Abstract
Objective
To conduct a randomized test comparing two multicomponent, contingency management interventions, one with and one without a full parent training curriculum, and an individual treatment for adolescent cannabis use disorders.
Method
153 adolescents who met DSM-IV criteria for cannabis abuse or dependence were randomized to motivational enhancement therapy/cognitive-behavioral therapy (MET/CBT), MET/CBT+abstinence-based contingency management (CM), or MET/CBT+CM+Parent Training (PT).
Results
Overall, during treatment, abstinence was greater for youth receiving clinic- and home-based CM without PT compared to those who received individual MET/CBT. There was no additional benefit of the full parent training curriculum on marijuana use, youth externalizing problems, or parenting.
Conclusion
These results suggest that clinic- plus home-based CM for cannabis use disorders can increase rates of abstinence during treatment over and above an evidence-based treatment (individual MET/CBT), but the addition of a comprehensive parenting training curriculum did not further enhance efficacy.
Keywords: Adolescent, cannabis, contingency management, parent training
INTRODUCTION
Cannabis is the most frequently misused illicit substance among youth and has substantial associated consequences. Cannabis use among teens far exceeds that of any other illicit substance1. Teens appear more vulnerable to the development of cannabis use disorders (CUD) than adults as indicated by more rapid development of CUD from time of initiation2. Teens who use cannabis regularly are at high risk for: poor academic performance; school dropout; delinquent behavior; arrest; other psychiatric problems; emergency room visits; other substance use disorders; drugged driving; and unprotected sex3. The impact of regular cannabis use on brain structure and function may also be cause for concern4.
More effective interventions and strategies are sorely needed for adolescents with cannabis and other substance use disorders. In the US, 76% of all youth admissions to substance use treatment report cannabis as the primary substance5. Overall, adolescents in treatment for substance abuse have better outcomes than those not in treatment, and well-specified types of stand-alone, individual, group, family, and integrated approaches demonstrate efficacy6. Comparing relative efficacy of treatment models is difficult given use of diverse outcomes across clinical trials; however, specific family-based approaches and packages combining more than one evidence-based approach appear most effective. That said, even with these interventions, treatment effects have much room for improvement. For example, in the multisite Cannabis Youth Treatment Study7 that compared 5 evidence-based interventions, less than 25% of adolescents were abstinent or in recovery at the end of treatment and across 12 months of follow-up, with little difference observed between treatments.
One candidate for enhancing outcomes is abstinence-based contingency management (CM). CM interventions attempt to modify the substance user’s environment such that (a) drug abstinence is carefully monitored, and (b) reinforcing events (e.g., tangible rewards) occur when abstinence is achieved. Robust data support CM for adult multiple types of substance use treatment outcomes among diverse populations with a mean effect size of d=.428. For cannabis, studies have shown that adding CM to behavioral therapy enhances abstinence outcomes (e.g.,9,10). Unfortunately, there are only a few studies testing CM for adolescent substance use. Several studies have shown positive effects of CM on adolescent tobacco use11,12. CM also increased abstinence rates relative to usual drug court services13 and relative to usual services after discharge from residential treatment14. Two outpatient clinical trials for cannabis and other substance use problems (one of which was a feasibility study) did not show clear evidence of CM effects on abstinence15,16.
We developed an outpatient CM intervention model to enhance motivation to engage in treatment and engender cannabis and other drug abstinence that utilizes a clinic-based abstinence reinforcement program17 and home-based CM that taught parents to use rewards and consequences contingent on substance testing results. In addition, adolescents received individual therapy (motivational enhancement therapy/cognitive-behavioral therapy: MET/CBT) training,18,19 and parents received a comprehensive parent training (PT) curriculum20. Results of an initial trial of this multicomponent intervention17 showed that MET/CBT+CM+PT enhanced abstinence outcomes relative to MET/CBT, engendering more weeks of continuous cannabis abstinence during treatment (d=.48, medium effect). However, the independent effects of CM vs. PT were not assessed.
The current study sought to replicate and extend these results in a more diverse population, comparing three treatment conditions: MET/CBT, MET/CBT+CM, and MET/CBT+CM+PT. We hypothesized that both CM conditions would engender more cannabis abstinence and less frequent cannabis use during treatment compared to MET/CBT. In addition, we hypothesized that PT would lead to greater abstinence, less frequent use, and greater improvements in parenting and youth externalizing than MET/CBT and MET/CBT+CM during the follow-up period.
METHOD
Participants
The study was conducted in compliance with the institutional review board of the University of Arkansas for Medical Sciences. Families were referred to our clinic located within an academic medical center by schools, the juvenile justice system, community therapists, or physicians, or were self-referred. All treatment services were funded by a National Institutes of Health (NIH) grant. Assessments were completed by research staff. Inclusion criteria were: 1) age 12–18 years (if 18, in high school); 2) reported use of cannabis during the prior 30 days or a cannabis-positive urine test; 3) met criteria for cannabis abuse or dependence; and 4) living with a parent/guardian who agreed to participate. Dependence on other substances or evidence of cognitive difficulty that would preclude participation in MET/CBT were exclusion criteria.
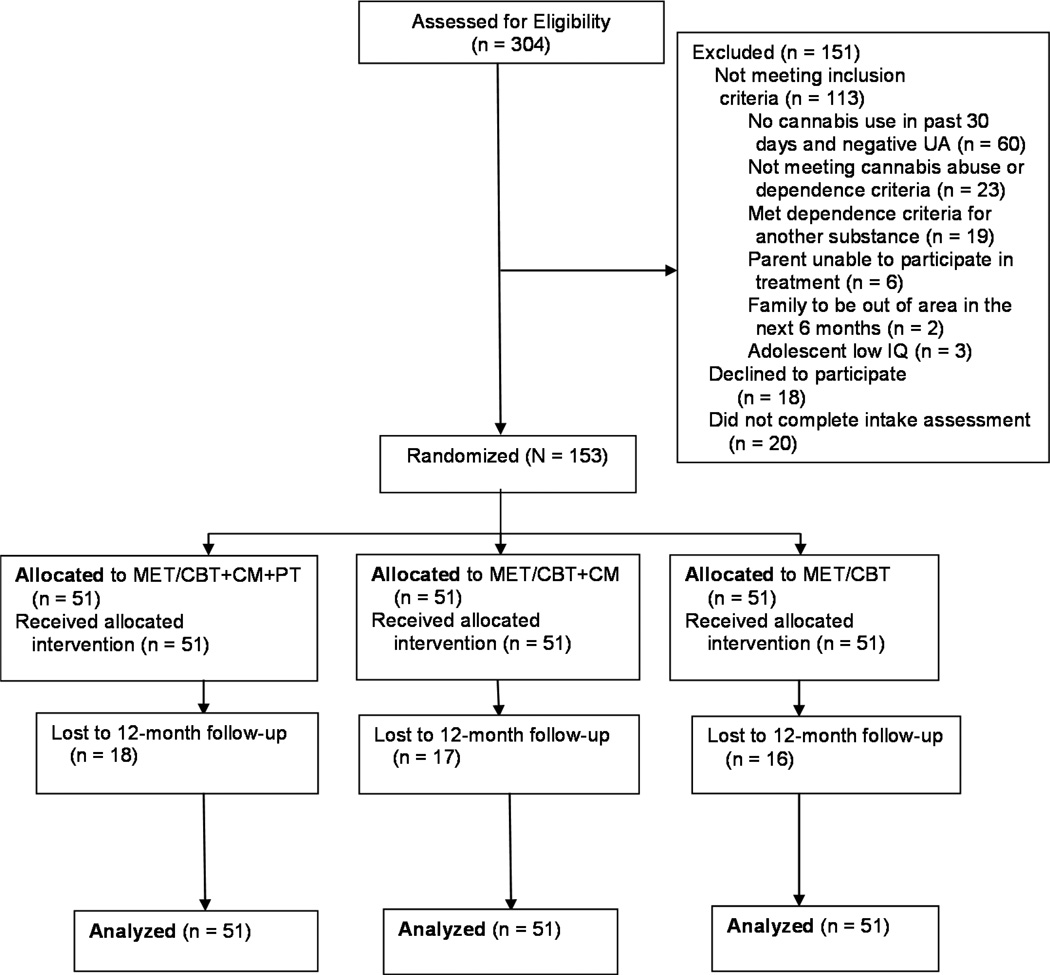
Of 304 youth assessed, 113 did not meet inclusion/exclusion criteria; 38 declined treatment or did not complete the intake; and 153 (136 male, 17 female) enrolled in the trial (Figure 1). Informed consent was obtained from the parent(s); assent was obtained from the adolescent (or consent if the participant was 18). Youth were enrolled between December 2007 and March 2011, and follow-up assessments were completed by July 2012.
Figure 1.
Consort diagram. Note: CBT = cognitive-behavioral therapy; CM = contingency management; MET = motivational enhancement therapy; PT = parent training; UA = urinalysis.
Minimum likelihood allocation21 was used to randomly assign participants sequentially to one of the three conditions while balancing across conditions on six characteristics that may influence outcome: abstinence prior to treatment (cannabis-negative urine test); gender; ≤10th grade level; conduct problems (T-score ≥64 on the externalizing subscale of the Child Behavior Checklist [CBCL]); ethnicity; and therapist.
Measures
Substance use
The Vermont Structured Diagnostic Interview (VSDI22) was administered to assess DSM-IV substance use disorders. The interview has good psychometric properties22. Frequency of cannabis, alcohol, and other substance use was assessed using the Time-Line Follow Back (TLFB23). Percentage of days used during each 3-month period prior to the TLFB was calculated as the number of reported days of use divided by the number of days for which data were provided. Incomplete data (fewer than 85% of possible days during or posttreatment) were coded as missing. Percentage of days used was highly skewed, and thus a four-level categorical variable was created: 0=0% days used; 1=greater than 0 and less than 15% of days used; 2=15% or more and less than 50% of days used; and 3=50% or more days used.
Urine toxicologies and alcohol breath tests were performed twice weekly in the clinic during the 14- week treatment period and at each follow-up assessment. Toxicology procedures are described in detail in a manual available online24. Observed urine specimens were tested via an onsite immunoassay semiquantitative drug testing (MCG 240 Benchtop Analyzer: Thermo Scientific, Fremont, CA) for cannabis, cocaine, opioids, benzodiazepines, amphetamines, and methamphetamines, and qualitative results (50ng/ml cutoff for cannabinoids) were provided to teens, parents, and therapists during the clinic visit. Invalid specimens (e.g., creatinine below 30 mg/dl) resulted in a request to provide a replacement specimen within 24 hours. Failure to submit a scheduled specimen during the treatment period was treated as a positive result. Longest period of continuous abstinence (LCA) during treatment was derived from the twice-weekly toxicology results. Failure to submit a specimen posttreatment was considered a missing observation in analyses.
Adolescent Externalizing
Parents completed the CBCL.25 T-scores for the CBCL’s Externalizing scale comprised of scores on the Aggressive Behavior and Rule-Breaking Behavior syndromes were used in analyses.
Parenting
The Alabama Parenting Questionnaire (APQ26,27) was completed by parents, and scores were obtained on three scales: Positive Involvement, Ineffective Discipline, and Deficient Monitoring. Mean item scores were used in analyses.
Follow-Up Assessments
At the end of treatment, and 3, 6, 9, and 12 months posttreatment, the adolescent and parent(s) participated in a comprehensive assessment. Research assistants not blinded to condition collected a urine specimen and a TLFB at each assessment; at the end of treatment, 6- and 12-month follow-ups, the CBCL and APQ were also administered. Table S1 (available online) shows the percentage of adolescents abstinent based on negative cannabis urine test results at each posttreatment assessment. Tables S2 and S3, available online, show mean TLFB, parenting, and externalizing scores at each assessment.
Intervention Conditions
Youth in all conditions received twice-weekly drug testing, and 40-minute, weekly individual therapy sessions for 14 consecutive weeks that covered the MET/CBT12 treatment18,19. All parents were informed of drug toxicology results.
MET/CBT
Youth in this condition received MET/CBT12 and $5 incentives for attending each clinic visit and providing a valid urine specimen ($5 × 28 visits=$140 maximum). Parents attended only the first session and were subsequently contacted twice weekly to collect their reports of youth substance use and to provide them with drug test results. They were not instructed on how to respond to the test results.
MET/CBT+CM
In addition to MET/CBT, families in this condition received a CM program that included incentives provided by staff for abstinence and a home-based substance use CM component delivered by parents17 (see manual for details24). The CM program provided an escalating schedule of earnings for documented abstinence at each twice-weekly clinic visit during weeks 3–14 (weeks 1–2 were considered a washout period because some cannabis users are likely to test positive for tetrahydrocannabinol [THC] for about 14 days after their last use). Documented abstinence was defined as a negative urinalysis for all substances, plus negative parent and adolescent reports of use. Adolescents consistently abstinent could earn incentives worth $590. Earnings were redeemed for gift cards selected by the teen and purchased by staff. In addition, to further reinforce early abstinence, a fishbowl28 component was added during weeks 1–4; this also allowed reinforcement of abstinence for those who tested negative during the washout period. The fishbowl contained tickets for good job (no prize; n=250); small ($1 prizes; n=209), medium ($20 gift cards; n=40), or large prizes. The number of pulls earned started at 1 and increased by 1 with each consecutive drug negative urine test, with a 5 pull bonus for each week of abstinence (two consecutive tests). Abstinence throughout weeks 1–4 would earn 56 pulls (approximately $135).
Home-based CM involved teaching parents to develop and use a substance monitoring contract (SMC) that specified positive and negative consequences to be implemented twice weekly in response to abstinence or substance use24. Each parent therapy session included attention to adherence with the SMC, evaluation of its impact, and modifications to the contract as necessary. Parents were provided with .02 saliva breath tests to use at home to document alcohol abstinence. To encourage parent participation, a fishbowl was used with the same winning slips as for the teen program. Parents earned pulls for session attendance, mid-week urine test attendance, and implementing the SMC and administering .02 breath tests. Complete adherence earned 111 pulls or approximately $27017,24.
MET/CBT+CM+PT
In addition to the above components, parents met with the therapist (without the adolescent) for an additional 30–40 minutes each week for behavioral parent training (PT) guided by Adolescent Transitions20, an evidence-based parent training program targeting conduct problems generally. Content involved developing contracts to target behaviors other than abstinence, identifying house rules and consequences for their violation, and instruction on parental monitoring, communication, and problem solving.
Continuing Care Components
At the end of the 14 weeks, therapists in all conditions recommended that families attend the clinic for an additional 12 weeks of once-weekly urine testing to facilitate ongoing parental monitoring, consistent with the rationale for use of this procedure during treatment. Youth in the MET/CBT condition earned 1 fishbowl pull for attending and providing a urine specimen, increasing by 1 pull up to 5 pulls for each consecutive sample provided, for a maximum of 50 pulls or approximately $120. In both MET/CBT+CM conditions, youth earned 5 pulls for abstinence, increasing by 1 for each consecutive week of abstinence up to 10, for a maximum of 105 pulls or approximately $255. Parents in both CM conditions were offered six sessions during this 12-week period. Sessions focused on reviewing the SMC, and parents who received PT also reviewed parenting strategies. No incentives were provided to parents.
Therapists, Treatment Integrity, and Fidelity
Three female master’s level clinicians served as therapists. To maintain treatment integrity: (a) therapists followed detailed manuals for each treatment and used a checklist during each session that detailed that session’s curriculum components; (b) treatment sessions were videotaped; and (c) active cases were discussed weekly in group supervision using a checklist to review each component.
Therapist adherence to the Family Management Curriculum was assessed using the Fidelity of Implementation Rating System (FIMP29). The FIMP provides a rating of adherence on a 9-point scale. Doctoral-level raters rated two randomly selected sessions for each MET/CBT+CM+PT family (84% of families had ≥1 rated session). Approximately 50% of sessions were rated by two doctoral-level raters with ≥80% agreement. Mean fidelity scores for overall quality were 3.78 (SD=.89), indicating scores in the upper end of the “needs work” range to the lower end of the “acceptable” range. These ratings are comparable to published reports30.
Therapist adherence to motivational interviewing (MI) and cognitive-behavioral therapy (CBT) was assessed using the Yale Adherence Competence Scale (YACS)31. The YACS provides a rating of frequency and extensiveness as well as competence on a 7-point scale. Raters were one undergraduate and two bachelor-level staff who were trained to ≥80% agreement with doctoral level staff. Two randomly selected sessions (one MI and one CBT) were rated for 50% of randomly selected participants. Approximately 33% of sessions were rated by two raters with ≥80% agreement. The mean rating for the frequency and extensiveness was 3.91 (SD = .39), and for the skill level of the therapist was 5.16 (SD=.80). Ratings were comparable or slightly higher than other published reports32.
Statistical methods
The primary outcome, cannabis abstinence during treatment, was compared between treatment conditions using zero-inflated Poisson models to accommodate the abundance of zeroes. In the LCA model, the odds of experiencing at least one cannabis-negative sample is compared between treatment conditions, followed by an estimation of the ratio of expected number of negative samples across conditions among participants who experienced at least one negative sample. Posttreatment abstinence (cannabis-negative specimens) was modeled using logistic generalized estimating equations (GEE). The model included treatment condition, linear and quadratic effects of time, as well as interactions between treatment and each time term. Cannabis use frequency (from the TLFB) was modeled using a piece-wise linear GEE in which a linear trajectory of use was modeled between intake and end of treatment, and a separate time trajectory was modeled starting at end of treatment and across all follow-up assessments. Each piece included a time by treatment interaction term. Youth externalizing and parenting were modeled using similar piece-wise models. All analyses were performed with SAS version 9.3.
RESULTS
Sample Characteristics
Table 1 shows demographic and substance use comparisons across the 3 conditions at intake. The conditions differed only on mean days of alcohol use in the past 30 days. Additional information about DSM mental health and other substance use diagnoses are presented in Table S4, available online. Overall, the sample was mostly male (89%), with a mean age of 15.8 (SD=1.3; range=12–18) and a large percentage of African American youth (62%).
Table 1.
Sample Characteristics for Each Treatment Condition
| MET/CBT+CM+PT (n = 51) |
MET/CBT+CM (n = 51) |
MET/CBT (n = 51) |
X2 or F | |
|---|---|---|---|---|
| Gender, n (%) | .96a | |||
| Male | 44 (86.3) | 45 (88.2) | 47 (92.2) | |
| Race, n(%) | .72a | |||
| Caucasian | 18 (35.3) | 18 (35.3) | 18 (35.3) | |
| African American | 31 (60.8) | 32 (62.7) | 32 (62.7) | |
| Other b | 2 (4.0) | 1 (2.0) | 1 (2.0) | |
| Ethnicity, n(%) | 1.23a | |||
| Hispanic | 1 (2.0) | 0 (.0) | 1 (2.0) | |
| SES,c mean (SD) | 5.0 (2.6) | 5.3 (2.3) | 4.8 (2.2) | .55 |
| Age, mean (SD) | 15.8 (1.4) | 15.9 (1.4) | 15.7 (1.2) | .40 |
| Two-parent participation, n(%) | 18 (35.3) | 15 (29.4) | 21 (41.2) | 1.54 |
| Female primary parent, n(%) | 42 (82.4) | 48 (94.1) | 42 (82.4) | 3.97 |
| Biological or adoptive primary parent, n(%) | 44 (86.3) | 46 (90.2) | 42 (82.4) | 1.32 |
| Tobacco user, n(%) | 29 (56.9) | 26 (51.0) | 31 (60.8) | 1.01 |
| Intake cannabis positive, n(%) | 34 (66.7) | 33 (64.7) | 37 (72.5) | .78 |
| Days used cannabis in past 30, mean (SD) | 10.0 (9.5) | 10.0 (9.3) | 11.9 (9.8) | .69 |
| Days used alcohol in past 30, mean (SD) | .35 (.8) | .22 (.5) | .75 (1.4) | 4.19 * |
| Drinks per drinking day, mean (SD) | 1.3 (3.6) | .37 (1.2) | 1.0 (1.7) | 1.98 |
| DSM Substance Use,d n(%) | ||||
| Cannabis dependence | 13 (25.5) | 16 (31.4) | 16 (31.4) | .57 |
| Cannabis abuse | 38 (74.5) | 35 (68.6) | 35 (68.6) | .57 |
| Alcohol abuse | 4 (7.8) | 1 (2.0) | 4 (7.8) | 2.23a |
Note: CBT=cognitive-behavioral therapy; CM = contingency management; MET=motivational enhancement therapy; PT=parent training; SES = socioeconomic status; THC = Δ-9-Tetrahydocannabinol.
”Value” represents Fisher’s exact test due to one or more cells with < 5 cases.
”Other” represents youth who identified as American Indian/Alaska Native or more than one race.
Hollingshead (1975) 9-step occupation scale.
Based on youth interview.
Endorsed by primary parent
p < .05 (MET/CBT > MET/CBT+CM and MET/CBT+CM+PT).
Retention, Participation, and Incentive Earnings
Participation was similar across conditions, as measured by the mean number of sessions attended (F[2,150]=1.03, p=0.36; M[SD]=9.0 [4.8], 10.2 [4.8], and 10.1 [4.2] for MET/CBT, MET/CBT+CM, and MET/CBT+CM+PT, respectively). Participation rates for follow-ups were also similar at end of treatment, 3, 6, 9, and 12 months, respectively: MET/CBT: 80%, 63%, 71%, 59% and 69%; MET/CBT+CM: 78%, 73%, 71%, 71% and 71%; and MET/CBT+CM+PT: 84%, 71%, 69%, 67% and 71%. There were no significant baseline differences across conditions on the variables in Table 1 for those with and without data at 12 months. Mean voucher earnings were: MET/CBT: $88.92 (SD=$46.81); MET/CBT+CM: $170.55 (SD=$167.26); MET/CBT+CM+PT: $139.19 (SD=$155.06). Mean week 1–4 fishbowl earnings in the CM conditions were: MET/CBT+CM: $48.86 (SD=$58.35); MET/CBT+CM+PT: $39.93 (SD=$53.00). Mean parent fishbowl earnings were: MET/CBT+CM: $134.30 (SD=$6.87); MET/CBT+CM+PT: $150.96 (SD=$6.26).
Attendance at the recommended urine tests during the maintenance period was generally poor, with mean attendance of: MET/CBT: 2.0 (SD=3.55); MET/CBT+CM: 2.9 (SD=4.1); MET/CBT+CM+PT: 3.5 (SD=4.0) samples. Mean attendance at the maintenance parent sessions was also low: MET/CBT+CM: 0.29 (SD=0.76); MET/CBT+CM+PT: 0.59 (SD=1.31) sessions.
Abstinence During Treatment
Results from the zero-inflated Poisson models of longest period of continuous cannabis abstinence (LCA) show that the likelihood of ≥1 negative tests during treatment (61%, 76%, and 76% of teens in MET/CBT, MET/CBT+CM, and MET/CBT+CM+PT, respectively) did not significantly differ between the three conditions (X2[2]=3.98, not significant). Among those with ≥1 negative tests, treatment condition was significantly associated with LCA (X2[2]=9.11, p<0.05). In pair-wise comparisons, MET/CBT+CM had significantly greater LCA than MET/CBT (mean[SD] 15.2[9.6] vs. 13.1[11.6]; OR=1.16, 95% CI=1.02,1.32, p<.05) and greater than MET/CBT+CM+PT (12.9[9.5]; OR=0.85, 95% CI=0.75,0.95, p<.01). MET/CBT, MET/CBT+CM, and MET/CBT+CM+PT, respectively, differed in the proportion of participants who achieved ≥2 weeks of abstinence (35% vs. 65% vs. 59%, X2[2]=9.92, p=0.007), and ≥4 weeks (31% vs. 53% vs. 43%, X2[2]=6.81, p=0.03), but not ≥6 weeks (29% vs. 47% vs. 33%, X2[2]=3.77, p=0.15).
Posttreatment Abstinence
Posttreatment abstinence was modeled via logistic GEE (Table 2, Figure S1, available online). At the end of treatment, there was a significant difference between treatment conditions (treatment condition main effect). In pair-wise comparisons, only MET/CBT+CM had a higher likelihood of a negative test at the end of treatment compared to MET/CBT. Abstinence rates decreased between end of treatment and the three-month follow-up, with similar abstinence rates in the three conditions at all follow-up assessments. There were marginally significant linear and quadratic time-by-treatment interactions (p≤0.08). Pairwise comparisons indicate that both the linear and quadratic time effects significantly differed between MET/CBT+CM and MET/CBT, reflecting the higher abstinence rate in MET/CBT+CM at end of treatment followed by similar abstinence rates for all conditions at subsequent follow-up assessments.
Table 2.
Posttreatment Cannabis Abstinence
| Marijuana Abstinence | ||
|---|---|---|
| Parameter Estimate (β) |
SE | |
| Intercept | −0.25 | 0.31 |
| Time | −0.16 | 0.11 |
| Time2 | 0.0030* | 0.0087 |
| Treatment Conditiona | X2(2)=7.51* | |
| MET/CBT+CM vs MET/CBT | 1.25** | 0.46 |
| MET/CBT+CM+PT vs MET/CBT | 0.76 | 0.45 |
| MET/CBT+CM+PT vs. MET/CBT+CM | −0.49 | 0.46 |
| Time × Treatment Condition a | X2(2)=5.78, ns | |
| Time × Time × MET/CBT+CM vs. Time × MET/CBT | −0.39* | 0.16 |
| Time × Time × MET/CBT+CM+PT vs. Time × MET/CBT | −0.22 | 0.16 |
| Time × MET/CBT+CM+PT vs. Time × MET/CBT+CM | 0.18 | 0.16 |
| Time2*Treatment Condition a | X2(2)=4.87, ns | |
| Time2*MET/CBT+CM vs. Time2*MET/CBT | 0.027* | 0.013 |
| Time2*MET/CBT+CM+PT vs. Time2*MET/CBT | 0.020 | 0.013 |
| Time2*MET/CBT+CM+PT vs. Time2*MET/CBT+CM | −0.007 | 0.01 |
Note: CBT = cognitive-behavioral therapy; CM = contingency management; MET = motivational enhancement therapy; NS = not significant; PT = parent training.
Generalized estimating equations (GEE) Chi-square score statistic for overall (type III) test of treatment effect zindicated in this row.
p≤0.05,
p≤0.01,
p≤0.001
Cannabis Use Frequency
The piece-wise model (during vs. posttreatment) results for self-report of cannabis use frequency (TLFB) are shown in Table 3 (and Figure S2, available online). There was a significant decrease in use during treatment for MET/CBT (time main effect) that was similar in MET/CBT+CM and MET/CBT+CM+PT (non-significant time × treatment condition interaction). Posttreatment, use in MET/CBT did not increase significantly (time main effect) and was similar in MET/CBT+CM and MET/CBT+CM+PT (non-significant time by treatment condition interaction).
Table 3.
Cannabis Use Frequency
| Marijuana Use Frequency | ||
|---|---|---|
| Parameter Estimate (β) |
SE | |
| During Treatment | ||
| Intercept | 2.12*** | 0.11 |
| Time | −0.22*** | 0.042 |
| Treatment Condition a | X2(2)=1.34, ns | |
| MET/CBT+CM vs. MET/CBT | −0.18 | 0.15 |
| MET/CBT+CM+PT vs. MET/CBT | −0.084 | 0.16 |
| MET/CBT+CM+PT vs. MET/CBT+CM | 0.095 | 0.16 |
| Time × Treatment Condition a | X2(2)=0.04, ns | |
| Time × MET/CBT+CM vs. Time × MET/CBT | −0.012 | 0.062 |
| Time × MET/CBT+CM+PT vs. Time × MET/CBT | −0.0079 | 0.059 |
| Time × MET/CBT+CM+PT vs. Time × MET/CBT+CM | 0.0045 | 0.063 |
| Posttreatment | ||
| Intercept | 1.33*** | 0.13 |
| Time | 0.024 | 0.013 |
| Treatment Condition a | X2(2)=1.22, ns | |
| MET/CBT+CM vs. MET/CBT | −0.22 | 0.20 |
| MET/CBT+CM+PT vs. MET/CBT | −0.11 | 0.20 |
| MET/CBT+CM+PT vs. MET/CBT+CM | 0.11 | 0.20 |
| Time × Treatment Conditiona | X2(2)=2.73, ns | |
| Time × MET/CBT+CM vs. MET/CBT | 0.031 | 0.021 |
| Time × MET/CBT+CM+PT vs. MET/CBT | −0.0021 | 0.020 |
| Time × MET/CBT+CM+PT vs. Time × MET/CBT+CM | −0.033 | 0.021 |
Note: CBT = cognitive-behavioral therapy; CM = contingency management; MET = motivational enhancement therapy; PT = parent training.
Generalized estimating equations (GEE) Chi-square score statistic for overall (type III) test of treatment effect indicated in this row.
p≤0.05,
p≤0.01,
p≤0.001
Parenting and Externalizing Psychopathology
Overall, there was significant improvement during treatment on all parenting scales (main effect of time in Table 4). During treatment, there was a significant treatment condition by time interaction only for positive involvement. In pair-wise comparisons, MET/CBT+CM+PT showed significantly less improvement than MET/CBT and MET/CBT+CM. However, positive involvement scores did not differ across conditions at the end of treatment (represented by the main effect of treatment condition in the posttreatment part of the model). There were no significant posttreatment changes on any parenting scale indicating similar maintenance of improvements across treatment conditions.
Table 4.
Parenting and Externalizing
| Positive Involvement | Deficient Monitoring | Negative Discipline | Externalizing | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter Estimate (β) |
SE | Parameter Estimate (β) |
SE | Parameter Estimate (β) |
SE | Parameter Estimate (β) |
SE | ||
| During Treatment | |||||||||
| Intercept | 3.7*** | 0.8 | 3.2*** | 0.1 | 1.4*** | 0.05 | 63.3*** | 1.5 | |
| Time | 0.04* | 0.02 | −0.09** | 0.03 | −0.03** | 0.01 | −1.5** | 0.5 | |
| Treatment Conditiona | F(2,148)=0.33 | F(2,148)=1.22 | F(2,148)=1.40 | F(2,138)=0.83 | |||||
| MET/CBT+CM | 0.03 | 0.1 | −0.2 | 0.2 | −0.1 | 0.07 | −1.4 | 2.2 | |
| vs. MET/CBT | |||||||||
| MET/CBT+CM+PT | 0.09 | 0.1 | 0.03 | 0.1 | −0.03 | 0.07 | 1.4 | 2.2 | |
| vs. MET/CBT | |||||||||
| MET/CBT+CM+PT | 0.06 | 0.1 | 0.2 | 0.1 | 0.08 | 0.07 | 2.8 | 2.2 | |
| vs. MET/CBT+CM | |||||||||
| Time × Treatment Conditiona | F(2,148)=3.89* | F(2,148)=0.22 | F(2,148)−0.09 | F(2,138)=0.48 | |||||
| Time × MET/CBT+CM | −0.007 | 0.03 | 0.008 | 0.04 | 0.003 | 0.02 | −0.1 | 0.7 | |
| vs. Time × MET/CBT | |||||||||
| Time × MET/CBT+CM+PT vs. | −0.07* | 0.03 | 0.03 | 0.04 | 0.007 | 0.02 | 0.5 | 0.7 | |
| Time × MET/CBT | |||||||||
| Time × MET/CBT+CM+PT | −0.07* | 0.03 | 0.02 | 0.04 | 0.006 | 0.02 | 0.6 | 0.7 | |
| vs. Time × MET/CBT+CM | |||||||||
| Posttreatment | |||||||||
| Intercept | 3.8*** | 0.08 | 2.9*** | 0.1 | 1.3*** | 0.05 | 58.0*** | 1.7 | |
| Time | −0.01 | 0.01 | 0.001 | 0.01 | −0.004 | 0.005 | −0.2 | 0.2 | |
| Treatment Conditiona | F(2,148)=1.03 | F(2,148)=1.47 | F(2,148)=1.39 | F(2,138)=2.21 | |||||
| MET/CBT+CM | 0.004 | 0.1 | −0.2 | 0.2 | −0.1 | 0.07 | −1.8 | 2.4 | |
| vs. MET/CBT | |||||||||
| MET/CBT+CM+PT | −0.2 | 0.1 | 0.1 | 0.2 | −0.01 | 0.07 | 3.2 | 2.4 | |
| vs. MET/CBT | |||||||||
| MET/CBT+CM+PT | −0.2 | 0.1 | 0.3 | 0.2 | 0.1 | 0.08 | 5.0* | 2.4 | |
| vs. MET/CBT+CM | |||||||||
| Time × Treatment Conditiona | F(2,148)=1.01 | F(2,148)=1.59 | F(2,148)=0.89 | F(2,138)=0.95 | |||||
| Time × MET/CBT+CM | 0.01 | 0.01 | 0.03 | 0.01 | 0.004 | 0.006 | −0.2 | 0.3 | |
| vs. Time × MET/CBT | |||||||||
| Time × MET/CBT+CM+PT vs. | 0.01 | 0.01 | 0.01 | 0.02 | −0.004 | 0.006 | −0.3 | 0.3 | |
| Time × MET/CBT | |||||||||
| Time × MET/CBT+CM+PT | −0.001 | 0.02 | 0.3 | 0.2 | −0.009 | 0.006 | −0.2 | 0.2 | |
| vs. Time × MET/CBT+CM | |||||||||
Note: CBT = cognitive-behavioral therapy; CM = contingency management; MET=motivational enhancement therapy; PT = parent training.
An F-statistic for overall (type III) test of treatment effect indicated in this row.
p≤0.05,
p≤0.01,
p≤0.001
Teen externalizing scores also improved overall during treatment (Table 4), with no significant treatment condition by time interaction. However, at the end of treatment, scores were significantly different across treatment conditions (significant main effect of treatment in the posttreatment model). Pair-wise comparisons indicated that externalizing scores were significantly higher for MET/CBT+CM+PT than for MET/CBT+CM. Posttreatment, there were no significant changes over time in the MET/CBT condition and no significant time by treatment interactions.
DISCUSSION
Overall, youth receiving clinic- and home-based CM with or without parent management training were more likely to achieve 4 weeks of continuous cannabis abstinence during treatment than were those who received individual MET/CBT plus regular drug testing with results shared with parents. About half (48%) of those receiving CM (with or without PT) achieved at least 4 weeks of continuous abstinence vs. 30% receiving MET/CBT only. Teens receiving clinic- and home-based CM without PT were also more likely to be abstinent at the end of treatment than those receiving individual MET/CBT. Similar during-treatment differences favoring CM were observed in our smaller prior randomized two-condition trial17; however, abstinence rates overall were lower in this lower socioeconomic status (SES), higher proportion, minority sample.
These results are in contrast with two recent studies that did not demonstrate the efficacy of an individual CM intervention in achieving greater abstinence during treatment with similar types of substance use disorders15,16. Those CM interventions used significantly lower magnitude incentives (<1/2 the value used in this study), and neither included home-based CM. Of note, the Henggeler at al.13 study that demonstrated efficacy for adolescent CM also used lower incentives (maximum $150) but involved parents implementing a home-based CM contract, suggesting that parent-delivered CM may be an important active component.
In some instances, results favored MET/CBT+CM over both MET/CBT and MET/CBT+CM+PT. We did not observe any additional benefit of the full PT curriculum on marijuana use, youth externalizing problems, or parenting. The failure of PT to improve outcomes may be due to several factors. First, it is possible that less than ideal therapist adherence and implementation skills limited outcomes. Second, the inclusion of some youth with low levels of conduct problems in the treatment sample may have limited the impact of PT. Third, the specific model used to implement PT in this study may not have been adequate or optimal. Fourth, it is important to note that the home-based CM delivered to those who did not receive PT is an evidence-based parenting intervention that focuses specifically on substance use. Therefore, the two CM conditions are most appropriately conceptualized as differing in the dose of PT, not the presence vs. absence of PT, and on the breadth of PT, including an exclusive focus on substance use vs. a broader focus on conduct problems and family communication, including substance use. Thus, these results do not suggest that parenting interventions fail to improve outcomes relative to individual interventions. Indeed, as noted above, teaching parents to implement CM at home may be an important active ingredient of CM for adolescents. However, the findings do suggest that the addition of a broad-spectrum parenting intervention, as delivered in this study, focusing on conduct problems did not boost outcomes over and above contingency contracting focusing on substance use more specifically. These results do not preclude the potential efficacy of PT with different samples or alternative models of implementation.
Despite significant improvements in reduction in marijuana use during treatment, maintenance of gains posttreatment was poor across conditions. There was a significant posttreatment decrease in cannabis abstinence and increase in self-reported cannabis use frequency, with similar rates of abstinence and use frequency across all three conditions in the follow-up period. This relapse occurred despite the availability of free continued substance testing, incentives for adolescents, and booster sessions for parents for three months after the end of counseling. Attendance at those visits was poor, suggesting that interventions targeted at helping parents maintain the use of home-based CM such as continuing parent incentives post-counseling may help decrease relapse. Additional targets of CM to motivate enduring change might also be considered, such as engagement in specific types of prosocial activities14.
There are some additional limitations that warrant mention. There was lower than ideal participation in the follow-up assessments, which negatively impacted statistical power. There was also a low percentage of females. In addition, assessments were conducted by staff not blind to treatment status.
Overall, these results suggest that the combination of clinic- and home-based CM for adolescent cannabis use disorders can increase rates of abstinence during treatment over and above an evidence-based treatment (individual MET/CBT). Additional comprehensive PT targeting conduct problems more broadly did not improve outcomes. Future studies should isolate the independent effects of twice-weekly testing with results provided to parents (a procedure not commonly used), clinic-based CM, and home-based CM. Despite these encouraging results, there remained a substantial percentage of teens who did not achieve abstinence, and the improvement in abstinence was not sustained after the end of the intervention. Thus, there remains a continued need to develop more effective interventions, particularly those that target maintenance of treatment gains.
Supplementary Material
Clinical Guidance.
-
*
The combination of clinic- and home-based contingency management for adolescent cannabis use disorders can increase rates of abstinence during treatment when combined with individual MET/CBT.
-
*
Home-based contingency management involves parents working with a therapist and their teen to identify specific rewards for abstinence and consequences for substance use based on the results of weekly drug testing at the clinic, teen self-reports, and observation of any substance use or intoxication at home. Parents meet weekly with the therapist to develop, implement, and modify this contract as necessary based on the teen's treatment response (abstinence or continued substance use).
-
*
Supplemental comprehensive parent training targeting conduct problems more broadly did not significantly improve outcomes over and above a parenting intervention focused specifically on substance use.
-
*
Posttreatment relapse remains a concern, and keeping families engaged in ongoing drug testing and monitoring proved difficult.
Acknowledgments
This research was funded by a National Institutes of Health (NIH) grant (DA015186).
Dr. Scherer served as the statistical expert for this research.
The authors would like to thank the families who participated in this project and our University of Arkansas for Medical Sciences staff including Eliza Bobyrshev, BA, RN, Zuzana Gubrij, MA, and Jonathan Young, MA, for their hard work and dedication.
Disclosure: Dr. Stanger has received research support from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Drug Abuse (NIDA), and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Dr. Ryan has received research support from NIDA. Dr. Scherer has received research support from NIDA, NIAAA, the National Center for Advancing Translational Sciences (NCATS), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute of Mental Health (NIMH), the Health Resources and Services Administration (HRSA), NICHD, and the McCarthy Foundation. Dr. Budney has received research support from NIDA, NIAAA, and NICHD. Ms. Norton has received research support from NIAAA, NIDA, and NICHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
Contributor Information
Catherine Stanger, Geisel School of Medicine at Dartmouth College, Lebanon, NH.
Stacy R. Ryan, University of Texas Health Science Center at San Antonio.
Emily A. Scherer, Geisel School of Medicine at Dartmouth College, Lebanon, NH.
Gray E. Norton, Geisel School of Medicine at Dartmouth College, Lebanon, NH.
Alan J. Budney, Geisel School of Medicine at Dartmouth College, Lebanon, NH.
References
- 1.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future: Marijuana use continues to rise among U.S. teens, while alcohol use hits historic lows. [Accessed October 12, 2012]; http://www.monitoringthefuture.org/data/11data.html#2011data-drugs. Published December 14, 2011. [Google Scholar]
- 2.Winters KC, Lee CY. Likelihood of developing an alcohol and cannabis use disorder during youth: association with recent use and age. Drug and alcohol dependence. 2008;92(1–3):239–247. doi: 10.1016/j.drugalcdep.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis M, Babor TF, Roebuck MC, Donaldson J. Changing the focus: the case for recognizing and treating cannabis use disorders. Addiction. 2002;97(Suppl 1):4–15. doi: 10.1046/j.1360-0443.97.s01.10.x. [DOI] [PubMed] [Google Scholar]
- 4.Lisdahl KM, Wright NE, Kirchner-Medina C, Maple KE, Schollenbarger S. Considering cannabis: The effects of regular cannabis use on neurocognition in adolescents and young adults. Current Addiction Reports. 2014;1(2):144–156. doi: 10.1007/s40429-014-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SAMHSA. Treatment episode data set (TEDS): 2002–2012. National admissions to substance abuse treatment services. Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; 2014. [Google Scholar]
- 6.Hogue A, Henderson CE, Ozechowski TJ, Robbins MS. Evidence base on outpatient behavioral treatments for adolescent substance use: updates and recommendations 2007–2013. J Clin Child Adolesc Psychol. 2014;43(5):695–720. doi: 10.1080/15374416.2014.915550. [DOI] [PubMed] [Google Scholar]
- 7.Dennis M, Godley SH, Diamond G, et al. The cannabis youth treatment (CYT) study: Main findings from two randomized trials. Journal of Substance Abuse Treatment. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- 9.Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. Journal of Consulting and Clinical Psychology. 2006;74(2):307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- 10.Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addictive Behaviors. 2007;32(6):1220–1236. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan-Sarin S, Cavallo DA, Cooney JL, et al. An exploratory randomized controlled trial of a novel high school based smoking cessation intervention for adolescent smokers using abstinence-contingent incentives and cognitive behavioral therapy. Drug and Alcohol Dependence. 2013;132:346–351. doi: 10.1016/j.drugalcdep.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds B, Dallery J, Shroff P, Patak M, Leraas K. A web-based contingency management program with adolescent smokers. J. Appl. Behav. Anal. 2008;41(4):597–601. doi: 10.1901/jaba.2008.41-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henggeler SW, McCart MR, Cunningham PB, Chapman JE. Enhancing the effectiveness of juvenile drug courts by integrating evidence-based principles. Journal of Consulting and Clinical Psychology. 2012;80(2):264–275. doi: 10.1037/a0027147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godley MD, Godley SH, Dennis ML, Funk RR, Pasetti LL, Petry NM. A randomized trial of assertive continuing care and contingency management for adolescents with substance use disorders. Journal of Consulting and Clinical Psychology. 2014;82(1):40–51. doi: 10.1037/a0035264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Killeen TK, McRae-Clark AL, Waldrop AE, Upadhyaya H, Brady KT. Contingency management in community programs treating adolescent substance abuse: A feasibility study. Journal of Child and Adolescent Psychiatric Nursing. 2012;25(1):33–41. doi: 10.1111/j.1744-6171.2011.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaminer Y, Burleson JA, Burke R, Litt MD. The efficacy of contingency management for adolescent cannabis use disorder: a controlled study. Subst Abus. 2014;35(4):391–398. doi: 10.1080/08897077.2014.933724. [DOI] [PubMed] [Google Scholar]
- 17.Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug and Alcohol Dependence. 2009;105(3):240–247. doi: 10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb C, Scudder M, Kaminer Y, Kadden R. The Motivational Enhancement Therapy and Cognitive Behavioral Therapy for Adolescent Cannabis Users. Vol. 2. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2001. [Google Scholar]
- 19.Sampl S, Kadden R. Motivational Enhancement Therapy and Cognitive Behavioral Therapy for Adolescent Cannabis Users: 5 Sessions. Vol. 1. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2001. [Google Scholar]
- 20.Dishion TJ, Kavanagh K. Intervening in Adolescent Problem Behavior: A Family-Centered Approach. New York: Guilford Press; 2003. [Google Scholar]
- 21.Aickin M. A program for balancing the allocation of subjects in a clinical trial. Computers and Biomedical Research. 1982;15:519–524. doi: 10.1016/0010-4809(82)90014-3. [DOI] [PubMed] [Google Scholar]
- 22.Hudziak JJ, Copeland W, Stanger C, Wadsworth M. Screening for DSM-IV externalizing disorders with the Child Behavior Checklist: A receiver-operating characteristic analysis. The Journal of Child Psychology and Psychiatry. 2004;45(7):1299–1307. doi: 10.1111/j.1469-7610.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- 23.Sobell LC, Sobell MB, Litten R, Allen J. Timeline follow-back: A technique for assessing selfreported alcohol consumption. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 24.Kamon J, Budney A, Stanger C. A contingency management intervention for adolescent marijuana abuse and conduct problems. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(6):513–521. doi: 10.1097/01.chi.0000159949.82759.64. [DOI] [PubMed] [Google Scholar]
- 25.Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 26.Frick PJ. The Alabama Parenting Questionnaire. Birmingham, AL: University of Alabama; 1991. [Google Scholar]
- 27.Hinshaw SP, Owens EB, Wells KC, et al. Family processes and treatment outcome in the MTA: Negative/ineffective parenting practices in relation to multimodal treatment. Journal of Abnormal Child Psychology. 2000;28:555–568. doi: 10.1023/a:1005183115230. [DOI] [PubMed] [Google Scholar]
- 28.Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: Contingency management for treatment of alcohol dependence. Journal of Consulting and Clinical Psychology. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- 29.Knutson NM, Forgatch MS, Rains LA. Fidelity of Implementation Rating System (FIMP): The training manual for PMTO. Eugene, OR: Oregon Social Learning Center; 2003. [Google Scholar]
- 30.Hukkelberg SS, Ogden T. Working alliance and treatment fidelity as predictors of externalizing problem behaviors in parent management training. Journal of Consulting and Clinical Psychology. 2013;81(6):1010–1020. doi: 10.1037/a0033825. [DOI] [PubMed] [Google Scholar]
- 31.Carroll KM, Nich C, Sifry RL, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 32.Gibbons CJ, Nich C, Steinberg K, et al. Treatment process, alliance and outcome in brief versus extended treatments for marijuana dependence. Addiction. 2010;105(10):1799–1808. doi: 10.1111/j.1360-0443.2010.03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.