Abstract
Perception, cognition, and social interaction depend upon coordinated neural activity. This coordination operates within noisy, overlapping, and distributed neural networks operating at multiple timescales. These networks are built upon a structural scaffolding with intrinsic neuroplasticity that changes with development, aging, disease, and personal experience. In this paper we begin from the perspective that successful interregional communication relies upon the transient synchronization between distinct low frequency (<80 Hz) oscillations, allowing for brief windows of communication via phase-coordinated local neuronal spiking. From this, we construct a theoretical framework for dynamic network communication, arguing that these networks reflect a balance between oscillatory coupling and local population spiking activity, and that these two levels of activity interact. We theorize that when oscillatory coupling is too strong, spike timing within the local neuronal population becomes too synchronous; when oscillatory coupling is too weak, spike timing is too disorganized. Each results in specific disruptions to neural communication. These alterations in communication dynamics may underlie cognitive changes associated with healthy development and aging, in addition to neurological and psychiatric disorders. A number of neurological and psychiatric disorders—including Parkinson’s disease, autism, depression, schizophrenia, and anxiety—are associated with abnormalities in oscillatory activity. Although aging, psychiatric and neurological disease, and experience differ in the biological changes to structural grey or white matter, neurotransmission, and gene expression, our framework suggests that any resultant cognitive and behavioral changes in normal or disordered states, or their treatment, is a product of how these physical processes affect dynamic network communication.
Keywords: neural oscillations, theta, gamma, coupling, coherence, network dynamics, depression, anxiety, Parkinson’s disease, autism, schizophrenia
Introduction
A complete model of cognitive function and dysfunction must account for a diversity of factors including, but not limited to, brain structure and genetics, rapid neuronal temporal dynamics and neuroplasticity, and developmental and sociological considerations (1; 2). Cognition is critically reliant upon the dynamic, parallel coordination of large groups of neurons separated by substantial neural distances. This coordination is surprisingly flexible but remarkably precise (3), especially considering behavior emerges in an inherently noisy electrochemical neuronal environment (4).
We propose a theory for how disruptions to dynamic network communication can lead to the neurocognitive changes observed during development and aging, as well as in neurological and psychiatric disorders. Our theory is predicated on evidence that neuronal oscillations bias the probability of spiking such that action potentials are more likely to occur during periods of interregional oscillatory coherence. We extend this by arguing that such oscillatory-mediated spike synchrony would feed back onto the local field potential (LFP), increasing LFP coherence. In the pathological case, we theorize that this mechanism results in an exaggerated state of overcoupling. In order to break cycles of LFP-induced spike synchrony causing reinforced LFP coherence causing even stronger spike synchrony, some controlling or interfering mechanism needs to be introduced. We posit that one mechanism may be neural noise, defined as temporally decorrelated spikes occurring during non-preferred LFP oscillatory phases.
This dynamic network communication framework differs from purely structural or neurochemical accounts in that, although neural architecture and neurochemistry play a critical role in cognitive functioning by providing the scaffolding upon which the dynamic communication system is built, structure and chemistry alone are insufficient for understanding neuronal dynamics (2; 5); ultimately cognition reduces to the dynamics of neural communication.
In this paper, we begin by reviewing the evidence for the causal role of oscillatory interregional communication in support of healthy cognition and present an accounting for the possible neurophysiological basis for such a communication mechanism. We then explore the consequences of alterations to network communication dynamics, including both failures to establish, and failures to break, an oscillatory communication network. We then argue that these changes might be observable in meso- or even macroscale electrophysiological recordings, reflected in specific components of the power spectral density (PSD). We conclude by presenting a theoretical framework for how these disruptions to network communication dynamics provide a unifying framework for understanding a variety of neurological and psychiatric disorders in terms of neuronal activity, using Parkinson’s disease, depression, anxiety, schizophrenia, and autism as exemplar cases. Thus, we argue that the resultant behavioral syndrome of any structural or neurochemical changes associated with development, aging, experience, and disease are manifested by their effect on dynamic network communication.
Oscillatory communication
Oscillations bias spiking activity
Neural oscillations are (usually) lower frequency (<80–100 Hz) and play a causal role in neural communication, cognition, and behavior (6; 7) (though there exists rhythmic activity at higher frequencies, such as hippocampal ripples). Research into the functional role of scalp electro-(EEG) and magneto-encephalography (MEG) oscillations shows that ongoing visual cortical oscillatory phase (8–10) and power (11; 12) predict performance on a variety of tasks (13–16). For example, people are more likely to correctly respond to target visual stimuli when those stimuli appear during the preferred phase or power of ongoing oscillatory activity, perhaps due to ongoing oscillatory phase biasing neuronal excitability (9).
In more causal direct tests on spiking activity, experiments have shown that applying relatively weak, low amplitude oscillatory electric fields bias neuronal firing. This finding, known as ephaptic coupling (6; 17), shows that subthreshold changes in extracellular electric fields affects the transmembrane voltage of nearby neurons. Experimental manipulations using exogenous oscillatory electric fields have found that low-frequency oscillatory stimulation (<2 Hz) can entrain cortical—or even hippocampal—neurons in proportion to the stimulation intensity and behavioral state of the animal (18). Similarly, oscillatory gamma (~30 Hz) hippocampal stimulation affects CA1 pyramidal spike timing, locking spikes to the phase of the stimulation oscillation (19). The authors of that report argue that a decoherent extracellular field could lead to less temporally correlated spiking, which could act as a “safety mechanism to prevent hypersynchronization.” This interaction between the coherence of the oscillatory field and spiking activity is a critical element in our framework, and we will refer to it throughout this paper.
Synchronization and neural communication
There are a variety of methods for assessing interregional neural communication depending on the measurement scale and signal source. The LFP, which is the extracellular electrical potential recorded invasively using penetrating electrodes, is influenced by a number of factors including neuronal geometry and laminar depth. Electrical activity recorded either on the cortical surface, such as with electrocorticography (ECoG), or from noninvasive scalp EEG, is dominated by postsynaptic potentials. Increases in local population firing rate are reflected by elevated broadband activity in the LFP and ECoG (20–23). This broadband shift is particularly evident in the high gamma (70–200 Hz) range and less in the low frequency range, possibly because the lower frequency portion of the PSD is masked by a simultaneous reduction in the oscillatory components of the LFP, which are less directly linked to neural firing rates and may reflect local excitatory-inhibitory circuit motifs (24) (See Fig. 1).
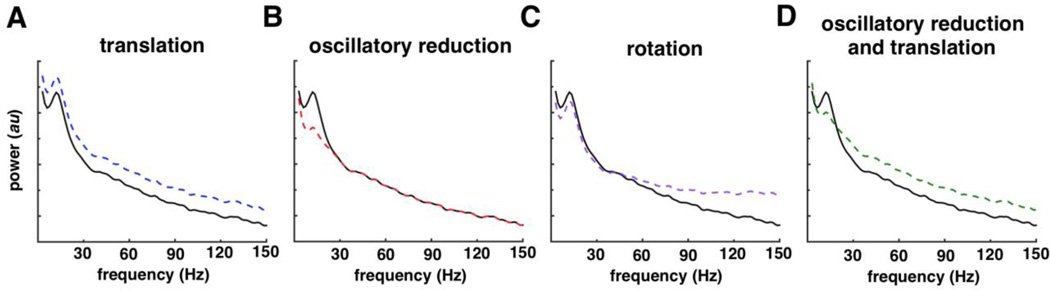
Fig. 1. Example power spectral changes.
All four plots contain an exemplar PSD consisting of a 1/f process plus an oscillation centered around 12 Hz (solid black lines). This PSD is then modified in four different ways (dashed colored lines). (A) In this example, the PSD in black has been modified by simple translation, adding power at all frequencies (blue line). (B) Here the background 1/f process remains unaffected, all that has changed is that the 12 Hz oscillation has been reduced in power (red line). (C) A single manipulation—rotation of the PSD about a pivot frequency (40 Hz)—results in a simultaneous decrease in low frequency power and an increase in high frequency power (purple line). (D) Here two separate effects—translation of the PSD and reduction of 12 Hz oscillatory power—has a similar effect as the single rotation process described in (C) in that low frequency power is reduced and high frequency power is increased. (c.f., Miller et al., 2013 (21))
Interregional communication can be inferred, at the level of the LFP, from the interregional oscillatory phase coherence between two regions (25; 26). In fMRI, the temporal profile of activity in a seed region may be correlated with the activity in other regions (27). Such methods can be generalized as a statistical assessment of how much information the activity in one region provides about the activity in a second region. These methods are used to index neural communication; interestingly, fMRI BOLD signal correlations may be driven by low frequency LFP oscillatory coupling, which in turn affect high gamma activity in the coupling regions (28).
A mechanism for neuronal communication through oscillatory coherence has been proposed (29–31). This theory, dubbed communication through coherence, is predicated on the fact that synaptic input arriving at a postsynaptic neuron is most likely to cause an action potential if the presynaptic activity arrives during the peak of the neuron’s excitability relative to an oscillatory membrane potential. In other words, the local oscillatory field biases the probability of neuronal firing, enhancing action potential precision (32) and/or computation (24). This means that the complex interrelationship between oscillatory coupling and local spiking may be necessary for coordinating brain dynamics (33; 34).
Phase/amplitude coupling
Although the communication through coherence theory is strictly referring to spike/field coupling, given that broadband high gamma likely reflects local population activity and is comodulated with the phase of oscillatory LFP, it’s plausible that this phase/amplitude coupling (PAC) may be a mesoscale analog of spike/field coupling and ephaptic coupling (when appropriate analytical considerations are accounted for (35)). We propose that PAC provides a bridge between local microscale (36; 37) and systems-level macroscale neuronal ensembles (29; 31; 38; 39) allowing for dynamic network communication (40–43). This assertion is supported by the fact that PAC tracks behavior, including learning (44; 45), working memory performance (46), attention (47), and reward processing (48; 49), across species (50) (see Johnson and Knight, 2015 (51) for a review). Recent evidence suggests that PAC might operate over short (< 500 ms) timescales, providing a mechanism for interregional coordination between the oscillatory local field and broadband gamma (52).
Low frequency oscillations may be nested within other frequency bands (38; 53–56) which themselves interact with one another. The “carrier” oscillation to which broadband high gamma activity is coupled depends on brain region and task (57; 58). Such complex multiplexing may allow for multiple parallel communication streams, providing a mechanistic basis for neural communication (7; 31; 59–62). Computational models suggest that this neural communication mechanism is susceptible to noise (63), and with relatively small changes in spiking statistics, such as the temporal autocorrelation of a single unit or synchronization across a local population, could lead to large, nonlinear effects on PAC.
Neural communication disruptions
Within the proposed dynamic network communication framework, disruptions to communication may arise in three ways: 1) failures to establish communication; 2) inaccuracies and/or noise in the communicated information; and 3) failures to terminate communication. Substantial neuroscientific research has focused on the presence of communication, using a variety of analytical methods such as functional connectivity, coupling/synchronization/coherence, mutual information, and other metrics. We hypothesize that, to communicate with fidelity, neuronal ensembles must enter a stage of oscillatory coherence, during which there is a brief window of facilitated spiking (31). Although the origin of LFP oscillations remains unclear, evidence suggests that LFP oscillations are not epiphenomena arising from neuronal synchrony, but may also reflect glial activity, which itself relates to the LFP (64). Given that the extracellular electric field alters neuronal membrane potential (ephaptic coupling), it is reasonable to assert that there is a complex interplay between spiking and LFP oscillatory coupling.
The LFP-mediated alignment of spiking probability itself feeds back onto the local field, increasing LFP coherence. In the pathological case, we argue that this mechanism results in a state of overcoupling detrimental to effective interregional communication. There is accumulating evidence that neurological and psychological disorders are associated with such pathological overcoupling (65), wherein two regions that are too strongly coupled are thought to also result in diminished information transfer. We posit that, in order to break this pathological overcoupling cycle, a controlling or interfering mechanism is needed. One such mechanism may be neural noise, defined as decreased spike/field coupling, or increased spiking during non-preferred phases of the carrier oscillation, which has been argued to be a plausible mechanism to prevent “hypersynchronization” (19). This temporal decorrelation feeds back onto the oscillatory activity, weakening LFP coherence, in turn disrupting neural synchrony, and so on. We argue that this controlling mechanism can also take a pathological form, resulting in a runaway state of undercoupling. In the non-pathological case, this push/pull relationship is a self-correcting mechanism that prevents pathological coupling on one end and temporally decorrelated spiking noise undercoupling on the other.
Measuring oscillations and spiking
Power Spectral Density
Given the importance that the distinction between oscillatory and spiking portions of the LFP plays in our conceptualization, we will outline the current understanding of the relationship between the LFP and spiking activity. First, the LFP power spectrum contains a mixture of signals, including a background, broadband 1/fα (69) process that can be described by two parameters: its slope and offset, (130) as well as narrowband peaks that rise above this background 1/f activity (70). This means that the oscillatory components must be measured not absolutely, but relative to this background 1/f activity, the offset and slope of which, we argue, contain meaningful information about the underlying neural activity.
This broadband 1/f is different from an “oscillation”(69), which are narrowband spectral processes within the PSD (70), and which may have a separate neurophysiological mechanism from spiking activity. Low frequency oscillations have high power and can be recorded at the level of scalp electroencephalography (EEG) (71). In contrast, high gamma activity has significantly lower amplitude and is more difficult to detect due to the lower relative signal-to-noise (71). The frequency of a narrowband oscillation can be different from the spike rate of individual neurons within that network (72; 73). Example PSDs are shown in Figures 1 and 2.
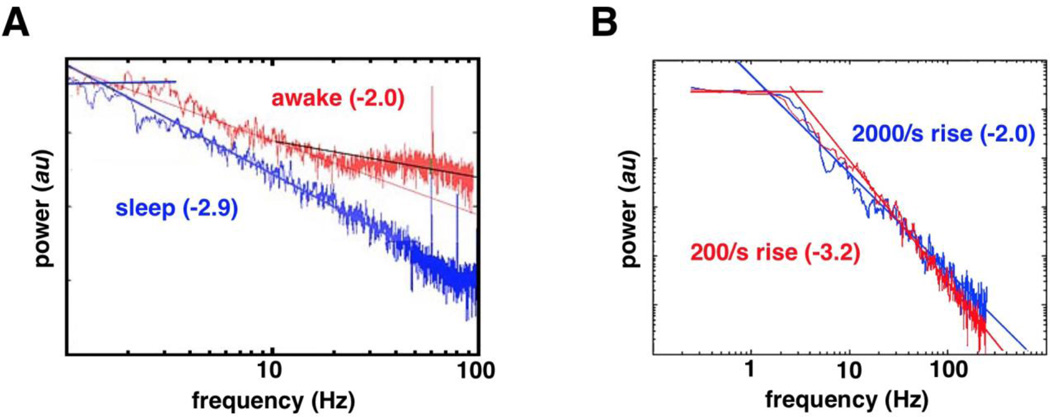
Fig. 2. Evidence for spectral slope changes.
(A) During sleep the human subdural ECoG PSD has a steep negative slope that flattens (whitens) during wakefulness. (B) Simulations of human ECoG PSD suggest that PSD slope changes arise as a function of the dendritic response to an input. As the strength of the input increases, more neurons fire simultaneously, forcing a greater local population to become refractory within a shorter time window, increasing the rise time. Thus, for weaker inputs with greater rise times the slope of the PSD is flatter (blue), whereas for stronger inputs with smaller rise times the slope of the PSD is steeper (red). Thus, within our framework, if the dendritic response is driven by ephaptic coupling, the stronger the coupling, the steeper the slope; with weaker ephaptic coupling, spike times are less temporally correlated and the slope is flatter. (From Freeman & Zhai, 2008)(68)
Inferring spiking from the power spectrum
Power in the low frequencies (< 20 Hz) is negatively correlated with both population spiking activity and fMRI BOLD signal, whereas increases in broadband high gamma activity are positively correlated with spiking and BOLD (74). Often, event-related decreases in low frequency power are often observed simultaneously with increases in broadband high gamma (21; 42; 52; 71; 75–78). While high gamma activity is difficult to detect at the level of non-invasive scalp EEG, task-related decreases in low frequency power are readily observed (71).
To give an example of how the 1/f and oscillatory components might interact, note that, with increased cortical excitation there is often a concomitant decrease in low frequency power. This suggests that there is either: 1) A “push/pull” relationship between broadband gamma and oscillatory power if these metrics are separable, or; 2) a direct inverse relationship if they reflect a core underlying phenomenon. The difference between these two theories is critical for understanding the physiology generating this phenomenon. By way of illustration, an apparent decrease in low frequency power can be caused by a drop in the amplitude of the underlying oscillation (Figure 1B, red line) or by a rotation of the entire PSD about a point (Figure 1C, purple line). In the former, the low frequency oscillatory power has dropped while the background 1/f remains the same, whereas in the latter the entire PSD shape has changed, with the rotation leading to a high gamma power increase and a low frequency power decrease. In the latter scenario, the magnitude of the narrowband oscillation remains unchanged relative to the background 1/f (21; 22; 79). This exemplifies the importance and need for measuring and modeling each of these electrophysiological processes independently: 1/f slope and offset, as well as narrowband oscillatory activity relative to the 1/f background. This is because the 1/f background will affect estimates of the oscillatory amplitude, and the oscillatory components will affect estimates of the 1/f slope and offset.
Oscillations and spiking “noise”
The fact that population spiking and the oscillatory frequency of a neuronal population can be different from the spiking frequency of individual neurons in the neural region generating the oscillation (72; 73), and that some oscillatory processes may have non-neuronal (glial) origins that affect behavior and neural communication (80; 81), suggests that spiking and oscillations are distinct. However, as noted above, oscillations and spiking interact with externally-applied electric fields to modulate network activity in humans (82), non-human primates (83), and rats (7).
While the 1/f offset likely reflects population spiking activity, there is little research on the neural origin of the spectral slope. However computational models provide evidence that the spectral slope reflects temporal correlations in the population spiking activity (84; 85) and that the slope changes with behavioral state (86). More specifically, Freeman & Zhai simulated the ECoG signal to identify plausible physiological mechanisms that lead to changes in power spectral slope and offset (68). As noted by Freeman & Zhai (68), EEG and ECoG signals are not simply a summation of action potentials, but rather the output of the dendrites that are synaptically driven by action potentials. This response can be characterized by an impulse function that has a rapid rise and prolonged return to background levels due to, they argue, “the reverberation of firing among the thousands of neurons transmitting and re-transmitting to each other,” in the local population. This is reflected by slowed rise rate and quickened decay rate. In their words, “when the strength of the driving impulse input is increased, [this] forces more neurons to fire and then become refractory.” They found that the PSD slope flattens with increasing rise rate, but is unaffected by decay rate. Thus, we argue that the flattened power spectral slope is related to higher background rates of firing decoupled from an oscillatory carrier frequency—noise—driven by greater local positive excitatory feedback possibly caused by increased excitation/inhibition ratio (neural noise as defined by, e.g., Rubenstein & Merzenich (87)).
This results shows that stronger inputs cause more neurons to fire within a shorter time window, putting a greater proportion of the population into a refractory period. This means that relatively weak inputs results in more temporal variability in the input response, resulting in a flatter spectrum (Figure 2B, blue). For stronger inputs, the PSD is steeper (Figure 2B, red). Therefore, within our framework, if the dendritic response is being modulated by ephaptic coupling, stronger ephaptic coupling would lead to a stronger dendritic response and thus a steeper PSD; the weaker the ephaptic coupling, the flatter the PSD. That is, local population spiking can be inferred from the PSD offset while spiking statistics and noise may be inferred from the PSD slope (67; 88; 89).
Neural communication pathology
Pathological overcoupling
In our theoretical framework, overcoupling, or hypersynchronization, is caused by an enhanced spike/field coupling process where the oscillatory local field causes the population spike timing to lock to the oscillation, which would feed back onto the local field. Recent research has demonstrated that patients with Parkinson’s disease, off medication, show pathologically strong motor cortical beta/high gamma PAC (90–92) (though the relationship between oscillatory beta and broadband high gamma activity through the frontal cortex, thalamus, and basal ganglia is more complex (93)). This pathological PAC is reduced when a subthalamic deep brain stimulator (DBS) is turned on, resulting in prompt motor improvement in the patient (92). DBS stimulation shuts off the subthalamic nucleus altering basal ganglia firing patterns. Here, we would argue that the loss of dopaminergic modulation of the basal ganglia-thalamocortical network causes a state of pathological network overcoupling (94). Thus, a possible mechanism of DBS is to induce enhanced high frequency neural activity of the basal ganglia-thalamocortical network to disrupt abnormal excessive coupling (95) and regularize neuronal firing (96), a phenomenon that has been called an “informational lesion”(97). We should also note that there is emerging evidence of the involvement of thalamic structures in control of cortical oscillations in both attention via the pulvinar (98) and memory via the anterior thalamic nucleus (45). These findings suggest that thalamic modulation might provide a venue for treatment of deficits outside the motor domain.
Of particular interest is promising new evidence for the use of DBS in treating psychiatric disorders such as depression, anxiety, or obsessive-compulsive disorder (99; 100). In the case of depression and anxiety, patients have a tendency to ruminate, a metacognitive processes wherein patients overly attend to negative or distressing emotions (101). From a dynamic network communication perspective, we argue that life events strengthen or weaken connections between brain regions and, via normal Hebbian learning mechanisms (102), networks that are more commonly activated are strengthened. In this framework, depression and anxiety might be conceived of as an overcoupling of the default mode network (103; 104), perhaps caused by reinforcement via experience and rumination.
From this perspective, we hypothesize that the underlying mechanism of DBS efficacy in these psychiatric diseases—which are quite different in etiology from Parkinson’s disease—is to some degree similar as to Parkinson’s: the introduction of “noisy” neural activity into the pathologically overcoupled network via DBS permits normal network communication by decoupling a pathologically overcoupled network (105). This DBS mechanism may “short-circuit” normal e.g., serotonergic modulation of network activity, which acts within the cortex to decrease functional network connectivity (106). Importantly, in Parkinson’s disease the anatomical target for DBS, the subthalamic nucleus, is clearly identifiable. In contrast, the key anatomical target for DBS in depression is the subgenual anterior cingulate cortex, which is not as well defined based on MRI alone. Thus, one could argue that pre-surgical mapping of the anterior cingulate to identify regions of pathological coupling could be used to guide DBS implantation for depression.
Pathological undercoupling
On the opposite end of the network communication disorder spectrum from pathological overcoupling is pathological undercoupling. In our framework, the precise spike timing relative to network oscillatory activity is degraded with “noisy” temporally decorrelated spikes that occur during non-facilitatory oscillatory phases. Here, one prominent example might be the “neural noise” hypothesis for age-related cognitive decline (107) wherein “noise” in this interpretation is operationalized as extraneous spikes not involved in encoding or communication. Viewed again from a dynamic network communication perspective, these noisy spikes serve to decouple the synchronization mechanism between brain regions, leading to communication and or representational errors that, in turn, result in age-related cognitive deficits.
From a neurobiological standpoint, excitatory/inhibitory balance helps control “runaway excitation”, and may lead to errors in encoding and/or interregional communication (108). Similar to the pathological overcoupling case, this hypothesis can be extended to account for the behavioral symptoms seen in psychiatric or neurological disorders. For example, both autism and schizophrenia have been shown to be associated with reduced neuronal signal-to-noise (109–111) or, more directly, increased neural noise possibly caused by disease-related alterations in the ratio of excitation to inhibition (87) or overall reduced inhibition (112). Given that the slope of the PSD may reflect the level of neural noise, we predict that schizophrenia and autism may show flattened LFP power spectra relative to controls.
A recent study showed that, in an autism spectrum disorder (ASD) group, alpha/gamma PAC was reduced compared to matched controls (113). Note that this is in contrast to Parkinson’s disease patients who show increased beta/high gamma PAC prior to DBS (90). This PAC reduction in autism supports the pathological noise hypothesis in that temporally decorrelated noise, by definition, means more spikes occurring during non-preferred phases of the low frequency oscillation. Importantly, within the dynamic network communication framework, increased neural noise (decreased PAC) should also result in decreased interregional oscillatory coherence, which is what was shown (113).
This noise-induced pathological undercoupling model may also explain some of the neurophysiological findings in relation to schizophrenia. Behaviorally, the majority of patients with schizophrenia show cognitive deficits in several domains, ranging from attention and working memory to social cognition (114). One proposal for the underlying cause for this broad array of cognitive impairments is that patients with schizophrenia suffer from a core working memory deficit (115). This has been referred to as a “rate limiting” deficit, in that a generalized inability to hold or maintain information in working memory results in a multitude of downstream cognitive effects. Within our proposed framework, such working memory deficits would result from an impairment of a prefrontal-dependent working memory network that may include sensory cortical and subcortical regions (116–118). This network dysfunction might arise from an inability to maintain oscillatory communication due to increased neural noise and deceased PAC.
Neurophysiologically, schizophrenia is associated with decreased frontal beta coherence (119; 120), decreased dopamine-mediated frontal lobe signal-to-noise (111), and broadband oscillatory decreases in signal-to-noise (121). Pharmacologically reducing (excitatory) glutamate release using mGlu2/3 receptor agonists improves schizophrenia symptoms (both positive and negative) (122), suggesting that reducing spiking activity has antipsychotic effects. Within our proposed framework, spurious, noisy spiking disrupts the ability for oscillatory communication networks to form via destructive resonance. As noted above, schizophrenia is associated with decreased frontal coherence and decreased signal-to-noise. Thus, mGlu2/3 receptor agonists, which reduce excitatory glutamate release, result in a reduction of temporally decorrelated spiking, which improves the spike/field coupling, resulting in improved oscillatory coherence and communication by improving signal-to-noise. In computational models, random noisy spiking has been shown to destabilize network dynamics, which has been proposed to underlie the behavioral symptoms of schizophrenia (123). These results align with our dynamic network communication hypothesis in that a reduction of “noisy” spiking activity would re-normalize pathological undercoupling and improve network communication and PAC.
Summary
Neuropsychiatric disorders have a long history of being viewed predominantly from a pharmacological perspective. We suggest that a electrophysiological perspective might provide a fuller understanding of the gamut of neuropsychiatric disorders and provide a path toward novel treatment interventions aimed at “normalizing” effective network communication. Recent advances in non-invasive stimulation methods, such as transcranial alternating current stimulation (tACS), random noise simulation (tRNS), and magnetic stimulation (TMS) (124; 125), as well as the introduction of “smart”, closed-loop invasive neurostimulators (126–128), may prove to be useful tools for dynamically modulating pathological network activity in disabling neuropsychiatric conditions (though their utility in this domain remains uncertain (129)).
Acknowledgments
This work was supported by the UCSD Qualcomm Institute Calit2 Strategic Research Opportunities program (BV) and a Sloan Research Fellowship (BV), as well as NINDS Grant R37 NS21135-27 (RTK) and DARPA SUBNET (RTK). The authors would like to thank Roemer van der Meij, Erik Peterson, Amitai Shenhav, Avgusta Shestyuk, and Timothy Verstynen for their comments and careful reading of earlier drafts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Wang X-J, Krystal JH. Neuron. Vol. 84. Elsevier; 2014. Computational psychiatry; pp. 638–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopell NJ, Gritton HJ, Whittington MA, Kramer MA. Beyond the Connectome: The Dynome. Neuron. 2014;83:1319–1328. doi: 10.1016/j.neuron.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sejnowski TJ, Paulsen O. J Neurosci. Vol. 26. Society for Neuroscience; 2006. Network oscillations: emerging computational principles; pp. 1673–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faisal AA, Selen LPJ, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy MM, Ching S, Whittington MA, Kopell N. Dynamical changes in neurological diseases and anesthesia. Current Opinion in Neurobiology. 2012;22:693–703. doi: 10.1016/j.conb.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anastassiou CA, Perin R, Markram H, Koch C. Ephaptic coupling of cortical neurons. Nat Neurosci. 2011;14:217–223. doi: 10.1038/nn.2727. [DOI] [PubMed] [Google Scholar]
- 7.Fröhlich F, Mccormick DA. Endogenous Electric Fields May Guide Neocortical Network Activity. Neuron. 2010;67:129–143. doi: 10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busch NA, Vanrullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proceedings of the National Academy of Sciences. 2010;107:16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. J Neurosci. Vol. 29. Society for Neuroscience; 2009. To see or not to see: prestimulus alpha phase predicts visual awareness; pp. 2725–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamagishi N, Callan DE, Anderson SJ, Kawato M. Attentional changes in pre-stimulus oscillatory activity within early visual cortex are predictive of human visual performance. Brain Research. 2008;1197:115–122. doi: 10.1016/j.brainres.2007.12.063. [DOI] [PubMed] [Google Scholar]
- 11.Van Dijk H, Schoffelen J-M, Oostenveld R, Jensen O. Prestimulus Oscillatory Activity in the Alpha Band Predicts Visual Discrimination Ability. Journal of Neuroscience. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palva S, Palva JM. New vistas for α-frequency band oscillations. Trends in Neurosciences. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Spaak E, de Lange FP, Jensen O. Local Entrainment of Alpha Oscillations by Visual Stimuli Causes Cyclic Modulation of Perception. Journal of Neuroscience. 2014;34:3536–3544. doi: 10.1523/JNEUROSCI.4385-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sokoliuk R, Vanrullen R. The Flickering Wheel Illusion: When Rhythms Make a Static Wheel Flicker. Journal of Neuroscience. 2013;33:13498–13504. doi: 10.1523/JNEUROSCI.5647-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landau AN, Fries P. Attention Samples Stimuli Rhythmically. Current Biology. 2012;22:1000–1004. doi: 10.1016/j.cub.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 17.Anastassiou CA, Koch C. Ephaptic coupling to endogenous electric field activity: why bother? Current Opinion in Neurobiology. 2014;31C:95–103. doi: 10.1016/j.conb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Ozen S, Sirota A, Belluscio MA, Anastassiou CA, Stark E, Koch C, Buzsaki G. Transcranial Electric Stimulation Entrains Cortical Neuronal Populations in Rats. Journal of Neuroscience. 2010;30:11476–11485. doi: 10.1523/JNEUROSCI.5252-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radman T, Su Y, An JH, Parra LC, Bikson M. Spike Timing Amplifies the Effect of Electric Fields on Neurons: Implications for Endogenous Field Effects. Journal of Neuroscience. 2007;27:3030–3036. doi: 10.1523/JNEUROSCI.0095-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning JR, Jacobs J, Fried I, Kahana MJ. J Neurosci. Vol. 29. Society for Neuroscience; 2009. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans; pp. 13613–13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller KJ, Honey CJ, Hermes D, Rao RP, denNijs M, Ojemann JG. Broadband changes in the cortical surface potential track activation of functionally diverse neuronal populations. NeuroImage. 2014;85:711–720. doi: 10.1016/j.neuroimage.2013.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winawer J, Kay KN, Foster BL, Rauschecker AM, Parvizi J, Wandell BA. Asynchronous Broadband Signals Are the Principal Source of the BOLD Response in Human Visual Cortex. Current Biology. 2013;23:1145–1153. doi: 10.1016/j.cub.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural Correlates of High-Gamma Oscillations (60–200 Hz) in Macaque Local Field Potentials and Their Potential Implications in Electrocorticography. Journal of Neuroscience. 2008;28:11526–11536. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Womelsdorf T, Valiante TA, Sahin NT, Miller KJ, Tiesinga P. Dynamic circuit motifs underlying rhythmic gain control, gating and integration. Nat Neurosci. 2014;17:1031–1039. doi: 10.1038/nn.3764. [DOI] [PubMed] [Google Scholar]
- 25.Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engel AK, Fries P, Singer W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 27.Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Saalmann YB, Pinsk MA, Arcaro MJ, Kastner S. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron. 2012;76:1010–1020. doi: 10.1016/j.neuron.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 30.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 31.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in Cognitive Sciences. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Schaefer AT, Angelo K, Spors H, Margrie TW. Plos Biol. Vol. 4. Public Library of Science; 2006. Neuronal Oscillations Enhance Stimulus Discrimination by Ensuring Action Potential Precision; p. e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tognoli E, Kelso JAS. The Metastable Brain. Neuron. 2014;81:35–48. doi: 10.1016/j.neuron.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. Science. Vol. 313. American Association for the Advancement of Science; 2006. High gamma power is phase-locked to theta oscillations in human neocortex; pp. 1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aru J, Aru J, Priesemann V, Wibral M, Lana L, Pipa G, et al. Untangling cross-frequency coupling in neuroscience. Current Opinion in Neurobiology. 2015;31:51–61. doi: 10.1016/j.conb.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 37.Quilichini P, Sirota A, Buzsáki G. J Neurosci. Vol. 30. Society for Neuroscience; 2010. Intrinsic circuit organization and theta-gamma oscillation dynamics in the entorhinal cortex of the rat; pp. 11128–11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends in Cognitive Sciences. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szczepanski SM, Crone NE, Kuperman RA, Auguste KI. Dynamic changes in phase-amplitude coupling facilitate spatial attention control in fronto-parietal cortex. Plos Biol. 2014 doi: 10.1371/journal.pbio.1001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K. Proceedings of the National Academy of Sciences. Vol. 101. National Acad Sciences; 2004. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep; pp. 5053–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Science. Vol. 320. American Association for the Advancement of Science; 2008. Entrainment of neuronal oscillations as a mechanism of attentional selection; pp. 110–113. [DOI] [PubMed] [Google Scholar]
- 42.Miller KJ, Hermes D, Honey CJ, Hebb AO. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS computational. 2012 doi: 10.1371/journal.pcbi.1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller KJ, Hermes D, Honey CJ, Sharma M, Rao RPN, Nijs den M, et al. Front Hum Neurosci. Vol. 4. Frontiers; 2010. Dynamic Modulation of Local Population Activity by Rhythm Phase in Human Occipital Cortex During a Visual Search Task. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tort ABL, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Proc Natl Acad Sci USA. Vol. 106. National Acad Sciences; 2009. Theta-gamma coupling increases during the learning of item-context associations; pp. 20942–20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sweeney-Reed CM, Zaehle T, Voges J, Schmitt FC, Buentjen L, Kopitzki K, et al. Corticothalamic phase synchrony and cross-frequency coupling predict human memory formation. eLife. 2014;4 doi: 10.7554/eLife.05352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. Proc Natl Acad Sci USA. Vol. 107. National Acad Sciences; 2010. Cross-frequency coupling supports multi-item working memory in the human hippocampus; pp. 3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szczepanski SM, Crone NE, Kuperman RA, Auguste KI, Parvizi J, Knight RT. Dynamic Changes in Phase-Amplitude Coupling Facilitate Spatial Attention Control in Fronto-Parietal Cortex. In: Posner M, editor. Plos Biol. Vol. 12. 2014. pp. e1001936–e1001914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Good Vibrations: Cross-frequency Coupling in the Human Nucleus Accumbens during Reward Processing. J Cogn Neurosci. 2009;21:875–889. doi: 10.1162/jocn.2009.21062. [DOI] [PubMed] [Google Scholar]
- 49.Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. J Neurosci. Vol. 29. Society for Neuroscience; 2009. Nuclei accumbens phase synchrony predicts decision-making reversals following negative feedback; pp. 7591–7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Canolty RT, Ganguly K, Kennerley SW, Cadieu CF, Koepsell K, Wallis JD, Carmena JM. Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies. Proceedings of the National Academy of Sciences. 2010;107:17356–17361. doi: 10.1073/pnas.1008306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson EL, Knight RT. Intracranial recordings and human memory. Current Opinion in Neurobiology. 2015;31:18–25. doi: 10.1016/j.conb.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voytek B, D'Esposito M, Crone N, Knight RT. A method for event-related phase/amplitude coupling. NeuroImage. 2013;64:416–424. doi: 10.1016/j.neuroimage.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phase correlation among rhythms present at different frequencies: spectral methods, application to microelectrode recordings from visual cortex and functional implications. Phase correlation among rhythms present at different frequencies: spectral methods, application to microelectrode recordings from visual cortex and functional implications. 1997;26:171–189. doi: 10.1016/s0167-8760(97)00763-0. [DOI] [PubMed] [Google Scholar]
- 54.Tort ABL, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proceedings of the National Academy of Sciences. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roopun AK, Kramer MA, Carracedo LM, Kaiser M, Davies CH, Traub RD, et al. Period concatenation underlies interactions between gamma and beta rhythms in neocortex. Front Cell Neurosci. 2008;2:1. doi: 10.3389/neuro.03.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roopun AK, Kramer MA, Carracedo LM, Kaiser M, Davies CH, Traub RD, et al. Temporal Interactions between Cortical Rhythms. Front Neurosci. 2008;2:145–154. doi: 10.3389/neuro.01.034.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voytek B, Canolty RT, Shestyuk A, Crone N, Parvizi J, Knight RT. Front Hum Neurosci. Vol. 4. Frontiers; 2010. Shifts in Gamma Phase-Amplitude Coupling Frequency from Theta to Alpha Over Posterior Cortex During Visual Tasks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foster BL, Parvizi J. Resting oscillations and cross-frequency coupling in the human posteromedial cortex. NeuroImage. 2012:1–8. doi: 10.1016/j.neuroimage.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nat Neurosci. 2013;16:349–356. doi: 10.1038/nn.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panzeri S, Brunel N, Logothetis NK, Kayser C. Sensory neural codes using multiplexed temporal scales. Trends in Neurosciences. 2010;33:111–120. doi: 10.1016/j.tins.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Koepsell K, Wang X, Hirsch JA, Sommer FT. Exploring the function of neural oscillations in early sensory systems. Front Neurosci. 2010;4:53. doi: 10.3389/neuro.01.010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujisawa S, Buzsáki G. A 4 Hz Oscillation Adaptively Synchronizes Prefrontal VTA, Hippocampal Activities. Neuron. 2011;72:153–165. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deco G, Jirsa V, McIntosh AR, Sporns O, Kotter R. Key role of coupling, delay, and noise in resting brain fluctuations. Proceedings of the National Academy of Sciences. 2009;106:10302–10307. doi: 10.1073/pnas.0901831106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mishima T, Hirase H. J Neurosci. Vol. 30. Society for Neuroscience; 2010. In vivo intracellular recording suggests that gray matter astrocytes in mature cerebral cortex and hippocampus are electrophysiologically homogeneous; pp. 3093–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Pritchard WS. The brain in fractal time: 1/f-like power spectrum scaling of the human electroencephalogram. International Journal of Neuroscience. 1992;66:119–129. doi: 10.3109/00207459208999796. [DOI] [PubMed] [Google Scholar]
- 67.Bédard C, Kröger H, Destexhe A. Does the 1/f Frequency Scaling of Brain Signals Reflect Self-Organized Critical States? Phys Rev Lett. 2006;97:118102. doi: 10.1103/PhysRevLett.97.118102. [DOI] [PubMed] [Google Scholar]
- 68.Freeman WJ, Zhai J. Simulated power spectral density (PSD) of background electrocorticogram (ECoG) Cogn Neurodyn. 2008;3:97–103. doi: 10.1007/s11571-008-9064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He BJ. Scale-free brain activity: past, present, and future. Trends in Cognitive Sciences. 2014;18:480–487. doi: 10.1016/j.tics.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopes da Silva F. EEG and MEG: Relevance to Neuroscience. Neuron. 2013;80:1112–1128. doi: 10.1016/j.neuron.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 71.Voytek B, Secundo L, Bidet-Caulet A, Scabini D, Stiver SI, Gean AD, et al. Hemicraniectomy: A New Model for Human Electrophysiology with High Spatio-temporal Resolution. J Cogn Neurosci. (2nd ed.) 2010;22:2491–2502. doi: 10.1162/jocn.2009.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whittington MA, Cunningham MO, LeBeau FEN, Racca C, Traub RD. Multiple origins of the cortical gamma rhythm. In: McBain CJ, Fishell G, editors. Devel Neurobio. Vol. 71. 2010. pp. 92–106. [DOI] [PubMed] [Google Scholar]
- 73.Brunel N, Wang X-J. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. Journal of Neurophysiology. 2003;90:415–430. doi: 10.1152/jn.01095.2002. [DOI] [PubMed] [Google Scholar]
- 74.Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Science. Vol. 309. American Association for the Advancement of Science; 2005. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex; pp. 951–954. [DOI] [PubMed] [Google Scholar]
- 75.Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998;121(Pt 12):2271–2299. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- 76.Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121(Pt 12):2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- 77.Edwards E. High Gamma Activity in Response to Deviant Auditory Stimuli Recorded Directly From Human Cortex. Journal of Neurophysiology. 2005;94:4269–4280. doi: 10.1152/jn.00324.2005. [DOI] [PubMed] [Google Scholar]
- 78.Jacobs J, Kahana MJ. J Neurosci. Vol. 29. Society for Neuroscience; 2009. Neural representations of individual stimuli in humans revealed by gamma-band electrocorticographic activity; pp. 10203–10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hermes D, Miller KJ, Wandell BA, Winawer J. Stimulus Dependence of Gamma Oscillations in Human Visual Cortex. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee HS, Ghetti A, Pinto-Duarte A, Wang X, Dziewczapolski G, Galimi F, et al. Astrocytes contribute to gamma oscillations and recognition memory. Proceedings of the National Academy of Sciences. 2014;111:E3343–E3352. doi: 10.1073/pnas.1410893111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuron. Vol. 43. Elsevier; 2004. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors; pp. 729–743. [DOI] [PubMed] [Google Scholar]
- 82.Ali MM, Sellers KK, Frohlich F. Transcranial Alternating Current Stimulation Modulates Large-Scale Cortical Network Activity by Network Resonance. Journal of Neuroscience. 2013;33:11262–11275. doi: 10.1523/JNEUROSCI.5867-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haegens S, Nácher V, Luna R, Romo R, Jensen O. Proc Natl Acad Sci USA. Vol. 108. National Acad Sciences; 2011. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking; pp. 19377–19382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Usher M, Stemmler M, Olami Z. Dynamic Pattern Formation Leads to 1f Noise in Neural Populations. Phys Rev Lett. 1995;74:326–329. doi: 10.1103/PhysRevLett.74.326. [DOI] [PubMed] [Google Scholar]
- 85.Pozzorini C, Naud R, Mensi S, Gerstner W. Temporal whitening by power-law adaptation in neocortical neurons. Nat Neurosci. 2013;16:942–948. doi: 10.1038/nn.3431. [DOI] [PubMed] [Google Scholar]
- 86.Podvalny E, Noy N, Harel M, Bickel S, Chechik G, Schroeder CE, et al. A unifying principle underlying the extracellular field potential spectral responses in the human cortex. Journal of Neurophysiology. 2015 doi: 10.1152/jn.00943.2014. jn.00943.2014–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanggi P, Jung P. Advances in chemical physics. Vol. 89. New York: Wiley [etc.]; 1995. Colored noise in dynamical systems; pp. 1958-239–1958-326. [Google Scholar]
- 89.Sosnoff JJ, Newell KM. Aging and Motor Variability: A Test of the Neural Noise Hypothesis. Experimental Aging Research. 2011;37:377–397. doi: 10.1080/0361073X.2011.590754. [DOI] [PubMed] [Google Scholar]
- 90.de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, et al. Proc Natl Acad Sci USA. Vol. 110. National Acad Sciences; 2013. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease; pp. 4780–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang AI, Vanegas N, Lungu C, Zaghloul KA. Beta-Coupled High-Frequency Activity and Beta-Locked Neuronal Spiking in the Subthalamic Nucleus of Parkinson's Disease. Journal of Neuroscience. 2014;34:12816–12827. doi: 10.1523/JNEUROSCI.1895-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Hemptinne C, Swann N, Ostrem JL, Ryapolova-Webb ES, Luciano M, Galifianakis NB, Starr PA. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci. 2015:1–10. doi: 10.1038/nn.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cannon J, McCarthy MM, Lee S, Lee J, Börgers C, Whittington MA, Kopell N. Neurosystems: brain rhythms and cognitive processing. Eur J Neurosci. 2013;39:705–719. doi: 10.1111/ejn.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P. Dopamine depletion increases the power and coherence of β-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci. 2005;21:1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- 95.McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clinical Neurophysiology. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 96.McConnell GC, So RQ, Hilliard JD, Lopomo P, Grill WM. Effective Deep Brain Stimulation Suppresses Low-Frequency Network Oscillations in the Basal Ganglia by Regularizing Neural Firing Patterns. Journal of Neuroscience. 2012;32:15657–15668. doi: 10.1523/JNEUROSCI.2824-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15:1137–1140. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- 98.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The Pulvinar Regulates Information Transmission Between Cortical Areas Based on Attention Demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holtzheimer PE, Mayberg HS. Deep Brain Stimulation for Psychiatric Disorders. Annu Rev Neurosci. 2011;34:289–307. doi: 10.1146/annurev-neuro-061010-113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 101.Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends in Neurosciences. 2011;34:1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.The Organizaiton of Behavior. The Organizaiton of Behavior. New York: John Wiley & Sons; 1949. [Google Scholar]
- 103.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Social Cognitive and Affective Neuroscience. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bar M. A cognitive neuroscience hypothesis of mood and depression. Trends in Cognitive Sciences. 2009;13:456–463. doi: 10.1016/j.tics.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brittain J-S, Sharott A, Brown P. The highs and lows of beta activity in cortico-basal ganglia loops. Eur J Neurosci. 2014;39:1951–1959. doi: 10.1111/ejn.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schaefer A, Burmann I, Regenthal R, Arélin K, Barth C, Pampel A, et al. Serotonergic Modulation of Intrinsic Functional Connectivity. Current Biology. 2014;24:2314–2318. doi: 10.1016/j.cub.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 107.Cremer R, Zeef EJ. What Kind of Noise Increases With Age? J Gerontol. 1987;42:515–518. doi: 10.1093/geronj/42.5.515. [DOI] [PubMed] [Google Scholar]
- 108.Haider B, Mccormick DA. Rapid Neocortical Dynamics: Cellular and Network Mechanisms. Neuron. 2009;62:171–189. doi: 10.1016/j.neuron.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, Behrmann M. Unreliable Evoked Responses in Autism. Neuron. 2012;75:981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weinger PM, Zemon V, Soorya L, Gordon J. Low-contrast response deficits and increased neural noise in children with autism spectrum disorder. Neuropsychologia. 2014;63:10–18. doi: 10.1016/j.neuropsychologia.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 111.Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends in Neurosciences. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 112.Vogels TP, Abbott LF. Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nat Neurosci. 2009;12:483–491. doi: 10.1038/nn.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khan S, Gramfort A, Shetty NR. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders Presented at the Proceedings of the …. 2013 doi: 10.1073/pnas.1214533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Keefe RSE, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012:11–37. doi: 10.1007/978-3-642-25758-2_2. [DOI] [PubMed] [Google Scholar]
- 115.Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. American Journal of Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- 116.Barcelo F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- 117.Szczepanski SM, Knight RT. Neuron. Elsevier Inc.; 2014. Insights into Human Behavior from Lesions to the Prefrontal Cortex; pp. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Voytek B, Knight RT. Proc Natl Acad Sci USA. Vol. 107. National Acad Sciences; 2010. Prefrontal cortex and basal ganglia contributions to visual working memory; pp. 18167–18172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Uhlhaas PJ, Linden DEJ, Singer W, Haenschel C, Lindner M, Maurer K, Rodriguez E. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010 doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 121.Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, Weinberger DR. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. American Journal of Psychiatry. 2004;161:490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- 122.Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 123.Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9:696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- 124.Paulus W. Transcranial electrical stimulation (tES – tDCS; tRNS, tACS) methods. Neuropsychological rehabilitation. 2011;21:602–617. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- 125.Antal A, Paulus W, Nitsche MA. Electrical stimulation and visual network plasticity. Restor Neurol Neurosci. 2011;29:365–374. doi: 10.3233/RNN-2011-0609. [DOI] [PubMed] [Google Scholar]
- 126.Sun FT, Morrell MJ. Closed-loop Neurostimulation: The Clinical Experience. Neurotherapeutics. 2014;11:553–563. doi: 10.1007/s13311-014-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Azodi-Avval R, Gharabaghi A. Front Comput Neurosci. Vol. 9. Frontiers; 2015. Phase-dependent modulation as a novel approach for therapeutic brain stimulation; p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chang EF. Neuron. Vol. 86. Elsevier Inc.; 2015. Towards Large-Scale, Human-Based, Mesoscopic Neurotechnologies; pp. 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Horvath JC, Forte JD, Carter O. Neuropsychologia. Elsevier; 2014. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects A systematic review; pp. 1–24. [DOI] [PubMed] [Google Scholar]
- 130.He BJ, Zempel JM, Snyder AZ, Raichle ME. The Temporal Structures and Functional Significance of Scale-free Brain Activity. Neuron. 2010;66:353–369. doi: 10.1016/j.neuron.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]