Abstract
Nuclear factor erythroid 2-related factor 2 (Nrf2) is an important redox-sensitive transcription factor that regulates the expression of several cytoprotective genes. More recently, genetic analyses of human tumors have indicated that Nrf2 may cause resistance to chemotherapy. In this study, we found that the expression levels of Nrf2 and its target genes GCLC, HO-1, NQO1 were significantly higher in cisplatin-resistant A549 (A549/CDDP) cells than those in A549 cells, and this resistance was partially reversed by Nrf2 siRNA. 3′,4′,5′,5,7-Pentamethoxyflavone (PMF), a natural flavonoid extracted from Rutaceae plants, sensitized A549/CDDP to CDDP and substantially induced apoptosis compared with that of CDDP alone treated group, and this reversal effect decreased when Nrf2 was downregulated by siRNA. Mechanistically, PMF reduced Nrf2 expression leading to a reduction of Nrf2 downstream genes, and in contrast, this effect was decreased by blocking Nrf2 with siRNA. Taken together, these results demonstrated that PMF could be used as an effective adjuvant sensitizer to increase the efficacy of chemotherapeutic drugs by downregulating Nrf2 signaling pathway.
Keywords: 3′,4′,5′,5,7-Pentamethoxyflavone; chemoresistance; cisplatin; lung cancer; Nrf2
INTRODUCTION
Lung cancer has become one of the main causes of cancer-related deaths in many countries. Non-small cell lung cancers (NSCLC) account for approximately 80% of all lung cancer cases (D’Addario et al., 2009). Cisplatin is widely used in the therapy of NSCLC and has a significant antitumor activity (Langer et al., 2002). However, one of the main factors contributing to the failure of cisplatin-based chemotherapy in NSCLC is the development of drug resistance (Longley and Johnston, 2005). Thus, it is a challenge to develop novel drug sensitizer that can enhance the efficacy of cisplatin-based chemotherapy and overcome chemoresistance.
Nuclear factor erythroid-related factor 2 (Nrf2) binds to anti-oxidant-response elements (AREs) and regulates the expression of several genes, including heme oxygenase (HO-1), NAD(P)H dehydrogenase quinine 1 (NQO1), UDP-Glucuronosyltrans- ferase (UGTs), cysteine ligase catalytic subunit (GCLC) and several ATP-dependent drug efflux pumps (Mrps) (Maher et al., 2008; Venugopal and Jaiswal, 1996). Therefore, Nrf2 plays an important role in protecting cells from oxidative stress and regulating cellular redox homeostasis (Jaiswal, 2004).
More recently, genetic analyses of human tumours have indicated that Nrf2-mediated defense responses may cause resistance to chemotherapy. (Hayashi et al., 2003; Maher et al., 2008; Wang et al., 2008). In human cancers such as lung and breast cancer, frequent mutations of Kelch-like ECH-associated protein 1 (Keap1), a repressor of Nrf2, have been identified and such mutations lead to constitutive activation of Nrf2 and its downstream genes (Hayes and McMahon, 2009). Highly expressed Nrf2 was found in doxorubicin-resistant MCF-7 cell line, and the chemoresistance could be reversed by blocking Nrf2 with siRNA (Shim et al., 2009). Therefore, discovery of compounds which can potently inhibit Nrf2-mediated responses is desirable and such small molecular inhibitors may be applied as an adjuvant to counteract chemoresistance.
Several natural products have been proved to be potent Nrf2 inhibitors, such as triptolide, EGCG, Brusato and procyanidins (Chen et al., 2013; Kweon et al., 2006; Ohnuma et al., 2011; Ren et al., 2011). Triptolide could enhance drug sensitivity of doxorubicin-resistant leukemia cell line HL60/A and imatinib-resistant cell line K562/G to doxorubicin and imatinib through downregulation of HIF-1α and Nrf2 (Chen et al., 2013). Cinnamomi Cortex extract reduced the expression of efflux transporters via Nrf2 suppression in A549 cells and LU99 cells and enhanced the cytotoxicity of doxorubicin and etoposide by increasing their intracellular concentrations (Ohnuma et al., 2011).
3′,4′,5′,5,7-Pentamethoxyflavone (PMF) (Fig. 1) is a polymethoxy-substituted flavonoid presented in plants of Rutaceae plant family such as Murraya paniculata and Neoraputia magnifica (Tomazelaa et al., 2000). Murraya paniculata is a widely used botanical drug with anti-inflammation, anti-obesity and antifungal activities (Lu et al., 2011). Neoraputia magnifica is proved to have anti-trypanosome activity (Soeiro and Castro, 2009). Previous reports showed that PMF could reduce adenoma development in a human gastrointestinal malignancies model ApcMin mouse through inhibiting prostaglandin production and interference with the COX pathway (Cai et al., 2009). However, whether PMF could sensitize chemoresistant cancer cells to chemotherapeutic drugs have not been studied yet.
Fig. 1.
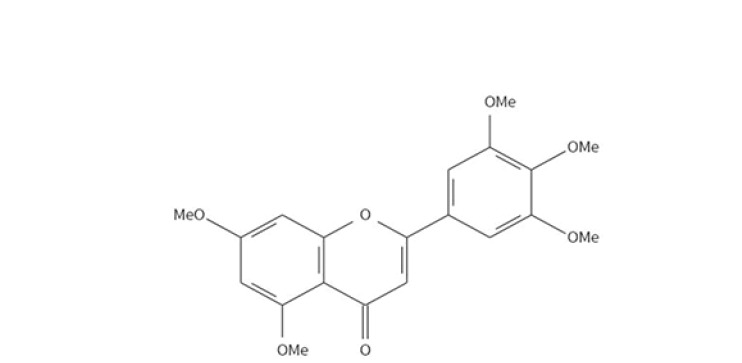
Chemical structure of 3′,4′,5′,5,7-Pentamethoxyflavone (PMF)
In this study, we demonstrated that PMF is a potent inhibitor of Nrf2 which sensitized CDDP-resistant A549 cells to CDDP, resulting in a reversal drug-resistant phenotype. Thus, PMF is a promising natural agent to sensitize cancer to therapeutic drugs and reverse chemoresistance.
MATERIALS AND METHODS
Materials
3′,4′,5′,5,7-Pentamethoxyflavone (PMF) was purchased from BioSun Sci & Tech Co., Ltd (China). Cis-diamminedichloroplatinum (II) (cisplatin, CDDP) was bought from Tokyo Chemical Industry (Japan) and kiton Red S (SRB) was purchased from Alfa Aesar (China). Primary antibody of Nrf2 was purchased from Santa Cruz Biotechnology (USA). NQO1, HO-1, ERK, p-ERK, PARP1 primary antibodies were got from Sangon (China). Caspase3, cleaved caspase3, β-actin, GAPDH, H3 and secondary antibody were obtained from CST (USA).
Cell culture
The A549 cell line and its CDDP-resistant cell line A549/CDDP cells were purchased from cell resource center of Institute of Basic Medical Sciences of Chinese Academy of Medical Sciences. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, HyClone, USA) supplemented with 10% fetal bovine serum (FBS, HyClone, USA) and antibiotics (50 U/mL of penicillin and 50 μg/mL streptomycin). The cells were grown in a humidified incubator with 5% CO2 at 37°C.
Cytotoxity assay
Exponentially growing A549 or A549/CDDP cells were seeded in 96-well plates at a density of 5 × 104 cells/well. Cells and siRNA-transfected cells were treated with the compounds for 48 h. Cell viability was assessed using SRB assay following the manufacturer’s protocol. Data were averaged from at least three independent experiments and are expressed as means ± SD.
Flow cytometric analysis of apoptotic cells
Cells were seeded in 6-well plates at a density of 1 × 106 /well and exposed to different treatments. After incubation for 24h, the cells were harvested and washed twice with ice-cold PBS. Afterwards the cells were incubated with Annexin V-FITC and PI successively for 15 min at room temperature in the dark according to the manufacturer’s instructions. Analyses were performed at FACSCalibur analyzer (Beckman Coulter EPICS XL, USA).
Transient transfection with Nrf2 siRNA or plasmids
The siRNA against human Nrf2 or negative control was synthesized by RiboBio (China) and the sequence of Nrf2 siRNA is 5′-GAGAAAGAAUUGCCUGUAAdTdT-3′;3′-dTdTCUCUUUCUUAACGGACAUU-5′. The plasmid pGL3-ARE was a kind gift from Dr. Athanassios Fragoulis (University Hospital Aachen) (Boesch-Saadatmandi et al., 2009). Cells were transfected with siRNA or plasmids using Lipofectamine 2000 (Invitrogen) according to the product specification. A dual-luciferase reporter assay (Promega, USA) was used to determine ARE-driven promoter activity.
Western blot assay
Cytoplasmic and nuclear extracts were extracted using a Nuclear Extract kit (Active Motif, USA) according to the manufacturer’s recommendations. Equivalent amounts of protein were separated by 12% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore Co., USA). Blocking was performed with 5% nonfat milk in TBST (TBS-1% Tween 20) for 1h, and then incubated with primary antibodies overnight at 4°C. Subsequently, a secondary horseradish peroxidase-conjugated anti-rabbit was applied, and then specific bands were visualized using ECL detection kit (Engreen Biosystem, China). The intensity of protein bands was analyzed using ImageJ software (National Institute of Health, USA).
Statistical analysis
All the experiments were repeated at least three times independently. SPSS v21.0 software (SPSS Inc., USA) was used for the statistical analysis. Values are presented as means ± SD. Statistical analysis was performed using Student’s t-test or one-way ANOVA. The level of significance was set at P < 0.05.
RESULTS
Nrf2-mediated defenses are upregulated in A549/DDP cells
To ensure the drug resistance phenotype of A549/CDDP cells, survival rates of cells exposed to CDDP were detected by SRB assay. Figure 2A showed that the IC50 of A549/CDDP cells (1136.3 μM) was significantly higher than that of A549 cells (317.9 μM, P < 0.05), which indicated that A549/CDDP cells were CDDP-resistance.
Fig. 2.
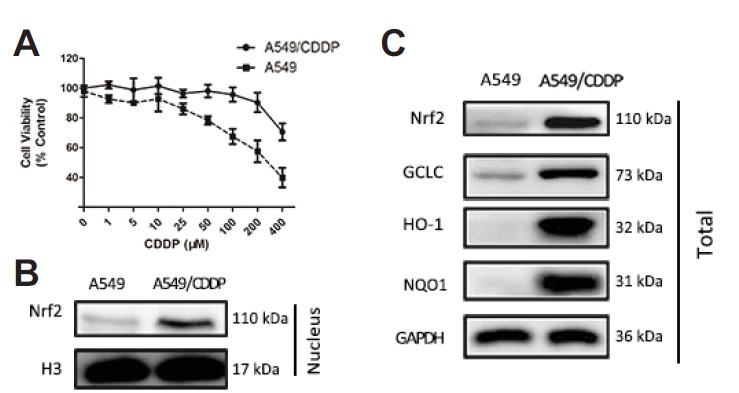
Nrf2 mediated defense was upregulated in A549/CDDP cells than in A549 cells. (A) The inhibitory effect of cisplatin on the viability of A549 and A549/CDDP cells. Cells were treated with series concentration of CDDP for 48 h and then viabilities were determined by SRB assay. (B–C) The expression of nuclear and total protein of Nrf2 and its target proteins GCLC, HO-1, NQO1 was assessed by western blot in A549 and A549/CDDP cells. Data are presented as means ± SD of three independent experiments and significant differences are indicated as *P < 0.05.
Up-regulation of Nrf2 is reported to play an important role in drug resistance. To evaluate whether Nrf2-mediate signaling pathway expression was different between A549 and A549/CDDP cells, western blot assay was performed. The expression of total and nuclear Nrf2 protein in A549 cells was significantly lower than those in A549/CDDP cells as well as its downstream target genes HO-1, NQO1 and GCLC (Figs. 2B–2C).
PMF sensitized A549/CDDP cells to CDDP
To determine whether PMF can enhance the sensitivity of A549/DDP cells to CDDP, SRB assay was carried out and IC50 was calculated. As shown in Fig. 3A, IC50 of PMF+CDDP treated group was significantly lower than that of the CDDP alone treated group, indicating that PMF reduced the resistance of A549/CDDP cells to CDDP. The effect of PMF on the cytotoxicity of CDDP was also investigated in A549 cells, which expressed lower Nrf2 than A549/DDP cells. The results showed that the effect of PMF was diminished in A549 cells compared with that in A549/CDDP cells, indicating that PMF might sensitize A549/CDDP cells to cisplatin through inhibition of Nrf2 (Fig. 3B). To investigate whether combination of PMF and CDDP would promote apoptosis of cells, A549/CDDP cells were exposed to CDDP (25 μM) or PMF (25 μM) or in combination for 24 h. Apoptotic cells were determined by PI/Annexin V staining and flow cytometric analysis. Figures 3C–3D showed that the ratio of apoptotic cells in the combined treatment group was significantly increased compared with that in the CDDP alone treated group (P < 0.05). Furthermore, apoptosis-related proteins were assayed by Western blotting. We found that the cleaved PARP1 and caspase3 levels induced by CDDP were significantly enhanced by PMF treatment (Fig. 3E). Taken together, these results illustrated that PMF enhanced the sensitivity of A549/CDDP cells to CDDP and promoted apoptosis.
Fig. 3.
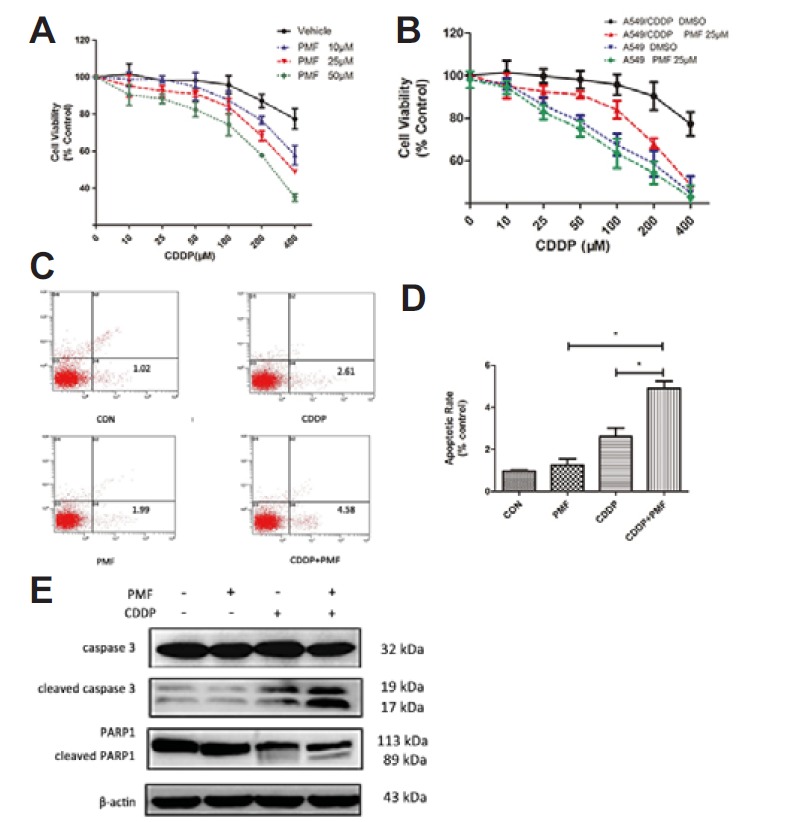
PMF sensitized A549/CDDP cells to CDDP. (A) A549/CDDP cells were exposed to PMF (10, 25, and 50 μM) and were incubated with increasing concentration of CDDP (5 – 400 μM) in culture for 48 h and SRB assay were taken. (B) Comparison of the suppression of PMF (25 μM) combined with different concentration of CDDP on A549 cells and A549/CDDP cells. (C–D) Apoptosis ratios were determined through flow cytometry. (E) The expression of cleaved Caspase 3 and PARP1were determined by Western blot. Data are presented as means ± SD of three independent experiments and significant differences are indicated as *P < 0.05.
PMF inhibited Nrf2 signaling pathway
To investigate whether the expression of Nrf2 and its target genes was regulated by PMF, A549/CDDP cells were incubated with PMF (10, 25 and 50 μM) for 24 h. Western blot analysis revealed that PMF treatment reduced the protein levels of Nrf2 and its downstream target genes, including HO-1, NQO1 and GCLC in a dose-dependent manner(Figs. 4B–4C).
Fig. 4.
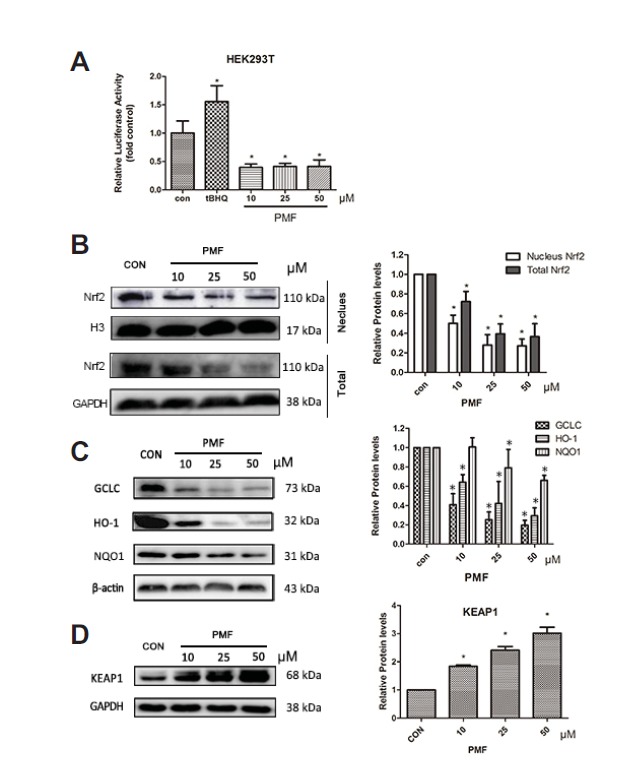
PMF inhibited Nrf2 signalling pathway. (A) HEK 293T cells were transfected with PGL3-ARE and pRL-TK plasmids., Twenty four hours after the transfection, the cells were treated with PMF (10, 25 and 50 μM) or tBHQ (10 μM) for 24 h. (B–D) A549/CDDP cells were treated with PMF (0–50 μM) for 24 hours. After treatment, nuclear fractions and total proteins were analyzed by western blot assay. Data are presented as means ± SD of three independent experiments and significant differences are indicated as *P < 0.05.
Activated Nrf2 binds to ARE sites and causes the up-regulation of its target genes. To further confirm the effect of PMF on Nrf2 transactivation, HEK293T cells were transiently transfected with ARE plasmid constructs. Treatment with PMF (10, 25 and 50 μM) produced a significant decrease in ARE transcriptional activity (Fig. 4A).
Keap1 is the major repressor of Nrf2 and facilitates the degradation of Nrf2 through the ubiquitin–proteasome pathway in cytosol (Itoh et al., 2010). The effect of PMF on the expression of Keap1 was also investigated. The expression of Keap1 was significantly increased by PMF in a dose dependent manner (Fig. 4D), which suggested that the inhibition of PMF on Nrf2 signaling pathway might be through Keap1.
In addition, Nrf2 activity has been implicated to be under the regulation of a number of protein kinases (Kim and Jang, 2014). To further explore the mechanisms underlying the inhibitory effect of PMF on Nrf2 activity, the phosphorylated Nrf2 and phosphatase ERK were investigated. The p-Nrf2 level and p-ERK were significantly decrease by PMF (Fig. 5).
Fig. 5.
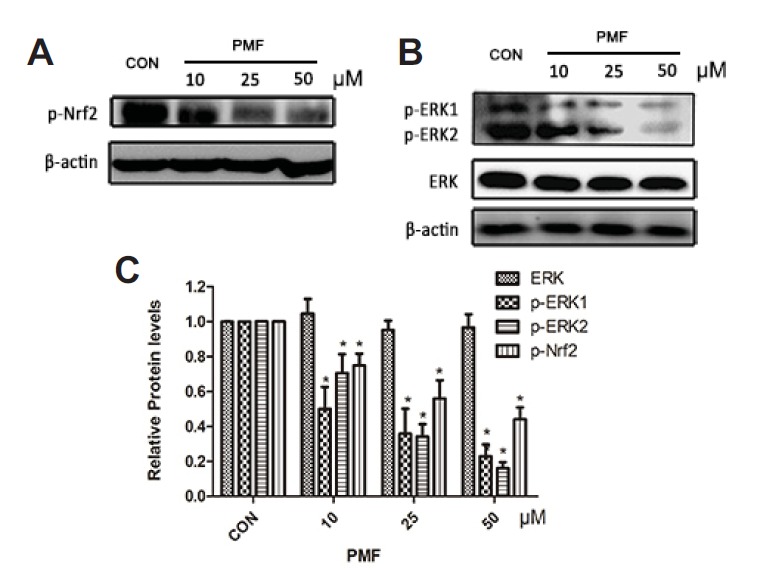
PMF reduced the expression of p-Nrf2 and p-ERK in A549/CDDP cells. After A549/CDDP cells were treated with PMF (10, 25 and 50 μM) for 48 h, the expression of p-Nrf2 (A) and protein kinases ERK (B) were determined using Western blot analysis. Data are presented as means ± SD of three independent experiments and significant differences are indicated as *P < 0.05.
PMF sensitized A549/CDDP cells to CDDP by inhibition of Nrf2
To confirm whether Nrf2 was involved in the chemoresistance of A549/CDDP cells, Nrf2 siRNA was transfected to knock down Nrf2 expression in A549/CDDP cells. The sensitivity of cells to CDDP was detected by SRB assay. Figure 6A showed that Nrf2 siRNA-transfected A549/CDDP cells were much more sensitive to CDDP than A549/CDDP cells.
Fig. 6.

PMF increased the sensitivity of A549/CDDP cells to CDDP by inhibition of Nrf2. (A–B) Effect of Nrf2 knockdown on the sensitivity to CDDP. Cells were treated with CDDP (10 – 400 μM) alone or combination with 50 μM PMF for 48 h. Cell viability was determined by SRB assay. (C–D) Effect of Nrf2 knockdown on the expression of Nrf2-mediated antioxidant genes. *P < 0.05, versus resistance. Data are presented as means ± SD of three independent experiments and significant differences are indicated as *P < 0.05.
To clarify whether Nrf2 signaling pathway was involved in PMF increasing sensitivity of A549/CDDP cells to CDDP, A549/CDDP cells were transfected with Nrf2 siRNA to knockdown the expression of Nrf2. As shown in Figs. 6B–6D, western blot analysis confirmed the successful knockdown of Nrf2 in A549/CDDP cells. The expression of Nrf2-target genes including HO-1, NQO1 and GCLC were also decreased in siRNA-transfected A549/CDDP cells. Furthermore, PMF did not significantly affect levels of Nrf2 and its target genes in A549/CDDP cells transfected with Nrf2 siRNA.
To investigate whether the sensitization by PMF was Nrf2 dependent, the sensitivity of A549/CDDP cells to CDDP was tested with Nrf2 knockdown. The effect of PMF was diminished in Nrf2 siRNA-transfected A549/CDDP (Fig. 6E). These results further demonstrated that PMF increased the sensitivity of A549/CDDP cells to CDDP by inhibition of Nrf2.
DISSCUSSION
Drug resistance during chemotherapy is considered as one of the major obstacle to the successful treatment of many cancers (El-Sheikh et al., 2013; Signore et al., 2013). Several studies have reported that Nrf2 played an important role in chemoresistance. (El-Sheikh et al., 2013; Maher et al., 2008; Na and Surh, 2014). The expression of Nrf2 and its target genes were significantly higher in tumor tissues than in their corresponding non-tumor tissues (Gao et al., 2013b). The proliferation of several cell lines was inhibited and became sensitive to anticancer drugs when Nrf2 expression was knocked down with siRNA (Ji et al., 2013; Singh et al., 2008). Both in vivo and in vitro studies demonstrated that Nrf2 was a potential target to increase drug sensitivity. In general, these findings indicated that up-regulation of Nrf2 and its target genes would increase resistance to the cytotoxic effects of chemotherapy drug.
In this study, the IC50 of A549/CDDP cells to CDDP was found to be higher than that of A549 cells (Fig. 1A), which suggested that A549/CDDP cells were CDDP-resistant. And higher levels of Nrf2 and Nrf2-mediated antioxidant enzymes were found in A549/CDDP cells than A549 cells. Transfection of A549/CDDP cells with Nrf2 siRNA decreased the Nrf2-mediated pathway, resulting in the partial recovery of sensitivity to CDDP in A549/CDDP cells (Fig. 6A). It is reported that HO-1 could potentiate tumor aggressiveness, by increasing tumor growth, angiogenesis and metastasis (Was et al., 2006). NQO1 could metabolically activate carcinogenic heterocyclic amines which present in smoke and higher levels of NQO1 expression have been detected in the lung tissues (Kiyohara et al., 2005). And GCLC is a subunit of a key enzyme in glutathione synthesis which was reported to enhance CDDP-resistance in NSCLC xenografts (Fujimori et al., 2004). Thus, we deduced that the up-regulation of Nrf2 and Nrf2-mediated antioxidant enzymes was one of the reasons that lead to resistance to CDDP in A549 cells, which was in consistent with previous studies (Tang et al., 2011). Our findings suggested that the activation of Nrf2-mediated signaling is responsible for, at least in part, CDDP resistance in A549 cells. In addition to these antioxidant enzymes, several transporters such as Mrps were also reported to be regulated by Nrf2, which could lead to chemoresistance. We will further investigate Nrf2-mediated transporters in the future work.
Several natural products have been reported to be inhibitors of Nrf2 and can reduce drug resistance (Gao et al., 2013b; Kweon et al., 2006; Tang et al., 2011; Zhong et al., 2013). For example, apigenin was reported to sensitize doxorubicin-resistance BEL-7402/ADM cells to doxorubicin by inhibiting Nrf2 pathway (Gao et al., 2013b). Luteolin was discovered to be a potent small-molecule inhibitor, which downregulated cytoprotective enzymes and sensitized NSCLC cells and colorectal cancer cell Lines to oxalipatin, blecomycin and doxorubicin (Chian et al., 2014; Tang et al., 2011). Wogonin was shown to suppress Nrf2 activity and reduce HO-1 and NQO1 expression in MCF-7/DOX cells, reversing their drug resistance (Zhong et al., 2013).
PMF is a natural polymethoxy-substituted flavonoid with anti-cancer potential (Kinoshita and Firman, 1997). In this study, we identified PMF as a potent Nrf2 inhibitor. PMF sensitized A549/CDDP cells to CDDP and suppressed the expression of Nrf2 as well as its target antioxidant genes. Furthermore, in siRNA-transfected A549/CDDP cells, PMF did not significantly affect levels of Nrf2 and its downstream genes, suggesting inhibition of Nrf2 was one of the reasons that PMF reduced chemoresistance.
The Nrf2 pathway is regulated at multiple levels, such as Keap1 and phosphorylation related pathways. To further understand the molecular mechanisms underlying the inhibition of PMF on Nrf2 pathway, the expression of Keap1 was investigated (Fig. 4D). We found that the expression of Keap1 was increased by PMF treatment. However, it is reportd that several genetic alterations of the Keap1 gene were found in both lung cancer cell lines and lung cancer patients. For example, a non-synonymous somatic mutation (G333C) was detected in the first repeat of the double glycine repeat/Kelch domain that could not repress the activity of Nrf2 (Singh et al., 2006). Therefore, further study is needed to explore the mechanisms underlying the effect of PMF on the function of Keap1. In addition to Keap1, Nrf2 activity has been implicated to be under the regulation of a number of protein kinases, such as ERK. The inhibition of phosphorylated ERK by PMF was observed in this study (Fig. 5). It is reported that Akt signaling pathways are involved in chemoresistance and regulating the phosphorylation of Nrf2 and ARE-mediated antioxidant gene. Chrysin and 4-metho- xychalcone were reported to decrease p-Akt and regulate the phosphorylation of Nrf2 and Nrf2-mediated gene expression (Gao et al., 2013a; Lim et al., 2013). Further studies are needed to investigate whether other pathways such as PI3K/Akt pathways are involved in PMF-mediated sensitization to cisplatin.
Taken together, this study demonstrated that higher level of Nrf2 expressed in A549/CDDP cells accounted for drug resistance and PMF inhibited the expression of Nrf2 and its target genes HO-1, NQO1 and GCLC, resulting in a reversal of drug-resistant phenotype and promoting apoptosis eventually. Further studies in animal models and clinical trials are necessary to exploit PMF as a possible natural agent for treatment of cancer.
Acknowledgments
The work was supported by the Natural Science Foundation of China (Grant Number. 81102886), National Science-technology Support Plan Projects (Grant Number: 2012BAI29B09) and Major Scientific and Technological Project of Guangdong Province (Grant Number: 2012A080202013), Guangdong Provincial Key Laboratory of Construction Foundation (2011A060901014).
REFERENCES
- Boesch-Saadatmandi C, Wagner AE, Graeser AC, Hundhausen C, Wolffram S, Rimbach G. Ochratoxin A impairs Nrf2-dependent gene expression in porcine kidney tubulus cells. J. Anim. Physiol. Anim. Nutr. 2009;93:547–554. doi: 10.1111/j.1439-0396.2008.00838.x. [DOI] [PubMed] [Google Scholar]
- Cai H, Sale S, Schmid R, Britton RG, Brown K, Steward WP, Gescher AJ. Flavones as colorectal cancer chemopreventive agents--phenol-o-methylation enhances efficacy. Cancer Prev. Res. 2009;2:743–750. doi: 10.1158/1940-6207.CAPR-09-0081. [DOI] [PubMed] [Google Scholar]
- Chen F, Liu Y, Wang S, Guo X, Shi P, Wang W, Xu B. Triptolide, a Chinese herbal extract, enhances drug sensitivity of resistant myeloid leukemia cell lines through downregulation of HIF-1a and Nrf2. Pharmacogenomics. 2013;14:1305–1317. doi: 10.2217/pgs.13.122. [DOI] [PubMed] [Google Scholar]
- Chian S, Li YY, Wang XJ, Tang XW. Luteolin sensitizes two oxaliplatin-resistant colorectal cancer cell lines to chemotherapeutic drugsvia inhibition of the Nrf2 pathway. Asian Pac. J. Cancer Prev. 2014;15:2911–2916. doi: 10.7314/apjcp.2014.15.6.2911. [DOI] [PubMed] [Google Scholar]
- D’Addario G, Felip E, ESMO Guidelines Working Group Non-small-cell lung cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2009;20:68–70. doi: 10.1093/annonc/mdp132. [DOI] [PubMed] [Google Scholar]
- El-Sheikh AA, Greupink R, Wortelboer HM, van den Heuvel JJ, Schreurs M, Koenderink JB, Masereeuw R, Russel FG. Interaction of immunosuppressive drugs with human organic anion transporter (OAT) 1 and OAT3, and multidrug resistance-associated protein (MRP) 2 and MRP4. Transl. Res. 2013;162:398–409. doi: 10.1016/j.trsl.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Fujimori S, Abe Y, Nishi M, Hamamoto A, Inoue Y, Ohnishi Y, Nishime C, Matsumoto H, Yamazaki H, Kijima H, et al. The subunits of glutamate cysteine ligase enhance cisplatin resistance in human non-small cell lung cancer xenografts in vivo. Int. J. Oncol. 2004;25:413–418. [PubMed] [Google Scholar]
- Gao AM, Ke ZP, Shi F, Sun GC, Chen H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem. Biol. Interact. 2013a;206:100–108. doi: 10.1016/j.cbi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Gao AM, Ke ZP, Wang JN, Yang JY, Chen SY, Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013b;34:1806–1814. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein1 in mouse embryo fibroblasts. Biochem. Biophs. Res. Commun; 2003. pp. 824–829. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem. Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid. Redox Signal. 2010;13:1665–1678. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Ji X, Wang H, Zhu J, Zhu L, Pan H, Li W, Zhou Y, Cong Z, Yan F, Chen S. Knockdown of Nrf2 suppresses glioblastoma angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. Int. J. Cancer. 2013;135:574–584. doi: 10.1002/ijc.28699. [DOI] [PubMed] [Google Scholar]
- Kim JK, Jang HD. Nrf2-mediated HO-1 induction coupled with the ERK signaling pathway contributes to indirect antioxidant capacity of caffeic acid phenethyl ester in HepG2 cells. Int. J. Mol. Sci. 2014;15:12149–12165. doi: 10.3390/ijms150712149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Firman K. Myricetin 5,7,3′,4′,5′-pentamethyl ether and other methylated flavonoids from Murraya Paniculata. Phytochemistry. 1997;45:179–181. [Google Scholar]
- Kiyohara C, Yoshimasu K, Takayama K, Nakanishi Y. NQO1, MPO, and the risk of lung cancer: A HuGE review. Genet. Med. 2005;7:463–478. doi: 10.1097/01.gim.0000177530.55043.c1. [DOI] [PubMed] [Google Scholar]
- Kweon MH, Adhami VM, Lee JS, Mukhtar H. Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J. Biol. Chem. 2006;281:33761–33772. doi: 10.1074/jbc.M604748200. [DOI] [PubMed] [Google Scholar]
- Langer CJ, Manola J, Bernardo P, Kugler JW, Bonomi P, Cella D, Johnson DH. Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of eastern cooperative oncology group 5592, a randomized trial. J. Natl. Cancer Inst. 2002;94:173–181. doi: 10.1093/jnci/94.3.173. [DOI] [PubMed] [Google Scholar]
- Lim J, Lee SH, Cho S, Lee IS, Kang BY, Choi HJ. 4-methoxychalcone enhances cisplatin-induced oxidative stress and cytotoxicity by inhibiting the Nrf2/ARE-mediated defense mechanism in A549 lung cancer cells. Mol. Cells. 2013;36:340–346. doi: 10.1007/s10059-013-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J. Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- Lu YQ, Wang LY, Luo YP. The antifungal activities and composition analysis of the essential oil from Murraya paniculata. Agrochemicals. 2011;50:443–446. [Google Scholar]
- Maher JM, Aleksunes LM, Dieter MZ, Tanaka Y, Peters JM, Manautou JE, Klaassen CD. Nrf2- and PPAR alpha-mediated regulation of hepatic Mrp transporters after exposure to perfluorooctanoic acid and perfluorodecanoic acid. Toxicol. Sci. 2008;106:319–328. doi: 10.1093/toxsci/kfn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na HK, Surh YJ. Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic. Biol. Med. 2014;67:353–365. doi: 10.1016/j.freeradbiomed.2013.10.819. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Matsumoto T, Itoi A, Kawana Ayako, Nishiyama T, Ogura K, Hiratsuka A. Enhanced sensitivity of A549 cells to the cytotoxic action of anticancer drugs via suppression of Nrf2 by procyanidins from Cinnamomi Cortex extract. Biochem. Biophys. Res. Commun. 2011;413:3623–3629. doi: 10.1016/j.bbrc.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, Zhang DD. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. USA. 2011;108:1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim GS, Manandhar S, Shin DH, Kim TH, Kwak MK. Acquisition of doxorubicin resistance in ovarian carcinoma cells accompanies activation of the NRF2 pathway. Free Radic. Biol. Med. 2009;47:1619–1631. doi: 10.1016/j.freeradbiomed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Signore M, Ricci-Vitiani L, De Maria R. Targeting apoptosis pathways in cancer stem cells. Cancer Lett. 2013;332:374–382. doi: 10.1016/j.canlet.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, et al. Dysfunctional KEAP1–NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:1865–1874. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F, Watson WH, et al. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68:7975–7984. doi: 10.1158/0008-5472.CAN-08-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro MN, Castro SL. Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert Opin. Ther. Targets. 2009;13:105–121. doi: 10.1517/14728220802623881. [DOI] [PubMed] [Google Scholar]
- Tang X, Wang H, Fan L, Wu X, Xin A, Ren H, Wang XJ. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic. Biol. Med. 2011;50:1599–1609. doi: 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Tomazela DM, Pupo MT, Passador EA, da Silva MF, Vieira PC, Fernandes JB, Fo ER, Oliva G, Pirani JR. Pyrano chalcones and a flavone from Neoraputia magnifica and their Trypanosoma cruzi glycosomal glyceraldehyde 3-phosphate dehydrogenase inhibitory activities. Phytochemistry. 2000;55:643–651. doi: 10.1016/s0031-9422(00)00248-x. [DOI] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Was H, Cichon T, Smolarczyk R, Rudnicka D, Stopa M, Chevalier C, Leger JJ, Lackowska B, Grochot A, Bojkowska K, et al. Overexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of mice. Am. J. Pathol. 2006;169:2181–2198. doi: 10.2353/ajpath.2006.051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Zhang F, Sun Z, Zhou W, Li ZY, You QD, Guo QL, Hu R. Drug resistance associates with activation of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by down-regulating Nrf2-mediated cellular defense response. Mol. Carcinog. 2013;52:824–834. doi: 10.1002/mc.21921. [DOI] [PubMed] [Google Scholar]


