Abstract
Background: Involvement of inflammatory processes in the atherogenesis is now well-recognized. The present study examines the relationship between a proinflammatory cytokine, interleukin-6 (IL-6), and atherosclerosis by meta-analyzing the correlation coefficients between IL-6 and intima media thickness reported in the relevant studies. Method: Relevant research articles were searched in several electronic databases by using most relevant MeSH terms and keywords. For the meta-analyses, correlation coefficients of individual studies were first converted into Fisher’s z scores and then overall effect size was transformed into correlation coefficient. Between-study hete rogeneity was tested by I2 index and subgroup analyses were performed. Quality of the included studies was assessed and tests for publication bias were carried out. Results: Thirty seven studies were selected for the meta-analysis from which data of 14832 participants including healthy persons, persons at risk of CVD and patients of various diseases is used. The effect size (correlation coefficient) with 95% confidence interval (CI) between IL-6 and intima media thickness was 0.336 (0.327 to 0.345); P < 0.0001 in the overall meta-analysis, 0.446 (0.422 to 0.47); P < 0.0001 in patients suffering from any pathological condition; 0.478 (0.446 to 0.508); P < 0.0001 in patients suffering either from a CVD or a disease which poses risk of CVD; 0.327 (0.264 to 0.388); P < 0.0001 in patients suffering from a disease with no CVD risk; and 0.31 (0.291 to 0.327); P < 0.0001 in participants of community-based surveys. There was no significant publication bias.Conclusion: Intima media thickness is significantly correlated with IL-6 levels in the patients with CVD or a disease posing risk of CVD as well as in apparently healthy populations.
Keywords: Atherosclerosis, intima media thickness, inflammation, interleukin-6, cardiovascular
Introduction
Atherosclerosis is characterized by the hardening of the artery walls because of the accumulation of cells, cholesterol, and extracellular matrix with the earliest detectable change as pathological intimal thickening [1]. This follows the appearance of fatty streaks of lipid-laden macrophages which triggers a series of changes in the vessel wall such as the endothelial activation and accumulation of smooth muscle and extracellular matrix which finally result in the formation of fibrofatty plaques. Such plaques can result in hazard either by causing stenosis at the site of formation or detaching to form a thrombus that can cause occlusion at any other place [2]. Dyslipidemia together with hypertension and diabetes poses major modifiable risk for atherogenesis, endothelial dysfunction, atherosclerosis and resulting cardiovascular disease (CVD) [3,4].
Research in the recent years has shown that inflammatory processes are involved in the pathogenesis of atherosclerosis [5]. It is postulated that injury or other insults such as infections, increased oxidative stress, shear stressors, molecular stressors such as angiotensin II, oxidized-lipoproteins, and cytokines activates monocytes/macrophages. Monocytes gather in the sub-endothelial space and excessively uptake low density lipoproteins and subsequently become the foam cells [6]. Interleukin (IL)-6 is a proinflammatory cytokine produced by the activated macrophages, endothelial cells and vascular smooth muscle cells and is capable of promoting the secretion of other cytokines. To promote atherogenesis, IL-6 is reported to stimulate monocyte chemoattractant protein 1 secretion from macrophages [7] and is associated with the increased expression of cell adhesion molecules [8,9]. Additionally, IL-6 has also been reported as a stimulator of the proliferation and migration of vascular smooth muscle cells [10].
Interleukin-6 has now been recognized as a predictor of CVD even after the adjustment for traditional risk factors and other inflammatory markers in a number of well-sized epidemiological studies involving apparently healthy individuals and patients [11-15]. A number studies have attempted to explore the relationship between inflammatory markers and atherosclerosis many of which have estimated statistical correlations between atherosclerosis markers and inflammatory markers. However, results are not always similar which provides impetus for a meta-analysis to attain a more reliable state of evidence. To our knowledge, there is no study to review the association of IL-6 with intima media thickness systematically. Therefore, the present study meta-analyzes the correlation coefficients between IL-6 and intima media thickness achieved in various studies which attempted to explore relationships between inflammatory markers and atherosclerotic markers either in cross sectional or longitudinal designs.
Method
Literature search
For the required original research articles (published before October 2014), electronic databases including EBSCO, Embase, PubMed, and Web of Science were searched. The MeSH terms and keywords used in logical combinations were interleukin-6, IL-6, intima media thickness, IMT, cIMT, ICA-IMT, BA-IMT, CCA-IMT, FA-IMT, carotid artery, brachial artery, femoral artery, atherosclerosis, arteriosclerosis, atherogenesis, and systemic sclerosis. Corroborations and cross references of important research papers were also explored. PRISMA guidelines were followed in carrying out this study. Quality assessment of the included studies was carried out by using a valid tool [16].
Inclusion and exclusion criteria
The inclusion criteria were: a) Cross sectional or longitudinal studies which carried out correlational analyses to investigate the associations between atherosclerosis and serum markers of inflammation and endothelial dysfunction; b) study reported correlation coefficient between intima media thickness (carotid/brachial/femoral artery) and serum IL-6 of the patients, persons at risk of CVD or healthy subjects. The exclusion criteria were: a) studies providing associational data that could be used to impute correlation coefficient indirectly e.g. from means and standard deviations/population size etc; b) studies mentioning the existence of association between IL-6 and intima media thickness but did not provide correlation coefficient.
Data extraction, synthesis and statistical analysis
Data regarding the participants’ demographic, clinical and pathological characteristics, and outcomes were obtained from the tabular, textual and graphic sources of the published research papers. Only raw values of the correlation coefficients provided in the studies were used in the meta-analyses. Imputation of correlation coefficients from data such as means and standard deviations or sample size were carried out for the rough estimates but were not included in the meta-analyses. For the meta-analysis, correlation coefficients reported in each of the included studies were first converted into Fisher’s z scores and then corresponding standard errors were calculated which were used to calculate the 95% confidence limits. Meta-analyses were performed under random effects model in Stata software (version 12; Stata Corporation, College Station, TX). The overall effect size was the weighted average of the inverse variance adjusted individual effect sizes (z scores). The effect sizes of the meta-analyses were then transformed intocorrelation coefficients which were then considered as the resultant effect sizes. Subgroup analyses were performed and between-study heterogeneity was tested by I2 index. For the assessment of publication bias, Begg-Mazumdar’s adjusted rank correlation test and the Egger’s regression asymmetry test were carried out.
Results
Thirty seven studies [17-54] were selected for the meta-analysis. Study screening and selection process is outlined in Figure 1 and important characteristics of the included studies are presented in Table 1. Of the included studies, data from 14832 participants including healthy, persons at risk of CVD and patients of various diseases is used to estimate overall effect size of the correlation coefficient between IL-6 and intima media thickness.
Figure 1.
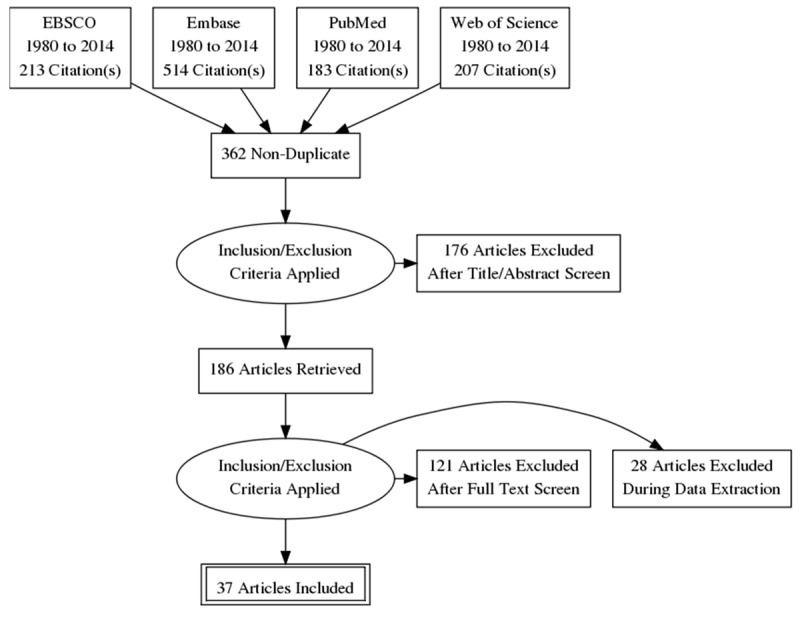
Flowchart of literature search, study screening and selection process.
Table 1.
Characteristics of the included studies
| Study/design | N | Age | Pathology/Atherosclerotic characteristics | % T2D | % HYPT | % DYSLIP | % MetS | % CVD |
|---|---|---|---|---|---|---|---|---|
| Amar et al 2006/C [17] | 953 | 49.4 ± 8.7 | Population survey | 3 | 13.6 | 13.6 | 13.6 | 0 |
| Bevc et al 2008/C [18] | 95 | 60 ± 13 | Hemodialysis patients | NA | NA | NA | NA | NA |
| Brucker et al 2014/C [19] | 58 | 49.5 ± 1.5 | Population survey | NA | 50 | NA | 0 | 0 |
| Cardellini et al 2007/C [20] | 176 | 36.1 ± 9.9 | Healthy children of Type 2 Diabetes (T2D) | 10 | 0 | 0 | NA | NA |
| Chapamn et al 2004/C [21] | 1092 | 53.3 ± 13 | Population survey | 2 | 24.3 | 7 | NA | 1 |
| Ciccone et al 2014/C [22] | 80 | 53 ± 10.5 | Obstructive sleep apnea | 0 | 0 | 0 | 0 | 0 |
| Elkind et al 2005/C [23] | 141 | 67.3 ± 8.6 | Population survey | 24.8 | 67.3 | 46.8 | NA | 17.7 |
| Esposito et al 2004/C [24] | 401 | 56 ± 9 | T2D | 100 | NA | NA | NA | 0 |
| Fagerberg et al 2008/C [25] | 98 | 58 years | Population survey | 0 | 0 | 0 | 0 | 0 |
| Fang et al 2010/C [26] | 86 | 10.5 ± 1.6 | Obese children/adolescent | 26 | NA | NA | 26 | 0 |
| Han et al 2010/C [27] | 852 | 59 ± 4 | Population survey | 0 | 0 | 0 | 0 | 0 |
| Hoshi et al 2007/C [28] | 226 | 67 ± 7.5 | CVD risk/primary stroke prevention/ILAA | 16.8 | 57.5 | 52.2 | NA | 20 |
| Kablak-Zeimb. 2011/L [29] | 227 | 64.5 ± 9.2 | Atherosclerotic occlusive disease | 35.6 | 91 | 100 | NA | 100 |
| Kapiotis et al 2006/C [30] | 145 | 12 ± 2.9 | Obese children | NA | NA | NA | 0 | 0 |
| Kato et al 2002/C [31] | 157 | 58 ± 1 | Hemodialysis patient | 11.4 | NA | NA | NA | 32 |
| Kim et al 2008/L [32] | 52 | 51.8 ± 10.8 | Continuous ambulatory peritoneal dialysis | 0 | 23.1 | NA | NA | 0 |
| Kobayashi et al 2010/C [33] | 393 | 59.6 ± 8.7 | Rheumatoid arthritis/healthy controls | 8.6 | 22.6 | 20.6 | NA | 0 |
| Lee et al 2007/C [34] | 392 | 63 ± 0.5 | Population survey | 8.6 | 39.2 | NA | NA | 12.5 |
| Leonsson et al 2003/C [35] | 102 | 54 ± 10 | Growth hormone deficiency | 0 | 0 | 0 | 0 | 0 |
| Li et al 2009/L [36] | 71 | 57 ± 4 | T2D | 100 | NA | NA | NA | NA |
| Lienonen et al 2005/C [37] | 78 | 61.6 ± 6.5 | Healthy controls | 0 | 0 | 0 | NA | 0 |
| Lind et al 2008/C [38] | 1016 | 70 years old | Population survey | 9 | 45 | NA | NA | 28 |
| Liu et al 2012/C [39] | 280 | 68.3 ± 6.5 | Atherosclerosis | 0 | 100 | 0 | NA | 0 |
| Martinic-Popovic 2014/C [40] | 45 | 74 (48 to 90) | Transient ischemic attack | 37.7 | 88.8 | 0 | NA | 100 |
| Minoguchi et al 2006/C [41] | 52 | 48.4 ± 3.1 | Obstructive Sleep apnea | 0 | 0 | 0 | 0 | 0 |
| Morillas et al 2012/C [42] | 126 | 56.5 ± 11.4 | Hypertensive | 23.8 | 100 | 57.1 | NA | 0 |
| Nakamura et al 2003/C [43] | 30 | 54.2 ± 5.5 | Hemodialysis-uremic patients | 50 | NA | NA | NA | 30 |
| Nishida et al 2007/C [44] | 254 | 48.6 ± 5.8 | Population survey | NA | NA | NA | NA | NA |
| Okazaki et al 2014/L [45] | 210 | 64 ± 8 | Individuals at risk of CVD | 11.9 | 79 | NA | NA | 30 |
| Porta et al 2007/C [46] | 85 | 57.5 ± 8.1 | Ischemic heart disease | 0 | 44.7 | 0 | NA | 100 |
| Ross et al 2010/C [47] | 27 | 10 (3 to 22) | HIV positive children | 0 | 0 | 0 | 0 | 0 |
| Ross et al 2009 [48] | 94 | 48 (21-70) | HIV positive patients | 0 | 0 | 0 | 0 | 0 |
| Rueda-Clausen 2009/C [49] | 102 | 55 (44-66) IQR | Dyslipidic patients | 0 | NA | 100 | NA | 33 |
| Sardo et al 2009/C [50] | 123 | 42 ± 9 | Hypertensive patients | 0 | 100 | 0 | 0 | 0 |
| Schipou et al 2013/C [51] | 46 | 48.6 ± 13.3 | Systemic sclerosis | 2 | 25 | NA | NA | 100 |
| Scuteri et al 2011/C [52] | 6123 | 43 ± 17 | Population survey | 7.3 | 36 | 9.6 | 69 | 0 |
| Stenvinkel et al 2002/C [53] | 45 | 51 ± 2 | Dialysis for end stage renal disease patients | 77.7 | 100 | NA | NA | 33 |
| Tarantino et al 2014/C [54] | 125 | 46 (34-53) IQR | Obese with non-alcoholic fatty liver disease | NA | NA | NA | NA | 0 |
C, cross sectional; CVD, cardiovascular disease DYSLIP, dyslipidemia; HYPT, hypertension; ILAA, intracranial large artery atherosclerosis; MetS (metabolic syndrome defined as the existence of at least 3 of 5 following conditions: 1) abdominal obesity; 2) a serum level of triglycerides ≥ 150 mg/dl; 3) a serum level of HDL-cholesterol 40 mg/dl; 4) systolic blood pressure ≥ 130 mm Hg, or diastolic blood pressure ≥ 85 mm Hg; 5) plasma level of glucose ≥ 100 mg/dl); L, longitudinal.
Among the included studies, 21 recruited persons either at risk of CVD including patients with T2D, obesity, hypertension, dyslipidemia and their respective controls, or patients suffering from a CVD; 11 were the general community-based surveys; remaining recruited patients with obstructive sleep apnea (2 studies), HIV positive patients (2 studies), rheumatoid arthritis patients (1 study), pituitary deficiency and growth hormone deficiency patients (1 study each), and their respective controls.
Average age of these participants ranged between 10 ± 4.7 and 74 ± 10.5 years with an average and standard deviation of 52.37 ± 7.45 years. In the overall population of this meta-analysis, the prevalence of cardiovascular conditions, T2D, dyslipidemia and hypertension was about 18%, 20%, 22% and 42%, respectively (exact prevalence may differ slightly because a few studies did not mention the prevalence as percentage in their respective research article/s).
There was no significant publication bias in this sample of studies as indicated by the Begg’s test and Egger’s test (Table 2; Figure 2).
Table 2.
Tests for publication bias assessment
| Begg’s test | ||||||
|
| ||||||
| Adjusted Kendall’s Score (P - Q) = 132 | ||||||
| Std. Dev. of Score = 89.02 (corrected for ties) | ||||||
| Number of dataset = 41 (Studies = 31) | ||||||
| z = 1.48; Pr > |z| = 0.138 | ||||||
| z = 1.47 (continuity corrected); Pr > |z| = 0.141 (continuity corrected) | ||||||
|
| ||||||
| Egger’s test | ||||||
|
| ||||||
| Standard Effect | Coefficient | Standard error | t | P > |t| | 95% confidence interval | |
|
| ||||||
| Slope | 0.3081291 | 0.0695739 | 4.43 | 0.000 | 0.1674027 | 0.4488556 |
| bias | 1.096172 | 1.306587 | 0.84 | 0.407 | −1.54665 | 3.738994 |
Figure 2.
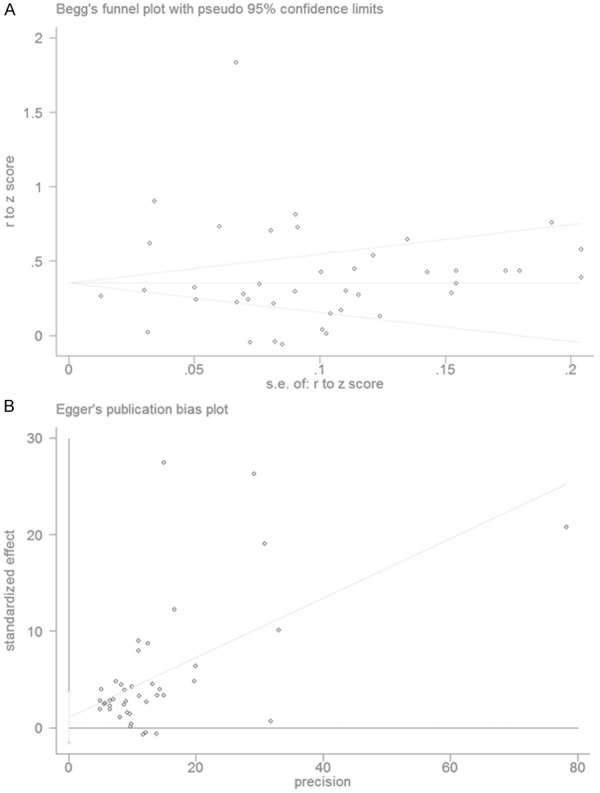
Plots showing the publication bias. A. Begg’s funnel plot. B. Eggers regression.
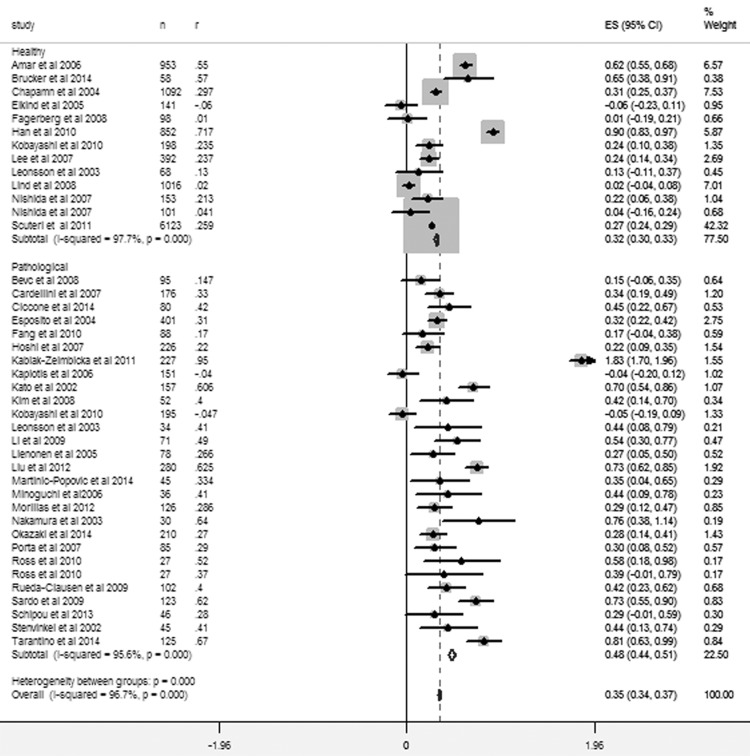
Quality assessment of the included studies is presented in Table 3 and the summary of the findings of the meta-analyses are presented in Table 4. The overall correlation coefficient (effect size of the meta-analysis of all included studies) between IL-6 and intima media thickness with 95% confidence interval [CI] was 0.336 [0.327, 0.345]; (P < 0.0001; Figure 3). In the subgroup analyses, meta-analysis of the studies which recruited patients suffering from various pathological conditions revealed the correlation coefficient of 0.446 [0.422, 0.470] (P < 0.0001) and in the submeta-analysis of studies involving healthy individuals correlation coefficient was 0.311 [0.292, 0.321] (P < 0.0001; Figure 3).
Table 3.
Assessment of quality of the included studies with Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [16]
| Criteria | Study reference | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||||||||||||||||||||||||
| 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | |
| 1. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 3. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 4. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 5. | N | N | N | N | N | N | Y | N | N | N | N | N | N | Y | N | N | N | N | Y | N | N | N | N | N | N | N | N | N | N | Y | N | N | N | N | N | N | N | N |
| 6. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 7. | NA | Y | NA | NA | NA | Y | NA | Y | NA | Y | NA | CD | Y | Y | Y | Y | Y | NA | Y | Y | Y | NA | Y | Y | Y | Y | Y | NA | CD | Y | Y | Y | Y | Y | Y | NA | Y | Y |
| 8. | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | Y | N | N | N | N | N | N | N | N | N | N | N | N | Y | N | N | N | N | N | N | N | N | N |
| 9. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 10. | N | N | N | N | N | N | N | N | N | N | N | N | Y | N | N | Y | N | N | N | Y | N | N | N | N | N | N | N | N | Y | N | N | N | N | N | N | N | N | N |
| 11. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 12. | N | N | N | N | N | N | Y | Y | N | N | Y | Y | N | Y | N | N | N | Y | Y | N | Y | N | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | N | N |
| 13. | NR | NR | NR | NR | NR | NR | NR | Y | NR | NR | Y | NR | NR | NR | NR | Y | NR | Y | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | Y | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 14. | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Criteria. 1. Was the research question or objective in this paper clearly stated? 2. Was the study population clearly specified and defined? 3. Was the participation rate of eligible persons at least 50%? 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study pre-specified and applied uniformly to all participants? 5. Was a sample size justification, power description, or variance and effect estimates provided? 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? 10. Was the exposure(s) assessed more than once over time? 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? 12. Were the outcome assessors blinded to the exposure status of participants? 13. Was loss to follow-up after baseline 20% or less? 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? *CD, cannot determine; NA, not applicable; NR, not reported.
Table 4.
Summary of the findings of the meta-analyses
| Group/subgroup | Studies | n | Effect size (z score) [95 CI] | Correlation coefficient [95% CI] | P | I2 |
|---|---|---|---|---|---|---|
| Overall | 37 | 14832 | 0.352 [0.336, 0.368] | 0.336 [0.327, 0.345] | P < 0.0001 | 96.7% |
| Healthy individuals | 12 | 266 | 0.316 [0.297, 0.334] | 0.311 [0.292, 0.321] | P < 0.0001 | 97.6% |
| Population surveys | 10 | 10979 | 0.319 [0.300, 0.337] | 0.310 [0.291, 0.327] | P < 0.0001 | 98.1% |
| Patients | 27 | 3587 | 0.480 [0.445, 0.514] | 0.446 [0.422, 0.470] | P < 0.0001 | 95.8% |
| CVD/CVD risk patients | 21 | 2861 | 0.518 [0.481, 0.555] | 0.478 [0.446, 0.508] | P < 0.0001 | 96.4% |
| Non-CVD risk patients | 6 | 726 | 0.341 [0.266, 0.415] | 0.327 [0.264, 0.388] | P < 0.0001 | 85.1% |
Figure 3.
Forest graph showing the overall meta-analysis and the submeta-analyses of the studies with healthy individuals and the studies which recruited patients suffering from various pathological conditions. Dual study data: Nishida et al., 2007 (men, women), and Ross et al., 2010 (internal carotid artery, common carotid artery).
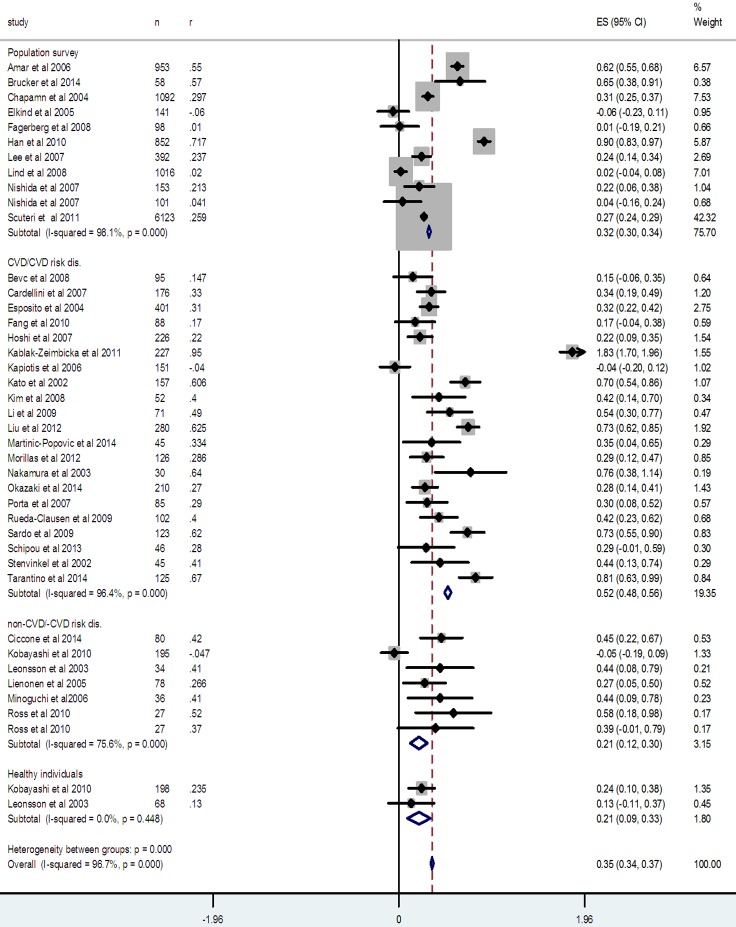
In the submeta-analysis of the studies which recruited patients suffering either from a CVD or a disease which poses risk of CVD, the correlation coefficient was 0.478 [0.446, 0.508]; P < 0.0001, whereas, it was 0.327 [0.264, 0.388]; P < 0.0001 in the studies with non-CVD/CVD risk disease patients (Figure 4). In the submeta-analysis of the studies which undertook cIMT and IL-6 measurements in the apparently healthy individuals (population surveys), the correlation coefficient was 0.310 [0.291 to 0.327]; P < 0.0001 and it was 0.21 [0.09, 0.32]; P < 0.0001 in the healthy controls of two studies (Figure 4).
Figure 4.
Forest graph of the meta-analysis of all included studies showing the overall effect size (z score) as well as the effects sizes of subgroups-population surveys, cardiovascular diseases (CVD) or diseases with CVD risk, non-CVD/CVD risk diseases, and healthy individuals.
In the submeta-analysis with regards to the design of the study, the correlation coefficient was 0.338 [0.323, 0.352]; P < 0.0001 for the studies which utilized cross sectional designs and it was 0.339 [0.240, 0.431]; P < 0.0001 for studies which utilized longitudinal designs.
Discussion
The present study finds a significant association between intima media thickness and IL-6 in a heterogeneous sample of human subjects with health status ranging from healthy participants to patients with chronic diseases. Moreover, the significance of this relationship persisted in subgroup; a) participants of community-based surveys with predominantly apparently healthy individuals, b) patients suffering from CVD or diseases which pose risk of CVD, or c) patients with other diseases such as AIDS, obstructive sleep apnea, hypophyseal deficiency, and rheumatoid arthritis.
It has been estimated that first myocardial infarction (MI) can happen with a carotid intima media thickness of above 0.882 mm and stroke above 0.75 mm. Moreover, an increase in carotid intima media thickness of more than 0.34 mm per year can lead to a cardiovascular event [55]. Thus, atherosclerosis and subsequent CVD risk prediction is of prime importance in which role of inflammatory agents is being increasing speculated. There are a number of studies which have examined the association of IL-6 with CVD and their risk factors and those which found a significant association are numerous. Ridker et al., [11] in a 6-year follow-up study of healthy middle-aged men, have reported that baseline IL-6 levels of > 2.28 pg/ml are associated with 2.3-fold increased risk of future myocardial infarction.
Among the proinflammatory cytokines, IL-6 is reported as a valuable predictor of the risk of a major adverse cardiovascular event in subjects with coronary heart disease (CHD) [56] and in hypercholesterolemic middle-aged men, on the basis of multivariate analyses including conventional CHD risk factors [57]. In a 6-year follow-up study of apparently healthy men, elevated IL-6 levels were associated with increased risk of future MI even after adjustments for baseline differences in total cholesterol, HDL-cholesterol, BMI, blood pressure, diabetes mellitus, family history of precocious CHD, alcohol use, or exercise frequency [11]. Moreover, in a study of MI and unstable angina patients, among others, IL-6, was also a significantly reliable predictor of mortality in the long-term and re-hospitalization for congestive heart failure but not for MI [14].
In a large prospective case-cohort study of about 400 CHD patients and 2000 controls, even after adjustment for traditional cardiovascular risk factors, elevated levels of IL-6, but not IL-18, were found to be independently associated with CHD risk, which was stronger in women [13]. Interleukin-6 has also been found to be a more reliable predictor of the risk of a prime CHD in a population-based prospective study of apparently healthy men. In this study, IL-6 was also associated with mortality related to MI and CHD but not with angina [58]. Elevated preoperative levels of IL-6 are also reported to be predictive of early graft occlusion after coronary artery bypass grafting [59].
Interleukin-6 is identified as a potential predictor of future cerebrovascular events in patients with cardiovascular risk factors, including hypertension, diabetes mellitus, hyperlipidemia, history of smoking, arteriosclerosis (transient ischemic attack), stroke, CHD, or peripheral artery disease (PAD) [15]. Similarly, in a study of elderly with no prior CVD (Health ABC Study) [60], IL-6 was found to be a predictor of stroke incidence. However, other studies such as PROSPER [61] and Caerphilly [62] could not find an independent association of IL-6 in discriminating stroke risk.
Persisting inflammation may lead to functional decline in patients with lower extremity PAD if it damages skeletal muscular physiology or promotes lower extremity atherosclerosis. Interleukin-6 is reported to be associated with faster decline in walk performance tests in PAD patients at three year follow-up [63]. Higher baseline IL-6 levels were also found to be associated with a higher PAD incidence at 5- and 12-year follow-up [12].
Association of IL-6 with atherosclerosis as well as with risk factors for atherosclerosis has not only been found in a number of adult population studies but also in those involving young participants [64].
Together with the literature cited above and the results of this meta-analysis, it is evident that the role of proinflammatory cytokine IL-6 in atherogenesis is pivotal. Interleukin-6 is released in response to acute infections, chronic inflammation, obesity, and physiological stress. Increased IL-6 levels initiate the synthesis of acute-phase reactants in the liver, endothelial cell activation, increased coagulation, stimulation of the hypothalamic-hypophyseal-adrenal axis, and promotion of lymphocyte proliferation and differentiation. Thus, targeting IL-6 in a therapeutic strategy can be useful. There is some evidence in this regard as individuals with IL-6 receptor variant that causes impaired IL-6 signaling have a decreased risk for CHD. Research is needed to test IL-6 receptor blockers such as tocilizumab in alleviating the inflammatory response [2].
Despite, a myriad number of reports providing evidence regarding the existence of a significant relationship between IL-6 and atherosclerosis/CVD risk, results of some studies did not conform to the existence of such a relationship. Among the included studies of this meta-analysis, population surveys of apparently healthy participants [23,25,38], studies in obese children and adolescents [26,30], in diabetic patients with or without CV events [37], in hemodialysis patients [18], in rheumatoid arthritis patients [33] and in HIV positive children [48], could not find a significant correlational relationship between IL-6 and intima media thickness. Nishida et al. [44] found a significant association in men but not in women in apparently healthy participants of a medical examination. Thus, there can be other factors to enforce or modify the actions of proatherogenic agents including IL-6 that should be explored and taken into account while testing the possible therapeutic role of IL-6 to control atherosclerosis.
In conclusion, inflammatory processes are importantly involved in the atherosclerosis. There exists a significant association between intima media thickness and interleukin-6 levels in patients with CVD or a disease posing risk of CVD as well as in apparently healthy populations including youth. Whether therapeutic strategies can focus on IL-6 to control atherosclerosis is subject to multi-dimensional research in future.
Disclosure of conflict of interest
None.
References
- 1.de Groot E, van Leuven SI, Duivenvoorden R, Meuwese MC, Akdim F, Bots ML, Kastelein JJ. Measurement of carotid intima-media thickness to assess progression and regression of atherosclerosis. Nat Clin Pract Cardiovasc Med. 2008;5:280–8. doi: 10.1038/ncpcardio1163. [DOI] [PubMed] [Google Scholar]
- 2.Hartman J, Frishman WH. Inflammation and Atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;22:149–151. doi: 10.1097/CRD.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 3.de Lombera Romero F, Fernandez Casares S, Gascuena Rubia R, Lazaro M, Hernandez Simon P, Saavedra Falero J, Sanchez Sanchez V, Velezquez Martín MT, Garcia Pascual J, Saenz de la Calzada C. Hypertension and dyslipidemia. Rev Esp Cardiol. 1998;51:24–35. [PubMed] [Google Scholar]
- 4.Lopez-Farre A, Farre J, Sanchez de Miguel L, Romero J, Gonzalez-Fernandez F, Casado S. Endothelial dysfunction: a global response. Rev Esp Cardiol. 1998;51:18–22. [PubMed] [Google Scholar]
- 5.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 6.Ikonomidis I, Stamatelopoulos K, Lekakis J, Vamvakou GD, Kremastinos DT. Inflammatory and non-invasive vascular markers: the multimarker approach for risk stratification in coronary artery disease. Atherosclerosis. 2008;199:3–11. doi: 10.1016/j.atherosclerosis.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Biswas P, Delfanti F, Bernasconi S, Mengozzi M, Cota M, Polentarutti N, Mantovani A, Lazzarin A, Sozzani S, Poli G. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood. 1998;91:258–265. [PubMed] [Google Scholar]
- 8.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, Bussolino F, Poli V, Ciliberto G, Mantovani A. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 9.van der Meer IM, de Maat MP, Bots ML, Breteler MM, Meijer J, Kiliaan AJ, Hofman A, Witteman JC. Inflammatory mediators and cell adhesion molecules as indicators of severity of atherosclerosis: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 2002;22:838–42. doi: 10.1161/01.atv.0000016249.96529.b8. [DOI] [PubMed] [Google Scholar]
- 10.Xiang S, Dong NG, Liu JP, Wang Y, Shi JW, Wei ZJ, Hu XJ, Gong L. Inhibitory effects of suppressor of cytokine signaling 3 on inflammatory cytokine expression and migration and proliferation of IL-6/IFN-γ-induced vascular smooth muscle cells. J Huazhong Univ Sci Technolog Med Sci. 2013;33:615–22. doi: 10.1007/s11596-013-1168-x. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 12.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur Heart J. 2007;28:354–362. doi: 10.1093/eurheartj/ehl441. [DOI] [PubMed] [Google Scholar]
- 13.Koenig W, Khuseyinova N, Baumert J, Thorand B, Loewel H, Chambless L, Meisinger C, Schneider A, Martin S, Kolb H, Herder C. Increased concentrations of C-reactive protein and IL-6 but not IL-18 are independently associated with incident coronary events in middle-aged men and women: results from the MONICA/KORA Augsburg case-cohort study, 1984-2002. Arterioscler Thromb Vasc Biol. 2006;26:2745–51. doi: 10.1161/01.ATV.0000248096.62495.73. [DOI] [PubMed] [Google Scholar]
- 14.Hartford M, Wiklund O, Mattsson Hulten L, Persson A, Karlsson T, Herlitz J, Caidahl K. C-reactive protein, interleukin-6, secretory phospholipase A2 group IIA and intercellular adhesion molecule-1 in the prediction of late outcome events after acute coronary syndromes. J Intern Med. 2007;262:526–36. doi: 10.1111/j.1365-2796.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- 15.Miwa K, Tanaka M, Okazaki S, Furukado S, Sakaguchi M, Mochizuki H, Kitagawa K. Association between interleukin-6 levels and first-ever cerebrovascular events in patients with vascular risk factors. Arterioscler Thromb Vasc Biol. 2013;33:400–5. doi: 10.1161/ATVBAHA.112.300350. [DOI] [PubMed] [Google Scholar]
- 16.National Heart, Lung and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. US Department of Health and Human Services. http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort.
- 17.Amar J, Fauvel J, Drouet L, Ruidavets JB, Perret B, Chamontin B, Boccalon H, Ferrieres J. Interleukin 6 is associated with subclinical atherosclerosis: a link with soluble intercellular adhesion molecule 1. J Hypertens. 2006;24:1083–8. doi: 10.1097/01.hjh.0000226198.44181.0c. [DOI] [PubMed] [Google Scholar]
- 18.Bevc S, Sabic S, Hojs R. Atherosclerosis in hemodialysis patients-the role of microinflammation. Ren Fail. 2008;30:1012–6. doi: 10.1080/08860220802406385. [DOI] [PubMed] [Google Scholar]
- 19.Brucker N, Charao MF, Moro AM, Ferrari P, Bubols G, Sauer E, Fracasso R, Durgante J, Thiesen FV, Duarte MM, Gioda A, Castro I, Saldiva PH, Garcia SC. Atherosclerotic process in taxi drivers occupationally exposed to air pollution and co-morbidities. Environ Res. 2014;131:31–8. doi: 10.1016/j.envres.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Cardellini M, Marini MA, Frontoni S, Hribal ML, Andreozzi F, Perticone F, Federici M, Lauro D, Sesti G. Carotid artery intima-media thickness is associated with insulin-mediated glucose disposal in nondiabetic normotensive offspring of type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2007;292:E347–52. doi: 10.1152/ajpendo.00291.2006. [DOI] [PubMed] [Google Scholar]
- 21.Chapman CM, Beilby JP, McQuillan BM, Thompson PL, Hung J. Monocyte count, but not C-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke. 2004;35:1619–24. doi: 10.1161/01.STR.0000130857.19423.ad. [DOI] [PubMed] [Google Scholar]
- 22.Ciccone MM, Scicchitano P, Zito A, Cortese F, Boninfante B, Falcone VA, Quaranta VN, Ventura VA, Zucano A, Di Serio F, Damiani MF, Resta O. Correlation between inflammatory markers of atherosclerosis and carotid intima-media thickness in Obstructive Sleep Apnea. Molecules. 2014;19:1651–62. doi: 10.3390/molecules19021651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elkind MS, Rundek T, Sciacca RR, Ramas R, Chen HJ, Boden-Albala B, Rabbani L, Sacco RL. Interleukin-2 levels are associated with carotid artery intima-media thickness. Atherosclerosis. 2005;180:181–7. doi: 10.1016/j.atherosclerosis.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Esposito K, Giugliano D, Nappo F, Marfella R Campanian Postprandial Hyperglycemia Study Group. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110:214–9. doi: 10.1161/01.CIR.0000134501.57864.66. [DOI] [PubMed] [Google Scholar]
- 25.Fagerberg B, Behre CJ, Wikstrand J, Hulten LM, Hulthe J. C-reactive protein and tumor necrosis factor-alpha in relation to insulin-mediated glucose uptake, smoking and atherosclerosis. Scand J Clin Lab Invest. 2008;68:534–41. doi: 10.1080/00365510701870898. [DOI] [PubMed] [Google Scholar]
- 26.Fang J, Zhang JP, Luo CX, Yu XM, Lv LQ. Carotid Intima-media thickness in childhood and adolescent obesity relations to abdominal obesity, high triglyceride level and insulin resistance. Int J Med Sci. 2010;7:278–83. doi: 10.7150/ijms.7.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han L, Bai X, Lin H, Sun X, Chen XM. Lack of independent relationship between age-related kidney function decline and carotid intima-media thickness in a healthy Chinese population. Nephrol Dial Transplant. 2010;25:1859–65. doi: 10.1093/ndt/gfp718. [DOI] [PubMed] [Google Scholar]
- 28.Hoshi T, Kitagawa K, Yamagami H, Furukado S, Hougaku H, Hori M. Relation between interleukin-6 level and subclinical intracranial large-artery atherosclerosis. Atherosclerosis. 2008;197:326–32. doi: 10.1016/j.atherosclerosis.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Kabłak-Ziembicka A, Przewłocki T, Stępień E, Pieniążek P, Rzeźnik D, Sliwiak D, Komar M, Tracz W, Podolec P. Relationship between carotid intima-media thickness, cytokines, atherosclerosis extent and a two-year cardiovascular risk in patients with arteriosclerosis. Kardiol Pol. 2011;69:1024–31. [PubMed] [Google Scholar]
- 30.Kapiotis S, Holzer G, Schaller G, Haumer M, Widhalm H, Weghuber D, Jilma B, Röggla G, Wolzt M, Widhalm K, Wagner OF. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol. 2006;26:2541–6. doi: 10.1161/01.ATV.0000245795.08139.70. [DOI] [PubMed] [Google Scholar]
- 31.Kato A, Odamaki M, Takita T, Maruyama Y, Kumagai H, Hishida A. Association between interleukin-6 and carotid atherosclerosis in hemodialysis patients. Kidney Int. 2002;61:1143–52. doi: 10.1046/j.1523-1755.2002.00215.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim DK, Kim HJ, Han SH, Lee JE, Moon SJ, Kim BS, Kang SW, Choi KH, Lee HY, Han DS. Chlamydia pneumoniae accompanied by inflammation is associated with the progression of atherosclerosis in CAPD patients: a prospective study for 3 years. Nephrol Dial Transplant. 2008;23:1011–8. doi: 10.1093/ndt/gfm696. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi H, Giles JT, Polak JF, Blumenthal RS, Leffell MS, Szklo M, Petri M, Gelber AC, Post W, Bathon JM. Increased prevalence of carotid artery atherosclerosis in rheumatoid arthritis is artery-specific. J Rheumatol. 2010;37:730–9. doi: 10.3899/jrheum.090670. [DOI] [PubMed] [Google Scholar]
- 34.Lee WY, Allison MA, Kim DJ, Song CH, Barrett-Connor E. Association of interleukin-6 and C-reactive protein with subclinical carotid atherosclerosis (the Rancho Bernardo Study) Am J Cardiol. 2007;99:99–102. doi: 10.1016/j.amjcard.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 35.Leonsson M, Hulthe J, Johannsson G, Wiklund O, Wikstrand J, Bengtsson BA, Oscarsson J. Increased Interleukin-6 levels in pituitary-deficient patients are independently related to their carotid intima-media thickness. Clin Endocrinol (Oxf) 2003;59:242–50. doi: 10.1046/j.1365-2265.2003.01832.x. [DOI] [PubMed] [Google Scholar]
- 36.Li GP, Li YB, Peng N, Li XX, Zhong SG, Zeng RH. The effects of atrovastatin on the levels of serum adiponectin and carotid intima media thickness in type 2 diabetes mellitus. Journal of Difficult and Complicated Cases. 2009;8:335–337. [Google Scholar]
- 37.Leinonen ES, Hiukka A, Hurt-Camejo E, Wiklund O, Sarna SS, Mattson Hultén L, Westerbacka J, Salonen RM, Salonen JT, Taskinen MR. Low-grade inflammation, endothelial activation and carotid intima-media thickness in type 2 diabetes. J Intern Med. 2004;256:119–27. doi: 10.1111/j.1365-2796.2004.01350.x. [DOI] [PubMed] [Google Scholar]
- 38.Lind L, Andersson J, Rönn M, Gustavsson T, Holdfelt P, Hulthe J, Elmgren A, Zilmer K, Zilmer M. Brachial artery intima-media thickness and echogenicity in relation to lipids and markers of oxidative stress in elderly subjects:--the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) Study. Lipids. 2008;43:133–41. doi: 10.1007/s11745-007-3125-6. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Lu F, Pan H, Zhao Y, Wang S, Sun S, Li J, Hu X, Wang L. Correlation of peripheral Th17 cells and Th17-associated cytokines to the severity of carotid artery plaque and its clinical implication. Atherosclerosis. 2012;221:232–41. doi: 10.1016/j.atherosclerosis.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 40.Martinic-Popovic I, Simundic AM, Dukic L, Lovrencic-Huzjan A, Popovic A, Seric V, Basic-Kes V, Demarin V. The association of inflammatory markers with cerebral vasoreactivity and carotid atherosclerosis in transient ischaemic attack. Clin Biochem. 2014;47:182–6. doi: 10.1016/j.clinbiochem.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, Okada S, Ohta S, Naito H, Adachi M. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:625–30. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 42.Morillas P, de Andrade H, Castillo J, Quiles J, Bertomeu-González V, Cordero A, Tarazón E, Roselló E, Portolés M, Rivera M, Bertomeu-Martínez V. Inflammation and apoptosis in hypertension. Relevance of the extent of target organ damage. Rev Esp Cardiol (Engl Ed) 2012;65:819–25. doi: 10.1016/j.recesp.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura T, Kawagoe Y, Matsuda T, Takahashi Y, Sekizuka K, Ebihara I, Koide H. Effects of LDL apheresis and vitamin E-modified membrane on carotid atherosclerosis in hemodialyzed patients with arteriosclerosis obliterans. Kidney Blood Press Res. 2003;26:185–91. doi: 10.1159/000071884. [DOI] [PubMed] [Google Scholar]
- 44.Nishida M, Moriyama T, Ishii K, Takashima S, Yoshizaki K, Sugita Y, Yamauchi-Takihara K. Effects of IL-6, adiponectin, CRP and metabolic syndrome on subclinical atherosclerosis. Clin Chim Acta. 2007;384:99–104. doi: 10.1016/j.cca.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Okazaki S, Sakaguchi M, Miwa K, Furukado S, Yamagami H, Yagita Y, Mochizuki H, Kitagawa K. Association of interleukin-6 with the progression of carotid atherosclerosis: a 9-year follow-up study. Stroke. 2014;45:2924–9. doi: 10.1161/STROKEAHA.114.005991. [DOI] [PubMed] [Google Scholar]
- 46.Porta B, Baldassarre D, Camera M, Amato M, Arquati M, Brusoni B, Fiorentini C, Montorsi P, Romano S, Tremoli E, Cortellaro M MIAMI Study Group. E-selectin and TFPI are associated with carotid intima-media thickness in stable IHD patients: the baseline findings of the MIAMI study. Nutr Metab Cardiovasc Dis. 2008;18:320–8. doi: 10.1016/j.numecd.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Ross AC, O’Riordan MA, Storer N, Dogra V, McComsey GA. Heightened inflammation is linked to carotid intima-media thickness and endothelial activation in HIV-infected children. Atherosclerosis. 2010;211:492–8. doi: 10.1016/j.atherosclerosis.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Ross AC, Rizk N, O’Riordan MA, Dogra V, El-Bejjani D, Storer N, Harrill D, Tungsiripat M, Adell J, McComsey GA. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–27. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rueda-Clausen CF, López-Jaramillo P, Luengas C, del Pilar Oubiña M, Cachofeiro V, Lahera V. Inflammation but not endothelial dysfunction is associated with the severity of coronary artery disease in dyslipidemic subjects. Mediators Inflamm. 2009;2009:469169. doi: 10.1155/2009/469169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sardo MA, Mandraffino G, Campo S, Saitta C, Bitto A, Alibrandi A, Riggio S, Imbalzano E, Saitta A. Biglycan expression in hypertensive subjects with normal or increased carotid intima-media wall thickness. Clin Chim Acta. 2009;406:89–93. doi: 10.1016/j.cca.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 51.Schiopu E, Au KM, McMahon MA, Kaplan MJ, Divekar A, Singh RR, Furst DE, Clements PJ, Ragvendra N, Zhao W, Maranian P, Khanna D. Prevalence of subclinical atherosclerosis is increased in systemic sclerosis and is associated with serum proteins: a cross-sectional, controlled study of carotid ultrasound. Rheumatology (Oxford) 2014;53:704–13. doi: 10.1093/rheumatology/ket411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scuteri A, Orru M, Morrell C, Piras MG, Taub D, Schlessinger D, Uda M, Lakatta EG. Independent and additive effects of cytokine patterns and the metabolic syndrome on arterial aging in the SardiNIA Study. Atherosclerosis. 2011;215:459–64. doi: 10.1016/j.atherosclerosis.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stenvinkel P, Heimburger O, Jogestrand T. Elevated interleukin-6 predicts progressive carotid artery atherosclerosis in dialysis patients: association with Chlamydia pneumoniae seropositivity. Am J Kidney Dis. 2002;39:274–82. doi: 10.1053/ajkd.2002.30546. [DOI] [PubMed] [Google Scholar]
- 54.Tarantino G, Costantini S, Finelli C, Capone F, Guerriero E, La Sala N, Gioia S, Castello G. Is serum Interleukin-17 associated with early atherosclerosis in obese patients? J Transl Med. 2014;12:214. doi: 10.1186/s12967-014-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aminbakhsh A, Mancini GB. Carotid intima media thickness measurements: What defines an abnormality? A systematic review. Clin Invest Med. 1999;22:149–157. [PubMed] [Google Scholar]
- 56.Lee KW, Lip GY, Tayebjee M, Foster W, Blann AD. Circulating endothelial cells, von Willebrand factor, interleukin-6, and prognosis in patients with acute coronary syndromes. Blood. 2005;105:526–32. doi: 10.1182/blood-2004-03-1106. [DOI] [PubMed] [Google Scholar]
- 57.Lowe GD, Rumley A, McMahon AD, Ford I, O’Reilly DS, Packard CJ West of Scotland Coronary Prevention Study Group. Interleukin-6, fibrin D-dimer, and coagulation factors VII and XIIa in prediction of coronary heart disease. Arterioscler Thromb Vasc Biol. 2004;24:1529–34. doi: 10.1161/01.ATV.0000135995.39488.6c. [DOI] [PubMed] [Google Scholar]
- 58.Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, Arveiler D, Fruchart JC, Ducimetiere P PRIME Study Group. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol. 2003;23:1255–61. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- 59.Hedman A, Larsson PT, Alam M, Wallen NH, Nordlander R, Samad BA. CRP, IL-6 and endothelin-1 levels in patients undergoing coronary artery bypass grafting. Do preoperative inflammatory parameters predict early graft occlusion and late cardiovascular events? Int J Cardiol. 2007;120:108–14. doi: 10.1016/j.ijcard.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 61.Stott DJ, Welsh P, Rumley A, Robertson M, Ford I, Sattar N, Westendorp RG, Jukema JW, Cobbe SM, Lowe GD. Adipocytokines and risk of stroke in older people: a nested case-control study. Int J Epidemiol. 2009;38:253–261. doi: 10.1093/ije/dyn215. [DOI] [PubMed] [Google Scholar]
- 62.Patterson CC, Smith AE, Yarnell JW, Rumley A, Ben-Shlomo Y, Lowe GD. The associations of interleukin-6 (IL-6) and downstream inflammatory markers with risk of cardiovascular disease: the Caerphilly Study. Atherosclerosis. 2010;209:551–557. doi: 10.1016/j.atherosclerosis.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 63.McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Tao H, Ridker PM, Criqui MH. Relation of interleukin-6 and vascular cellular adhesion molecule-1 levels to functional decline in patients with lower extremity peripheral arterial disease. Am J Cardiol. 2011;107:1392–8. doi: 10.1016/j.amjcard.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haddy N, Sass C, Droesch S, Zaiou M, Siest G, Ponthieux A, Lambert D, Visvikis S. IL-6, TNF-alpha and atherosclerosis risk indicators in a healthy family population: the STANISLAS cohort. Atherosclerosis. 2003;170:277–83. doi: 10.1016/s0021-9150(03)00287-9. [DOI] [PubMed] [Google Scholar]