TEA at ST-36 [18,23,29,48-50], little is known about the effect of EA and TEA on intestinal motility. Luo, et al and Iwa et al reported that EA at ST-36 exerted a stimulatory effect on the contractility of the distal colon in conscious rats, and the stimulatory effect might be mediated via the cholinergic pathway [5,7]. In another study, Iwa et al found that the number of c-Fos-immunopositive cells at Barrington’s nucleus was significantly increased in response to EA at ST-36 and Barrington’s nucleus played an important role in mediating EA-induced distal colonic motility in conscious rats [7]. In our laboratory, EA at ST36 was found to improve glucagon-induced small intestinal hypomotility and accelerate intestinal transit via the opioid and cholinergic pathway in dogs [28]. One recent study in healthy volunteers reported that acupuncture at ST36 is able to improve upper abdominal symptoms and impaired vagal activity induced by RD [45]. In the present study, both EA and TEA were shown to have ameliorating effects on RD-induced impairment in small bowel transit and these effects were blocked by atropine, suggesting the involvement of the vagal pathway. We also found EA and TEA ameliorate RD-induced impairment in small bowel contractions. Most importantly, for the first time, the needleless method of TEA was demonstrated to be as effective as EA.
While dysrhythmia has been frequently reported in the stomach, little is known on slow waves and dysrhythmia in the small intestine, partly attributed to the lack of non-invasive method for the assessment of intestinal slow waves. Effects of EA on gasrtic slow waves have been studied in both animals and humans. In dogs with duodenal or rectal distension, it was found that EA at ST-36 increased the regularity of gastric slow waves [23,37]. Lin et al and Liu et al reported EA and acupuncture and at ST36 resulted in a significant increase in the percentage of normal gastric slow waves in healthy volunteers [24,45]. One recent study showed TEA at ST-36 improved gastric slow waves, lowered plasma VIP, and IL-6 levels, and improved sympathovagal balance in patients with systemic sclerosis [51]. However, effects of EA and TEA on intestinal slow waves have rarely been studied. In an earlier study performed in our lab, RD at a volume of 120 ml inhibited intestinal contractions but did not alter intestinal slow [12]. In the present study, RD at a volume of 140 ml substantially reduced the percentage of normal slow waves. Both EA and TEA improved RD-induced impairment in intestinal slow waves. Interestingly, the enhancing effects of EA and TEA persisited to the period when RD was ceased, suggesting that both EA and TEA are capable of improving intestinal slow waves even under normal physiological conditions. This was similar to the effect of EA on gastric slow waves observed in healthy volunteers (23). More interestingly, however, the needleless method of TEA was noted to be as potent as EA. Mechanistically, the improvement in intestinal slow waves was believed to be attributed to enhancement in vagal activity, as concurrently, there was a signficant increase in vagal activity during RD with EA and TEA in comparison with the control session. Similar to their effects on intestinal slow waves, the vagal enhancement with EA and TEA also persisited in the period when RD was terminated. This was in agreement with previous studies reporting similar vagal mechanisms involved in the improvement of gastric slow waves with EA and improvement of glucagon-induced intestinal dysrhythmia with EA [33,52].
Assessment of vagal activity from spectral analysis of heart rate variability is a well-established method and has been previously applied to assess the vagal mechanism of EA and TEA at ST-36 [23,29,37,53]. In dogs, EA was reported to accelerate gastric emptying in association with increased vagal activity [41]. In patients with functional dyspepsia, TEA at ST36 and PC6 was shown to markedly improve dyspepsia symptoms and enhance vagal activity [29]. In healthy volunteers, RD was found to decrease vagal activity and manual acupuncture at ST36 was reported to improve vagal activity [45]. In rats, it was reported that acupuncture on the hind limbs increased vagal discharges, whereas, acupuncture on the abdomen increased sympathetic discharges [54]. In the present study, we found that RD dramatically suppressed vagal activity (HF in Figure 5), and EA and TEA substantially increased vagal activity. In the mean time, we also found that atropine blocked the accelerative effects of EA and TEA on small bowel transit. Taking together, we believe that EA and TEA improve small bowel motilty via the vagal mechanism. The exact pathway involved in the improvemnent of vagal activity with EA and TEA remains unknown. However, in rats, it was reported that acupuncture at ST-36 increased the number of c-Fos immunopositive cells in the caudal nucleus tractus solitarius and the dorsal motor nucleus trctus of the vagus (DMV), suggesting that acupuncture at ST-36 stimulates the DMV through the nucleus tractus solitarius, resulting in enhanced vagal efferent activity [47,55].
Clinical perspectives
The findings of the present study suggest 1) EA and TEA may have a therapeutic potential for treating intestinal motility. The lack of medical therapies for treating intestinal dysmotility makes the EA and TEA therapies more attractive. Further clinical studies are needed to prove such a therapeutic potential. 2) TEA is a more attractive therapy than the traditional method of EA. TEA has been shown in this study to be as effective as EA. However, TEA can be performed without needles and thus can be self-administrated by patients at home. This would be well accepted by patients and physicians as it has following advantages: 1) it can be performed at home without visiting a doctor or an acupuncturist; this would reduce the cost of the therapy; 2) since it can be performed at home, the therapy can be applied more frequently than EA, such as daily or a few times daily. This would make the therapy more efficacious.
In conclusion, rectal distention inhibits postprandial small bowel motility. Both EA and TEA at ST-36 improve RD-induced impairment in intestinal contractions, transit and slow waves mediated via the vagal mechanisms. In addition, the needleless method TEA is as effective as EA in treating RD-induced intestinal dysmotility and may have a great therapeutic potential for treating intestinal hypomotility disorders.
Acknowledgements
Jun Song performed the research, analyzed the data and drafted the manuscript. Jieyun Yin performed the research, interpreted the data and revised the manuscript. Jiande Chen designed the experiments, analyzed the data and finalized the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Boccia G, Buonavolonta R, Coccorullo P, Manguso F, Fuiano L, Staiano A. Dyspeptic symptoms in children: the result of a constipation-induced cologastric brake? Clin Gastroenterol Hepatol. 2008;6:556–560. doi: 10.1016/j.cgh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Bassotti G, Stanghellini V, Chiarioni G, Germani U, De Giorgio R, Vantini I, Morelli A, Corinaldesi R. Upper gastrointestinal motor activity in patients with slow-transit constipation. Further evidence for an enteric neuropathy. Dig Dis Sci. 1996;41:1999–2005. doi: 10.1007/BF02093603. [DOI] [PubMed] [Google Scholar]
- 3.Abo M, Kono T, Wang Z, Chen JD. Impairment of gastric and jejunal myoelectrical activity during rectal distension in dogs. Dig Dis Sci. 2000;45:1731–1736. doi: 10.1023/a:1005590413490. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds JC, Ouyang A, Lee CA, Baker L, Sunshine AG, Cohen S. Chronic severe constipation. Prospective motility studies in 25 consecutive patients. Gastroenterology. 1987;92:414–420. [PubMed] [Google Scholar]
- 5.Mollen RM, Hopman WP, Kuijpers HH, Jansen JB. Abnormalities of upper gut motility in patients with slow-transit constipation. Eur J Gastroenterol Hepatol. 1999;11:701–708. doi: 10.1097/00042737-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 6.van der Sijp JR, Kamm MA, Nightingale JM, Britton KE, Granowska M, Mather SJ, Akkermans LM, Lennard-Jones JE. Disturbed gastric and small bowel transit in severe idiopathic constipation. Dig Dis Sci. 1993;38:837–844. doi: 10.1007/BF01295909. [DOI] [PubMed] [Google Scholar]
- 7.Seidl H, Gundling F, Pehl C, Pfeiffer A, Schepp W, Schmidt T. Small bowel motility in functional chronic constipation. Neurogastroenterol Motil. 2009;21:1278–e1122. doi: 10.1111/j.1365-2982.2009.01364.x. [DOI] [PubMed] [Google Scholar]
- 8.Shafik A. Effect of rectal distension on the small intestine with evidence of a recto-enteric reflex. Hepatogastroenterology. 2000;47:1030–1033. [PubMed] [Google Scholar]
- 9.Coremans G, Geypens B, Vos R, Tack J, Margaritis V, Ghoos Y, Janssens J. Influence of continuous isobaric rectal distension on gastric emptying and small bowel transit in young healthy women. Neurogastroenterol Motil. 2004;16:107–111. doi: 10.1046/j.1365-2982.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- 10.Kellow JE, Gill RC, Wingate DL. Modulation of human upper gastrointestinal motility by rectal distension. Gut. 1987;28:864–868. doi: 10.1136/gut.28.7.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bojo L, Cassuto J. Gastric reflex relaxation by colonic distension. J Auton Nerv Syst. 1992;38:57–64. doi: 10.1016/0165-1838(92)90216-4. [DOI] [PubMed] [Google Scholar]
- 12.Qi H, Brining D, Chen JD. Rectal distension inhibits postprandial small intestinal motor activity partially via the adrenergic pathway in dogs. Scand J Gastroenterol. 2007;42:807–813. doi: 10.1080/00365520601127257. [DOI] [PubMed] [Google Scholar]
- 13.Guinet A, Jousse M, Damphousse M, Hubeaux K, Le Breton F, Sheikh Ismael S, Amarenco G. Modulation of the rectoanal inhibitory reflex (RAIR): qualitative and quantitative evaluation in multiple sclerosis. Int J Colorectal Dis. 26:507–513. doi: 10.1007/s00384-010-1109-0. [DOI] [PubMed] [Google Scholar]
- 14.Karamanolis G, Tack J. Promotility medications--now and in the future. Dig Dis. 2006;24:297–307. doi: 10.1159/000092883. [DOI] [PubMed] [Google Scholar]
- 15.Sarna SK. Molecular, functional, and pharmacological targets for the development of gut promotility drugs. Am J Physiol Gastrointest Liver Physiol. 2006;291:G545–555. doi: 10.1152/ajpgi.00122.2006. [DOI] [PubMed] [Google Scholar]
- 16.Quigley EM. Prucalopride: safety, efficacy and potential applications. Therap Adv Gastroenterol. 2012;5:23–30. doi: 10.1177/1756283X11423706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans PR, Bak YT, Kellow JE. Effects of oral cisapride on small bowel motility in irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:837–844. doi: 10.1046/j.1365-2036.1997.00240.x. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Song G, Yin J, Chen J, Chen JH, Song J, Chen JD. Effects and mechanisms of electroacupuncture on glucagon-induced small intestinal hypomotility in dogs. Neurogastroenterol Motil. 2010;22:1217–1223. e1318. doi: 10.1111/j.1365-2982.2010.01565.x. [DOI] [PubMed] [Google Scholar]
- 19.Morey SS. NIH issues consensus statement on acupuncture. Am Fam Phys. 1998;57:2545–2546. [PubMed] [Google Scholar]
- 20.Fireman Z, Segal A, Kopelman Y, Sternberg A, Carasso R. Acupuncture treatment for irritable bowel syndrome. A double-blind controlled study. Digestion. 2001;64:100–103. doi: 10.1159/000048847. [DOI] [PubMed] [Google Scholar]
- 21.Chan J, Carr I, Mayberry JF. The role of acupuncture in the treatment of irritable bowel syndrome: a pilot study. Hepatogastroenterology. 1997;44:1328–1330. [PubMed] [Google Scholar]
- 22.Broide E, Pintov S, Portnoy S, Barg J, Klinowski E, Scapa E. Effectiveness of acupuncture for treatment of childhood constipation. Dig Dis Sci. 2001;46:1270–1275. doi: 10.1023/a:1010619530548. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Song GQ, Yin J, Koothan T, Chen JD. Electroacupuncture improves impaired gastric motility and slow waves induced by rectal distension in dogs. Am J Physiol Gastrointest Liver Physiol. 2008;295:G614–620. doi: 10.1152/ajpgi.90322.2008. [DOI] [PubMed] [Google Scholar]
- 24.Lin X, Liang J, Ren J, Mu F, Zhang M, Chen JD. Electrical stimulation of acupuncture points enhances gastric myoelectrical activity in humans. Am J Gastroenterol. 1997;92:1527–1530. [PubMed] [Google Scholar]
- 25.Ouyang H, Xing J, Chen J. Electroacupuncture restores impaired gastric accommodation in vagotomized dogs. Dig Dis Sci. 2004;49:1418–1424. doi: 10.1023/b:ddas.0000042240.05247.01. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang H, Chen JD. Review article: therapeutic roles of acupuncture in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20:831–841. doi: 10.1111/j.1365-2036.2004.02196.x. [DOI] [PubMed] [Google Scholar]
- 27.Xu S, Hou X, Zha H, Gao Z, Zhang Y, Chen JD. Electroacupuncture accelerates solid gastric emptying and improves dyspeptic symptoms in patients with functional dyspepsia. Dig Dis Sci. 2006;51:2154–2159. doi: 10.1007/s10620-006-9412-x. [DOI] [PubMed] [Google Scholar]
- 28.Tatewaki M, Strickland C, Fukuda H, Tsuchida D, Hoshino E, Pappas TN, Takahashi T. Effects of acupuncture on vasopressin-induced emesis in conscious dogs. Am J Physiol Regul Integr Comp Physiol. 2005;288:R401–408. doi: 10.1152/ajpregu.00344.2004. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Peng S, Hou X, Ke M, Chen JD. Transcutaneous electroacupuncture improves dyspeptic symptoms and increases high frequency heart rate variability in patients with functional dyspepsia. Neurogastroenterol Motil. 2008;20:1204–1211. doi: 10.1111/j.1365-2982.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 30.Song J, Yin J, Chen JD. Acute and chronic effects of desvenlafaxine on gastrointestinal transit and motility in dogs. Neurogastroenterol Motil. 2013;25:824–e637. doi: 10.1111/nmo.12190. [DOI] [PubMed] [Google Scholar]
- 31.Yin J, Chen J. Excitatory effects of synchronized intestinal electrical stimulation on small intestinal motility in dogs. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1190–1195. doi: 10.1152/ajpgi.00092.2007. [DOI] [PubMed] [Google Scholar]
- 32.Shafik A, Shafik AA, El SO, Shafik IA. Study of the effect of ileal distension on the motor activity of the jejunum, and of jejunal distension on the motor activity of the ileum. Hepatogastroenterology. 2007;54:2007–2010. [PubMed] [Google Scholar]
- 33.Yin J, Chen JD. Electroacupuncture improves rectal distension-induced delay in solid gastric emptying in dogs. Am J Physiol Regul Integr Comp Physiol. 2011;301:R465–472. doi: 10.1152/ajpregu.00271.2010. [DOI] [PubMed] [Google Scholar]
- 34.Shafik A. Effect of duodenal distension on the pyloric sphincter and antrum and the gastric corpus: duodenopyloric reflex. World J Surg. 1998;22:1061–1064. doi: 10.1007/s002689900517. [DOI] [PubMed] [Google Scholar]
- 35.Lin X, Peters LJ, Hayes J, Chen JD. Entrainment of segmental small intestinal slow waves with electrical stimulation in dogs. Dig Dis Sci. 2000;45:652–656. doi: 10.1023/a:1005466904380. [DOI] [PubMed] [Google Scholar]
- 36.Lin X, Hayes J, Peters LJ, Chen JD. Entrainment of intestinal slow waves with electrical stimulation using intraluminal electrodes. Ann Biomed Eng. 2000;28:582–587. doi: 10.1114/1.294. [DOI] [PubMed] [Google Scholar]
- 37.Ouyang H, Yin J, Wang Z, Pasricha PJ, Chen JD. Electroacupuncture accelerates gastric emptying in association with changes in vagal activity. Am J Physiol Gastrointest Liver Physiol. 2002;282:G390–396. doi: 10.1152/ajpgi.00272.2001. [DOI] [PubMed] [Google Scholar]
- 38.Wang ZS, Chen JD. Robust ECG R-R wave detection using evolutionary-programming-based inference system (EPFIS) and its application to assessing brain-gut interaction. IEEE Proceed-Sci Measure Tech. 2000;147:351–356. [Google Scholar]
- 39.Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li Z, Chen JJ, Murphy DL, Gershon MD. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G685–695. doi: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- 40.Zagorodnyuk VP, Kyloh M, Gregory SJ, Peiris H, Brookes SJ, Nan Chen B, Spencer NJ. Loss of visceral pain following colorectal distension in an endothelin-3 deficient mouse model of Hirschsprung’s disease. J Physiol. 2011;589:1691–1706. doi: 10.1113/jphysiol.2010.202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi H, Chen JD. Effects of intestinal electrical stimulation on postprandial small-bowel motility and transit in dogs. Am J Surg. 2006;192:e55–60. doi: 10.1016/j.amjsurg.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Xu X, Pasricha PJ, Sallam HS, Ma L, Chen JD. Clinical significance of quantitative assessment of rectoanal inhibitory reflex (RAIR) in patients with constipation. J Clin Gastroenterol. 2008;42:692–698. doi: 10.1097/MCG.0b013e31814927ba. [DOI] [PubMed] [Google Scholar]
- 43.Bajwa A, Thiruppathy K, Trivedi P, Boulos P, Emmanuel A. Effect of Rectal Distension on Voluntary External Anal Sphincter Function in Healthy Subjects. Colorectal Dis. 2011;13:1173–1179. doi: 10.1111/j.1463-1318.2010.02420.x. [DOI] [PubMed] [Google Scholar]
- 44.Lei Y, Zhu H, Xing J, Chen JD. Rectal distension modulates canine gastric tone and accommodation. Dig Dis Sci. 2005;50:2134–2140. doi: 10.1007/s10620-005-3020-z. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Huang H, Xu X, Chen JD. Effects and possible mechanisms of acupuncture at ST36 on upper and lower abdominal symptoms induced by rectal distension in healthy volunteers. Am J Physiol Regul Integr Comp Physiol. 2013;303:R209–217. doi: 10.1152/ajpregu.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao SS, Kuo B, McCallum RW, Chey WD, DiBaise JK, Hasler WL, Koch KL, Lackner JM, Miller C, Saad R, Semler JR, Sitrin MD, Wilding GE, Parkman HP. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537–544. doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Zarate N, Knowles CH, Yazaki E, Lunnis PJ, Scott SM. Clinical presentation and patterns of slow transit constipation do not predict coexistent upper gut dysmotility. Dig Dis Sci. 2009;54:122–131. doi: 10.1007/s10620-008-0324-9. [DOI] [PubMed] [Google Scholar]
- 48.Tatewaki M, Harris M, Uemura K, Ueno T, Hoshino E, Shiotani A, Pappas TN, Takahashi T. Dual effects of acupuncture on gastric motility in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R862–872. doi: 10.1152/ajpregu.00715.2002. [DOI] [PubMed] [Google Scholar]
- 49.Sato A, Sato Y, Suzuki A, Uchida S. Neural mechanisms of the reflex inhibition and excitation of gastric motility elicited by acupuncture-like stimulation in anesthetized rats. Neurosci Res. 1993;18:53–62. doi: 10.1016/0168-0102(93)90105-y. [DOI] [PubMed] [Google Scholar]
- 50.Liu JH, Yan J, Yi SX, Chang XR, Lin YP, Hu JM. Effects of electroacupuncture on gastric myoelectric activity and substance P in the dorsal vagal complex of rats. Neurosci Lett. 2004;356:99–102. doi: 10.1016/j.neulet.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Koothan T, Chen JD. Synchronized gastric electrical stimulation improves vagotomy-induced impairment in gastric accommodation via the nitrergic pathway in dogs. Am J Physiol Gastrointest Liver Physiol. 2009;296:G310–318. doi: 10.1152/ajpgi.90525.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNearney TA, Sallam HS, Hunnicutt SE, Doshi D, Chen JD. Prolonged treatment with transcutaneous electrical nerve stimulation (TENS) modulates neuro-gastric motility and plasma levels of vasoactive intestinal peptide (VIP), motilin and interleukin-6 (IL-6) in systemic sclerosis. Clin Exp Rheumatol. 2013;31:140–150. [PubMed] [Google Scholar]
- 53.Sallam H, McNearney TA, Doshi D, Chen JD. Transcutaneous electrical nerve stimulation (TENS) improves upper GI symptoms and balances the sympathovagal activity in scleroderma patients. Dig Dis Sci. 2007;52:1329–1337. doi: 10.1007/s10620-006-9257-3. [DOI] [PubMed] [Google Scholar]
- 54.Li YQ, Zhu B, Rong PJ, Ben H, Li YH. Neural mechanism of acupuncture-modulated gastric motility. World J Gastroenterol. 2007;13:709–716. doi: 10.3748/wjg.v13.i5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwa M, Tateiwa M, Sakita M, Fujimiya M, Takahashi T. Anatomical evidence of regional specific effects of acupuncture on gastric motor function in rats. Auton Neurosci. 2007;137:67–76. doi: 10.1016/j.autneu.2007.08.001. [DOI] [PubMed] [Google Scholar]


