Abstract
Tumor endothelial marker 8 (TEM8) is an endothelial-specific marker that is upregulated during tumor angiogenesis. We previously demonstrated that DNA-based vaccine encoding xenogeneic TEM8 can potentiate anti-angiogenesis immunotherapy of malignancy; nevertheless, it remains to be improved in minimizing immune tolerance. Recently, it has been reported that murine beta-defensin 2 (MBD2) is chemotactic for immature dendritic cells and plays a pivotal role in breaking immune tolerance. Herein, we constructed a genetic fusion vaccine encoding murine TEM8 and MBD2 to investigate whether the novel vaccine preferentially elicits therapeutic antitumor immune responses and suppresses cancerous angiogenesis in mouse models. The anti-angiogenesis effect was determined by microvessel density (MVD) using immunohistochemical staining. The efficacy of the fusion vaccine was primarily assessed by detecting cytotoxic T lymphocyte activity (51Cr-release assay). Enzyme-linked immunosorbent spot (ELISpot) assay was used to detect TEM8-specific INF-γ production, and the activity of CTL was further verified by a depletion of CD8+ T cells via anti-CD8 monoclonal antibody. Our results showed that the DNA fusion vaccine possessed an enhanced therapeutic antitumor immunity through anti-angiogenesis in BALB/c mice inoculated with CT26 cells, and this effect was generally attributed to stimulation of an antigen specific CD8+ T-cell response against mTEM8. In conclusion, our study demonstrated that the fusion vaccine based on mTEM8 and MBD2 induced autoimmunity against endothelial cells, resulting in deceleration of tumor growth, and could be potential therapeutical application in clinic.
Keywords: MBD2-mTEM8, fusion vaccine, anti-tumor effect, immunotherapy
Introduction
Tumor angiogenesis, a pathological process that involves the proliferation of a network of blood vessels, represents a fundamental step in tumor growth, progression and metastasis [1]. Thus, anti-angiogenesis therapy targeting pro-angiogenic proteins has become one of the major strategies for cancer treatment [2-4]. Many molecules have been ascribed as specific biomarkers of angiogenesis and one such protein is tumor endothelial marker 8 (TEM8) [5-8].
TEM8 is a recently described protein that is predominantly expressed in tumor endothelium [7,9]. In vitro studies indicate that TEM8 has a role in various endothelial cell functions, including adhesion and migration [10,11]. Elevated TEM8 expression was also detected in breast cancer tissues especially invasive ones, with an association of poor survival outcome [12]. A further study established that tumor growth was delayed in TEM8 knock-out mice challenged with B16 melanoma [13]. Additionally, TEM8 overexpression appears to be correlated with nodal involvement and disease progression and has a prognostic value of advanced colorectal cancer [14,15].
Immunological targeting of tumor endothelial cells is a proven cure for several tumor models [16-18]. The common strategies of anti-angiogenic active immunotherapy involve passive immunotherapy [19], adoptive cell transfer therapy [20] and vaccination based on cell or DNA vaccine encoding xenogeneic/non-xenogeneic homologous molecules [21-24]. It is noticed that administration of TEM8 DNA vaccine alone had no effect on tumor growth, while DNA vaccine encoding syngeneic TEM8 with tumor antigens enhanced the immunity to breast and melanoma tumors, respectively [25]. Poor presentation of TEM8 MHC I epitopes by dendritic cells pulsed with TEM8 recombinant protein may contribute to the up-mentioned phenomenon. Therefore, the discovery of new stimulators of dendritic cells for the development of the TEM8 vaccines for the immunotherapy of tumors will be of great significance.
Defensin is a class of small, cationic antimicrobial peptides which are divided into α-, β-, and θ-defensin subfamilies [26]. β-defensins are productions of the epithelial cells from skin, lung, kidney, pancreas, uterus, eye, nasal and oral mucosa. Recently, it is demonstrated that β-defensins can modulate immune responses. Murine β-defensin 2 (MBD2) is thought to activate dendritic cells via a Toll-like receptor 4 (TLR4)-dependent mechanism [27-31]. Given that dendritic cells are professional antigen presenting cells, their activation should induce a primary T-cell response. Actually, previous studies have shown that DNA immunizations with fusion constructs encoding for low immunogenic lymphoma antigen with MBD2 or with MBD2-expressing inactivated leukemia cells induces strong T cell immunity against lymphoma and acute lymphoid leukemia, respectively [32,33].
In the present study, we have described a novel strategy for achieving an antitumor angiogenesis immune response with a DNA vaccine encoding syngeneic TEM8 and murine beta-defensin 2 in the hope of overcoming the potential immune tolerance. It was observed that vaccination of mice decelerated tumor growth in a murine colon cancer cell model. And our vaccine caused the collapse of tumor vessels by evoking a T cell-mediated immune response. Thus, our study identifies the fusion DNA vaccine can halt tumor angiogenesis by hacking TEM8 with the aid of MBD2 and may act as a promising approach for further development toward clinical trials.
Material and methods
Animals and cell lines
Experiments using the animals were conducted with the approval of the Animal Care and Use Committee of Third Military Medical University (Approval ID: SCXK (Military) 2007015), according to the State Science and Technology Commission Regulations for the Administration of Affairs Concerning Experimental Animals (1988, China). Female BALB/c mice, 7~8 weeks of age, were purchased from the Experimental Animal Center of Chinese Academy of Medical Science (Beijing, China). African green monkey kidney cell COS-7 and murine colon cancer cell CT-26 were purchased from American Type Culture Collection (Manassas, VA, USA). Cell lines were maintained in Dulbecco’s Modified Eagle’s medium or RPMI 1640 medium containing 10% fetal bovine serum in a 37°C, 5% CO2 humidified incubator. Cell culture reagents were purchased from Gibco-Invitrogen (Carlsbad, CA, USA).
Construction of expression vector encoding MBD2 and murine TEM8
MBD2 was synthesized by Life Technologies (Carlsbad, CA, USA) and the MBD2 mature region (GI: 4808311) was as follows: GAACTTGACCACTGCCACACCAATGGAGGGTACTGTGTCAGAGCCATTTGTCCTCCTTCTGCCAGGCGTCCTGGGAGCTGTTTCCCAGAGAAGAACCCCTGTTGCAAGTACATGAAA. The sequences of mTEM8 (AF378762) was cloned by PCR techniques from a murine cDNA library. The following PCR primers were used: mTEM8 forward, 5’-GGCCGCCGCGAGGATGGG-3’; mTEM8 reverse, 5’-GCACAGCAAATAAGTGTCTTCCAC-3’. MBD2-mTEM8-linker-reverse, GCCAGAGCCACCTCCGCCTGAACCGCCTCCACCTTTCATGTACTTGCAACAG; MBD2-mTEM8linker-forward, GTTCAGGCGGAGGTGGCTCTGGCGGTGGCGGATCGGGCCGCCGCGAGGATGGG. MBD2 and mTEM8 were fused by overlapping PCR containing a 20-amino-acid [(G4S) ×4] linker sequence. The linked construct was inserted into the pcDNA3.1 vector (Life Technologies) between the restriction sites KpnI and EcoRI. All constructs were verified by DNA sequencing (Life Technologies).
In vitro characterization of the fusion vaccine
Plasmid DNA from the reconstructed plasmid or empty pcDNA3.1 vector was transfected into COS-7 cells using FuGENE 6 (Roche, Indianapolis, IN, USA) according to the manufacturer’s instruction. Genetic expression of plasmid DNA was detected in transfected COS-7 cells by RT-PCR with following primers: MBD2-mTEM8 forward, CATGGTCCTGCTGGAGTTCGTG; MBD2-mTEM8 reverse, CTTGAGCAATATCTGGTGACTG.
By conducting Western Blot analyse, the cell lysate was centrifuged and the supernatant was harvested to analyze protein expression with an anti-mTEM8 mouse monoclonal antibody (Santa Cruz biotechnology, Santa Cruz, CA, USA).
Immunization and tumor cell challenge
Mice were immunized by intramuscular injection of cationic nanoliposomes loaded with DNA vaccine in both later quadriceps (100 μg per mouse) once a week for four weeks. The mice were challenged subcutaneously with 5×105 tumor cells in the right flank one day before the first vaccination. Tumor volume was measured every three days with a caliper, and tumor volumes were calculated according to the formula: volume =0.5× length × width2.
A 2 months follow-up was performed with the tested mice. The data was recorded in a database.
Vessel density quantification
To explore whether the antitumor immunity involved the inhibition of angiogenesis, detection of vessel density in tumor tissue and angiogenesis in vivo was conducted. Three days after the last vaccination, the mice were sacrificed, then the tumor tissues were fixed in acetone, and stained with an anti-CD31 antibody (Santa Cruz biotechnology) as described for microvessel density analysis. The sections were then stained with labeled streptavidin biotin reagents (Dako, Carpinteria, CA, USA). Vessel density was determined by counting the number of microvessels per high-power field in the sections.
51Cr-release assay
Specific cytotoxicity mediated by cytotoxic T lymphocyte (CTL) was accessed by 51Cr-release assay described as follows: BALB/c mice were immunized with 100 μg DNA as mentioned above. Spleens were collected on day 7 after the last immunization. T lymphocytes were isolated from single-cell suspensions with MACS (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) as CTL effector cells; CT-26 colon carcinoma cells, which were transfected with pmTEM8, were used as target cells [24]. Effector and target cells were seeded into the 96-well microtiter plate at various effector/target ratios. The percentage of specific lysate was calculated as 100× (experimental release-spontaneous release)/(maximum release-spontaneous release).
CTL activity assay
The ELISpot assay was used to quantify epitope specific IFN-γ releasing effector cells. Briefly, nitrocellulose bottomed 96-well plates (Millipore, Bedford, MA, USA) were coated with an anti-IFN-γ antibody. CD8+ T cells, purified via aforementioned method, were incubated overnight with target cells (CT-26-mTEM8, 1×104/well). The plates were washed two times, and the biotinylated detection antibody was added. Specific binding was visualized using alkaline phosphatase-avidin (Dako) together with the respective substrate. The reaction was terminated on the appearance of dark purple spots, which were quantitated using the AlphaImager System (Alpha Innotech, San Leandro, CA).
In vivo depletion of CD8+ T cells
BALB/c mice were injected intraperitoneally with anti-CD8 monoclonal antibody (Santa Cruz biotechnology) at 250 μg/mouse before each immunization and then immunized with 100 μg of plasmid. Controls included non-depleted animals either immunized with pmTEM8 or pcDNA3.1.
Statistical analysis
The data collected were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA).
Kaplan-Meier surivial curve was used to detect the overall survival rate of the BALB/c mice.
The statistical significance of differential findings between experimental groups and controls were determined by one-way analysis of variance (ANOVA) and considered significant if P<0.05.
Results
Genetic and protein expression of the DNA constructs
As shown in Figure 1A, recombinant MBD2-mTEM8 plasmid, with a theoretical length of 918 bp, presented its PCR product ~1 kb which was confirmed by DNA sequencing.
Figure 1.

A. PCR products of fusion gene. Lane 1, marker; lane 2, PCR products of MBD2-mTEM. B. Expression of the fusion gene by Western Blot. The plasmids DNA were transfected into COS-7 cells with different plasmids. Lane 1, COS-7 cells transfected with pmTEM8, (MW, 71kD); lane 2, COS-7 cells transfected with pcDNA3.1; lane 3, COS-7 cells transfected with pMBD2-mTEM8 (MW, 80 kD).
Protein expression of the fusion DNA vaccine was verified by Western blotting. The proteins in the lysate of the cells transfected with pMBD2-mTEM8, pmTEM8 and pcDNA3.1 plasmids were detected using mTEM8 mAb. The bands in lane 2 and lane 3 were in concert with the theoretic molecular weights of mTEM8 protein and MBD2-mTEM8 fusion protein (Figure 1B).
pMBD2-mTEM8 vaccine elicits therapeutic antitumor effects
Tumor volumes were evaluated as predictive of a specific in vivo immune response in CT-26-bearing BALB/c mice vaccinated with pMBD2-mTEM8, a cocktail containing pmTEM8 plus pMBD2, pmTEM8, vehicle vector or PBS. Tumors grew predominantly in nonvaccinated mice, namely those with vehicle and PBS. Conversely, there was an obvious suppression of tumor growth in mice immunized with pMBD2-mTEM8, nevertheless, no significant tumor shrinkage was detected in mice immunized with pmTEM8 and the cocktail regimen (Figure 2A, 2C). Moreover, we found the death occurrence only happened after 49 days of tumor challenge in the pMBD2-mTEM8 group. Furthermore, the mean lifespan of mice in the fusion gene group was prolonged remarkably (Figure 2B, P<0.05). Taken together, these data suggested that the fusion vaccine pMBD2-mTEM8 potentiates the antitumor immunity.
Figure 2.

The examination of antitumor efficiency. BALB/c mice (n=10) were inoculated with s.c. injection of CT-26 cells (5×105), and 7 d later they received vaccination of PBS, pcDNA3.1, pmTEM8, mix, or pMBD2-mTEM8 vaccine once a week for four times. The tumor volume and the lifespan of mice were observed to assess antitumor efficiency (A); The average tumor growth volume was calculated as 0.5× length× width2. There was a significant difference in tumor volume (P<0.01) in BALB/c mice with pMBD2-mTEM8 immunized when compared with other controls (C); The mean lifespan of mice in the pMBD2-mTEM8 group was prolonged remarkably (B).
pMBD2-mTEM8 vaccine inhibits tumor angiogenesis
To explore whether immunization with pMBD2-TEM8 halts tumor angiogenesis, the microvessel density of tumor tissue was evaluated via CD31 staining. The results showed that the average number of vessels per high power field was lower in the fusion DNA vaccine group compared with control ones (Figure 3), indicating that vaccination with pMBD2-TEM8 vaccine potentially inhibits tumor angiogenesis.
Figure 3.
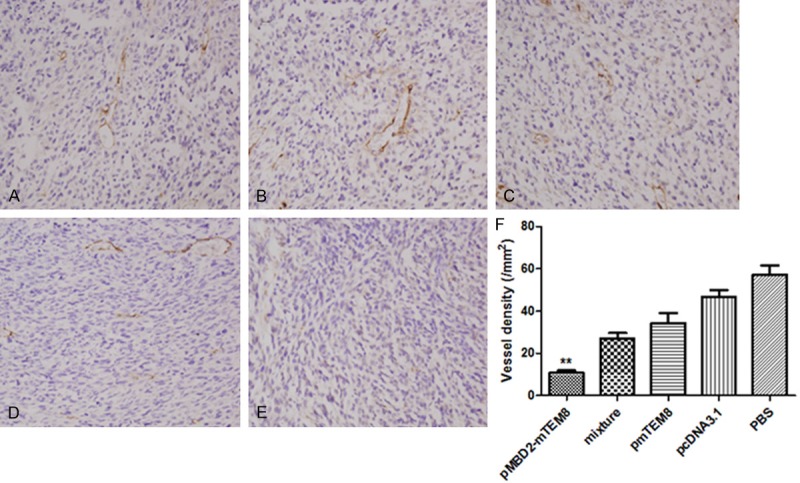
Inhibition of angiogenesis of tumors. BALB/c mice were immunized with PBS (A), pcDNA3.1 vector (B), pmTEM8 (C), mixture (D) or pMBD2-mTEM8 (E). (E) Three days after the last vaccination, the tumor tissues were fixed in acetone, and stained with an antibody reactive to CD31. (F) Vessel density of tumor tissues from pMBD2-mTEM8 immunized mice indicated a significant decrease compared with control groups (**, P<0.01).
CD8+ T lymphocytes possess a role in antitumor response of pMBD2-mTEM8 plasmid
IFN-γ ELISpot and a 51Cr-release cytolytic assay was used to evaluate the T cell responses against mTEM8 positive CT-26 target cells. As shown in Figure 4A, immunization of pMBD2-mTEM8 resulted in significantly higher CTL activity than that of the four control groups, with a ~60% of specific lysis at an effect-to-target ratio of 100:1. However, pmTEM8 alone or combined with pMBD2 could not stimulate a strong response of CTL.
Figure 4.

Decreased antitumor activity by the depletion of CD8+ T lymphocytes via corresponding mAbs in BALB/c mice. BALB/c mice were treated and immunized as described in materials and methods. Depletion of CD8+ T lymphocytes impaired the antitumor activity of the pMBD2-mTEM8 vaccine in CT26 model. The immunized group of pMBD2-mTEM8 resulted in significantly high CTL activity with a ~60% of specificlysis at an effect-to-target ratio of 100:1 (A). Similarly, the mice vaccinated with pMBD2-mTEM8 showed the highest production of IFN-γ among all the groups (B). Compared with the control groups, the antitumor effect of pMBD2-mTEM8 obviously decreased by depleting of CD8+ T cells (C) (*, P<0.05).
IFN-γ ELISpot assay has been taken as a surrogate measure of CTL activity [13]. The number of mTEM8 epitope specific IFN-γ releasing CD8+ T cells was elevated in the spleens of mice immunized with the combinatorial delivery of MBD2 and mTEM8, compared with that in controls. As expected, pMBD2-mTEM8 vaccinated mice resulted in the highest production of IFN-γ among all the groups (Figure 4B).
To further confirm whether CD8+ T lymphocytes played a role in antitumor response of pMBD2-mTEM8 vaccine, CD8 antibody was injected to mice before immunization to block the activity of CTL. It was noted that depletion of CD8+ T cells obviously abrogated antitumor effect of pMBD2-mTEM8 (Figure 4C), indicating a critical role of CTL.
Discussion
Therapeutic cancer vaccination has been extensively developed over the past few years through exploiting a wide variety of vaccine modalities. In light of clinical benefit with targeted therapies using inhibitors of angiogenesis, especially the approval of bevacizumab in 2004, it had started a hot pursuit of anti-angiogenic active immunotherapy of which effectiveness is verified in a series of experiments [16].
TEM8 is identified as a biomarker of newly-formed vasculature especially tumor vessels. It was observed that administration of TEM8 DNA alone had no effect on tumor growth [25]. This is in concordance with findings in our previous and present studies. This phenomena is probably associated with the fact that certain isoforms, such as TEM8/ATR, are also expressed in some normal somatic tissues [34], which eventually leads to failures to mount an immune response to TEM8.
To conquer immune tolerance, first of all, tumor vaccine should be optimized to attract professional antigen-presenting cells, e.g. immature dendritic cells (iDC), to the site of antigen presentation. Recently, it is reported that a small antimicrobial peptide, MBD2, may act as a chemotactic factor for iDC through chemokine receptor CCR6. MBD2 also acts directly on iDC as an endogenous ligand for Toll-like receptor 4 (TLR4), inducing upregulation of costimulatory molecules and DC maturation [28]. What’s more, a receptor-mediated process has also been uncovered for MBD2-mediated antigen cross-presentation, which induces T-cell-dependent antitumor immunity [35].
Given that MBD2 contributes to overcome immune tolerance, we thus designed a fusion DNA vaccine encoding TEM8 and MBD2 to explore whether the novel vaccine preferentially elicits therapeutic antitumor immune responses and suppresses tumor angiogenesis. In the present study, we provided the evidence that the fusion DNA vaccine inhibited tumor angiogenesis and growth, and expended lifespan of tumor-bearing mouse models. We previously discovered that the antitumor effect of pTEM8 carried by attenuated Salmonella typhimurium was abrogated in CD8-depleted mice but not in CD4-depleted mice [36]. Thus, we focus our experimental designs on detecting the activation of CD8+ T cells. Similarly, the activity of CTL and the production of IFN-γ in mice immunized with pMBD2-mTEM8 was shown to be upregulated, which was abrogated in CD8-depleted mice, indicating a TEM8-specific CD8 cytotoxic T-cell response following vaccination. However, a recent study demonstrated that both humoral and cellular immunity are engaged in the protective immune response vaccinated with fusion vaccine pMBD2-mFlk-1 [37], hence, further studies need to be conducted to analyze the involvement of CD4+ T-cell responses as well as certain cytokine profile in our fusion DNA vaccination.
Acknowledgements
This work was supported by the Natural Science Foundation of Chongqing (CSTC2007B135041).
Disclosure of conflict of interest
None.
References
- 1.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 2.Grothey A, Galanis E. Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol. 2009;6:507–518. doi: 10.1038/nrclinonc.2009.110. [DOI] [PubMed] [Google Scholar]
- 3.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 4.Marx J. Cancer. Encouraging results for second-generation antiangiogenesis drugs. Science. 2005;308:1248–1249. doi: 10.1126/science.308.5726.1248. [DOI] [PubMed] [Google Scholar]
- 5.Blagosklonny MV. Antiangiogenic therapy and tumor progression. Cancer Cell. 2004;5:13–17. doi: 10.1016/s1535-6108(03)00336-2. [DOI] [PubMed] [Google Scholar]
- 6.Hofmeister V, Vetter C, Schrama D, Brocker EB, Becker JC. Tumor stroma-associated antigens for anti-cancer immunotherapy. Cancer Immunol Immunother. 2006;55:481–494. doi: 10.1007/s00262-005-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 8.Winder T, Lenz HJ. Vascular endothelial growth factor and epidermal growth factor signaling pathways as therapeutic targets for colorectal cancer. Gastroenterology. 2010;138:2163–2176. doi: 10.1053/j.gastro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11:539–554. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotchkiss KA, Basile CM, Spring SC, Bonuccelli G, Lisanti MP, Terman BI. TEM8 expression stimulates endothelial cell adhesion and migration by regulating cell-matrix interactions on collagen. Exp Cell Res. 2005;305:133–144. doi: 10.1016/j.yexcr.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Rmali KA, Puntis MC, Jiang WG. TEM-8 and tubule formation in endothelial cells, its potential role of its vW/TM domains. Biochem Biophys Res Commun. 2005;334:231–238. doi: 10.1016/j.bbrc.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 12.Davies G, Rmali KA, Watkins G, Mansel RE, Mason MD, Jiang WG. Elevated levels of tumour endothelial marker-8 in human breast cancer and its clinical significance. Int J Oncol. 2006;29:1311–1317. [PubMed] [Google Scholar]
- 13.Cullen M, Seaman S, Chaudhary A, Yang MY, Hilton MB, Logsdon D, Haines DC, Tessarollo L, St Croix B. Host-derived tumor endothelial marker 8 promotes the growth of melanoma. Cancer Res. 2009;69:6021–6026. doi: 10.1158/0008-5472.CAN-09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rmali KA, Puntis MC, Jiang WG. Prognostic values of tumor endothelial markers in patients with colorectal cancer. World J Gastroenterol. 2005;11:1283–1286. doi: 10.3748/wjg.v11.i9.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rmali KA, Watkins G, Harrison G, Parr C, Puntis MC, Jiang WG. Tumour endothelial marker 8 (TEM-8) in human colon cancer and its association with tumour progression. Eur J Surg Oncol. 2004;30:948–953. doi: 10.1016/j.ejso.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Pan J, Jin P, Yan J, Kabelitz D. Anti-angiogenic active immunotherapy: a new approach to cancer treatment. Cancer Immunol Immunother. 2008;57:1105–1114. doi: 10.1007/s00262-008-0452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei YQ, Wang QR, Zhao X, Yang L, Tian L, Lu Y, Kang B, Lu CJ, Huang MJ, Lou YY, Xiao F, He QM, Shu JM, Xie XJ, Mao YQ, Lei S, Luo F, Zhou LQ, Liu CE, Zhou H, Jiang Y, Peng F, Yuan LP, Li Q, Wu Y, Liu JY. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nat Med. 2000;6:1160–1166. doi: 10.1038/80506. [DOI] [PubMed] [Google Scholar]
- 18.Wei YQ, Huang MJ, Yang L, Zhao X, Tian L, Lu Y, Shu JM, Lu CJ, Niu T, Kang B, Mao YQ, Liu F, Wen YJ, Lei S, Luo F, Zhou LQ, Peng F, Jiang Y, Liu JY, Zhou H, Wang QR, He QM, Xiao F, Lou YY, Xie XJ, Li Q, Wu Y, Ding ZY, Hu B, Hu M, Zhang W. Immunogene therapy of tumors with vaccine based on Xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci U S A. 2001;98:11545–11550. doi: 10.1073/pnas.191112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Mahony D, Bishop MR. Monoclonal antibody therapy. Front Biosci. 2006;11:1620–1635. doi: 10.2741/1909. [DOI] [PubMed] [Google Scholar]
- 20.Gattinoni L, Powell DJ Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bequet-Romero M, Ayala M, Acevedo BE, Rodriguez EG, Ocejo OL, Torrens I, Gavilondo JV. Prophylactic naked DNA vaccination with the human vascular endothelial growth factor induces an anti-tumor response in C57Bl/6 mice. Angiogenesis. 2007;10:23–34. doi: 10.1007/s10456-006-9062-9. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Mizutani N, Mizutani M, Luo Y, Zhou H, Kaplan C, Kim SW, Xiang R, Reisfeld RA. Endoglin (CD105) is a target for an oral DNA vaccine against breast cancer. Cancer Immunol Immunother. 2006;55:1565–1574. doi: 10.1007/s00262-006-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu JY, Wei YQ, Yang L, Zhao X, Tian L, Hou JM, Niu T, Liu F, Jiang Y, Hu B, Wu Y, Su JM, Lou YY, He QM, Wen YJ, Yang JL, Kan B, Mao YQ, Luo F, Peng F. Immunotherapy of tumors with vaccine based on quail homologous vascular endothelial growth factor receptor-2. Blood. 2003;102:1815–1823. doi: 10.1182/blood-2002-12-3772. [DOI] [PubMed] [Google Scholar]
- 24.Niethammer AG, Xiang R, Becker JC, Wodrich H, Pertl U, Karsten G, Eliceiri BP, Reisfeld RA. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med. 2002;8:1369–1375. doi: 10.1038/nm1202-794. [DOI] [PubMed] [Google Scholar]
- 25.Felicetti P, Mennecozzi M, Barucca A, Montgomery S, Orlandi F, Manova K, Houghton AN, Gregor PD, Concetti A, Venanzi FM. Tumor endothelial marker 8 enhances tumor immunity in conjunction with immunization against differentiation Ag. Cytotherapy. 2007;9:23–34. doi: 10.1080/14653240601048369. [DOI] [PubMed] [Google Scholar]
- 26.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 27.Bowdish DM, Davidson DJ, Hancock RE. Immunomodulatory properties of defensins and cathelicidins. Curr Top Microbiol Immunol. 2006;306:27–66. doi: 10.1007/3-540-29916-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, Oppenheim JJ. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 29.Navid F, Boniotto M, Walker C, Ahrens K, Proksch E, Sparwasser T, Muller W, Schwarz T, Schwarz A. Induction of regulatory T cells by a murine beta-defensin. J Immunol. 2012;188:735–743. doi: 10.4049/jimmunol.1100452. [DOI] [PubMed] [Google Scholar]
- 30.Mei HF, Jin XB, Zhu JY, Zeng AH, Wu Q, Lu XM, Li XB, Shen J. beta-defensin 2 as an adjuvant promotes anti-melanoma immune responses and inhibits the growth of implanted murine melanoma in vivo. PLoS One. 2012;7:e31328. doi: 10.1371/journal.pone.0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahrens K, Schunck M, Podda GF, Meingassner J, Stuetz A, Schroder JM, Harder J, Proksch E. Mechanical and metabolic injury to the skin barrier leads to increased expression of murine beta-defensin-1, -3, and -14. J Invest Dermatol. 2011;131:443–452. doi: 10.1038/jid.2010.289. [DOI] [PubMed] [Google Scholar]
- 32.Biragyn A, Surenhu M, Yang D, Ruffini PA, Haines BA, Klyushnenkova E, Oppenheim JJ, Kwak LW. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167:6644–6653. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- 33.Ma XT, Xu B, An LL, Dong CY, Lin YM, Shi Y, Wu KF. Vaccine with beta-defensin 2-transduced leukemic cells activates innate and adaptive immunity to elicit potent antileukemia responses. Cancer Res. 2006;66:1169–1176. doi: 10.1158/0008-5472.CAN-05-2891. [DOI] [PubMed] [Google Scholar]
- 34.Bonuccelli G, Sotgia F, Frank PG, Williams TM, de Almeida CJ, Tanowitz HB, Scherer PE, Hotchkiss KA, Terman BI, Rollman B, Alileche A, Brojatsch J, Lisanti MP. ATR/TEM8 is highly expressed in epithelial cells lining Bacillus anthracis’ three sites of entry: implications for the pathogenesis of anthrax infection. Am J Physiol-cell Ph. 2005;288:C1402–1410. doi: 10.1152/ajpcell.00582.2004. [DOI] [PubMed] [Google Scholar]
- 35.Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol. 1999;17:253–258. doi: 10.1038/6995. [DOI] [PubMed] [Google Scholar]
- 36.Ruan Z, Yang Z, Wang Y, Wang H, Chen Y, Shang X, Yang C, Guo S, Han J, Liang H, Wu Y. DNA vaccine against tumor endothelial marker 8 inhibits tumor angiogenesis and growth. J Immunother. 2009;32:486–491. doi: 10.1097/CJI.0b013e3181a1d134. [DOI] [PubMed] [Google Scholar]
- 37.Wang YS, Wang GQ, Wen YJ, Wang L, Chen XC, Chen P, Kan B, Li J, Huang C, Lu Y, Zhou Q, Xu N, Li D, Fan LY, Yi T, Wu HB, Wei YQ. Immunity against tumor angiogenesis induced by a fusion vaccine with murine beta-defensin 2 and mFlk-1. Clin Cancer Res. 2007;13:6779–6787. doi: 10.1158/1078-0432.CCR-07-1587. [DOI] [PubMed] [Google Scholar]


