Abstract
NF-E2-related factor 2 (Nrf2), a basic leucine zipper transcription factor, has recently received a great deal of attention as an important molecule that enhances antioxidative defenses and induces resistance to chemotherapy or radiotherapy. In this study, we investigated the apoptosis-inducing and Nrf2-upregulating effects of quercetin on malignant mesothelioma (MM) MSTO-211H and H2452 cells. Quercetin treatment inhibited cell growth and led to upregulation of Nrf2 at both the mRNA and protein levels without altering the ubiquitination and extending the half-life of the Nrf2 protein. Following treatment with quercetin, analyses of the nuclear level of Nrf2, Nrf2 antioxidant response element-binding assay, Nrf2 promoter-luc assay, and RT-PCR toward the Nrf2-regulated gene, heme oxygenase-1, demonstrated that the induced Nrf2 is transcriptionally active. Knockdown of Nrf2 expression with siRNA enhanced cytotoxicity due to the induction of apoptosis, as evidenced by an increase in the level of proapoptotic Bax, a decrease in the level of antiapoptotic Bcl-2 with enhanced cleavage of caspase-3 and PARP proteins, the appearance of a sub-G0/G1 peak in the flow cytometric assay, and increased percentage of apoptotic propensities in the annexin V binding assay. Effective reversal of apoptosis was observed following pretreatment with the pan-caspase inhibitor Z-VAD. Moreover, Nrf2 knockdown exhibited increased sensitivity to the anticancer drug, cisplatin, presumably by potentiating the oxidative stress induced by cisplatin. Collectively, our data demonstrate the importance of Nrf2 in cytoprotection, survival, and drug resistance with implications for the potential significance of targeting Nrf2 as a promising strategy for overcoming resistance to chemotherapeutics in MM.
Keywords: apoptosis, chemoresistance, cisplatin, quercetin, mesothelioma, Nrf2
INTRODUCTION
Reactive oxygen species (ROS), such as superoxide (O2−), hydrogen peroxide (H2O2) and hydroxyl radical (OH−), are generated as byproducts of intracellular oxygen metabolism, or in response to various exogenous stimuli including chemotherapeutics, ionizing radiation, ultraviolet, growth factors, cytokines and hypoxia. ROS play critical roles in normal physiological pathways and vital functions; however, the overproduction of ROS can have toxic impacts on cells by inducing apoptosis and causing damage to cellular proteins, lipids, polysaccharides, and DNA. To counterbalance the oxidative damage and protect themselves against ROS insults, cells possess a variety of antioxidant defense systems which maintain the redox balance.
Increased oxidative stress in conjunction with reduced levels of antioxidants has been observed in many types of human cancer (Jiang et al., 2013; Oberley and Oberley, 1997; Sharma et al., 2009). Because diverse anticancer drugs rely on free radical mechanisms to carry out their effects, it is possible that the reduced antioxidant defenses can initially make the cells susceptible to chemotherapeutics. However, as the tumor progresses, cancer cells lose their preferential sensitivity to therapeutic regimens by acquiring resistance to them and developing into more aggressive tumors. Although antioxidants can alleviate the toxic effects of free radical-producing drugs and help maintain the health of normal tissues in cancer patients, much earlier work has shown that the upregulation of antioxidant systems may afford the same protection to tumor cells against oxidative damage and subsequently promote tumor progression by increasing aggressiveness and drug resistance (Ceccarelli et al., 2008; Kim et al., 2005).
The transcription factor Nrf2 plays an essential role in antioxidant response element (ARE)-mediated expression of phase II detoxifying enzymes (e.g. glutathione S-transferase), antioxidant enzymes [e.g. NADPH quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), and aldo-keto reductase family 1, member C1], and drug-transporting proteins (e.g., multidrug resistance protein (MRP) 1 and multidrug resistance associated proteins 1/3). Upon exposure to oxidants or electrophiles, Kelch-like ECH-associated protein 1 (Keap1), a cytosolic repressor protein that binds to Nrf2, is inactivated by the direct modification of crucial cysteine residues. This results in dissociation of the Nrf2-Keap1 complex or in conformational changes in the associated motif of Nrf2-Keap1. Consequently, Nrf2 translocates to the nucleus, where it recruits the small musculoaponeurotic fibrosarcoma protein (sMaf). The Nrf2-sMaf heterodimer then binds to ARE, present in Nrf2-regulated genes, thereby inducing their transcription (Slocum and Kensler, 2011). Therefore, the Nrf2/ARE signaling pathway is of great importance in the prevention of cell dysfunction when subjected to oxidative or electrophilic stress by maintaining cellular redox homeostasis (Wakabayashi et al., 2004).
A role of Nrf2 in drug resistance has been suggested based on its property to induce downstream detoxifying and antioxidant enzymes or drug efflux pumps, as mentioned above. In this regard, the Nrf2 pathway has somewhat of a double-edged sword in nature, providing both benefits and risks to cells. Nrf2 activation appears to be beneficial for cancer chemoprevention in normal and premalignant tissues. However, in some cancers it prevents apoptosis while enhancing the survival of damaged cells, tumorigenesis, and drug resistance during chemotherapy (Niture and Jaiswal, 2013; Wang et al., 2008). The Nrf2 pathway was found to be dysregulated in many cancers, including those in the lung (Ohta et al., 2008), gall bladder (Shibata et al, 2008), and head and neck (Stacy et al., 2006), through mechanisms which result in increased levels of Nrf2 and impaired Keap1. Somatic mutations in the Nrf2-Keap1 axis, leading to constitutive activation of the Nrf2 pathway along with a loss of Nrf2 repression, are considered to be the main cause of Nrf2 dysregulation (Kim et al., 2011; Stacy et al., 2006). Stable overexpression of Nrf2 was demonstrated to enhance the resistance of cancer cells to chemotherapeutic agents (Wang et al., 2008), whereas its depletion or the overexpression of Keap1 enhanced sensitivity (Qu et al., 2010). Similarly, endometrial cancer cells with high levels of Nrf2 expression were more resistant to toxic effects of anticancer drugs, whereas silencing of Nrf2 could increase the efficacy of chemotherapy (Jiang et al., 2010). Interestingly, these responses have been demonstrated in studies using several chemotherapeutic agents, such as cisplatin, etoposide and doxorubicin, which may trigger the production of ROS for the killing of cancer cells (Chung et al., 2014; Shim et al., 2009; Wang et al., 2008). Considering the direct relationship between Nrf2 signaling and protection from oxidative insults, the activators of Nrf2 during chemotherapy may not only attenuate the efficacy of anticancer drugs by scavenging ROS, but also enhance drug resistance.
The Nrf2/ARE signaling pathway is a common molecular target for a variety of natural products, which exert their chemopreventive activities against a wide spectrum of cancer types. Quercetin, a flavonoid, is one of the most abundant polyphenolic groups in fruits and vegetables, and is known to exert potential anticancer activities including the inhibition of tumor initiation (Khanduja et al., 1999), antioxidative activity (Saw et al., 2014), antiproliferative activities against tumor cells (Borska et al., 2012), and activating apoptosis in different cancer cell lines (Granado-Serrano et al., 2006). Interestingly, quercetin has a unique ability to act as either an antioxidant or a pro-oxidant, presumably depending on the concentration and duration of exposure. It has been reported that higher doses of quercetin (40–100 μM) decreased cell survival and reduced the levels and activities of cellular antioxidants, contributing to beneficial anti-tumor effects by exerting cytotoxic effects, whereas low doses (5–30 μM) increased the total antioxidant capacity of cancer cells and antagonized the cytotoxic effects of antineoplastic agents in lung cancer A549, colorectal cancer HCT116, and ovarian cancer cells (Li et al., 2014; Robaszkiewicz et al., 2007; Samuel et al, 2012). Similarly, short-term treatment with quercetin was found to induce antioxidative and antiapoptotic effects, while long-term treatment resulted in pro-oxidative and proapoptotic effects (Ferraresi et al., 2005). Therefore, the uncontrolled consumption of quercetin may interfere with and weaken the effects of anticancer therapy rather than help it.
Malignant mesothelioma (MM) is a lethal asbestos-associated cancer with poor responsiveness to current chemotherapeutic drugs, which is often attributable to its high resistance to apoptosis. Accordingly, this study aimed to investigate the efficacy of quercetin as an activator of Nrf2 in MM cells, and to evaluate the effects of inhibiting Nrf2 expression on the viability of MM cells and their sensitivity to cisplatin through use of an siRNA-based technique.
MATERIALS AND METHODS
Reagents and cell culture
Quercetin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrozolum bromide (MTT), 2′,7′-dichlorofluorescein diacetate (DCF-DA), sodium dodecyl sulfate (SDS), phosphate buffered saline (PBS), cycloheximide (CHX), cisplatin, and antibodies to ubiquitin and β-actin were obtained from Sigma-Aldrich Co. (USA). Trizol reagent, cell culture media and reagents were purchased from Invitrogen (USA). Antibodies to Bcl-2, Bcl-xL, Bax, caspase-3, cleaved caspase-3, poly (ADP-ribose) polymerase (PARP), and cleaved PARP were from Cell Signaling Technologies (USA). Antibodies to Nrf2, lamin A/C, and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (USA). Anti-human HO-1 antibody was obtained from Stressgen Biotechnologies (USA). The human mesothelioma cell lines, MSTO-211H and H2452, were obtained from the American Type Culture Collection (ATCC, USA). Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 1 mM glutamine, 100 units of penicillin/ml and 100 μg of streptomycin/ml.
Cell viability assay
The cells were seeded in 96-well microtiter plates, and were treated with drugs or chemicals at different concentrations for the indicated times, after which they were exposed to MTT (final 0.1 mg/ml) for additional 4 h. The formazan crystals, formed by the reduction of MTT in living cells, were solubilized in 200 μl of DMSO and were measured with spectrophotometry at 560 nm. The results were expressed as percentage, based on the ratio of the absorbance between the treated cells and the controls (100%).
Western blotting
Western blot analyses were performed using cell lysate as described previously (Lee et al. 2012). Cell lysate containing 40 μg of protein was separated on NuPAGE 4–12% bis-tris polyacrylamide gels (Invitrogen, USA) and were electrophoretically transferred to Immuno-Blot PVDF membrane. The membranes were incubated with primary antibodies overnight at 4°C, and washed and incubated with HRP-conjugated secondary antibody for 1 h. The signal was visualized by an ECL detection kit. The blots were then stripped with a stripping buffer (100 mM β-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl, pH 6.7) and were reprobed with anti-β-actin and lamin A/C antibodies as loading controls.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using Trizol reagent (Invitrogen). Reverse transcription was performed with 1 μg of the total RNA, oligo(dT)15 primer, and a AMV reverse transcriptase (iNtRON Biotechnology, Seongnam, Korea). Each single-stranded cDNA was then diluted and subjected to PCR amplification using i-MAX II™ DNA polymerase (iNtRON Biotechnology). The PCR conditions were as follows: initial denaturation at 94°C for 2 min followed by 28 cycles of 94°C for 30 s, 57°C for 30 s and 72°C for 30 s. The following primers were used to amplify the fragments of genes. Specific primers for RT-PCR amplification of various genes were as follows: (a) Nrf2 (124-bp): sense 5′cttggcctcagtgattctgaagtg-3′ and antisense 5′-cctgagatggtgacaagggttgta-3′, (b) HO-1 (249-bp): sense 5′-gcaacccgacagcatgc-3′ and antisense 5′-tgcggtgcagctcttctg-3′, (c) GAPDH (247-bp): sense 5′-acctgacctgccgtctagaa-3′ and antisense 5′-tccaccaccctgttgctgta-3′. The PCR products were separated on 1.5% aga-rose gels.
Immunoprecipitation
Immunoprecepitation was done by using a Universal Magnetic Co-IP kit according to the manufacturer’s protocol (Active Motif, USA). Briefly, 500 μg of protein from cell lysates were immunoprecipitated using anti-Nrf2 or anti-ubiquitin antibody, and protein G magnetic beads, after which immunoprecipitated proteins were resolved on NuPAGE 4–12% bis-tris polyacrylamide gels, as above. Immunodetection was carried out by using anti-ubiquitin or anti-Nrf2 antibody, respectively.
Preparation of nuclear and cytoplasmic extracts
Nuclear extracts were prepared according to the instructions of the NE-PER nuclear and cytoplasmic extraction kit (Pierce, USA). Briefly, cells were resuspended in 10 vol of CER I solution, after which they were incubated in CER II solution on ice for 1 min and homogenized. Nuclei were pelleted by centrifugation at 14,000 rpm for 5 min, and the supernatant was kept as the cytoplasmic extract. The nuclear fraction was lysed for 40 min on ice in NER solution. The insoluble pellet was removed by centrifugation at 14,000 rpm for 10 min. The supernatant was used as the nuclear extract.
DNA binding activity of Nrf2
The DNA-binding capacity of Nrf2 was determined by using a TransAM Nrf2 Transcription Factor Assay Kit according to the manufacturer’s instruction (Active Motif, USA). The DNA binding of activated Nrf2 protein with specific oligonucleotide sequence (5′-GTCACAGTGACTCAGCAGAATCTG-3′) coated onto a microtitre plate was revealed by the addition of anti-Nrf2 antibody, washing, and incubation with HRP-conjugated secondary antibody. Absorbance was finally read at 450 nm and the results were expressed as a percentage, based on the ratio of the absorbance of treated cells to that of controls (100%).
Luciferase reporter assay
Cells were transfected with Nrf2-luciferase plasmids (pNrf2RE-luc) or pSV-β-galactosidae plasmids using lipofectamine plus reagent according to manufacturer’s protocol (Invitrogen). Briefly, transfected cells were treated with quercetin for 48 h and then lysed in a reporter assay lysis buffer (Promega). Relative luciferase activity in the cell lysates was calculated by dividing luciferase activity by β-galactosidae activity.
siRNA-mediated gene silencing
RNA interference of Nrf2 was performed using an Nrf2-specific siRNA duplex from Invitrogen (Cat# 12990). Briefly, cells were seeded in 6-well and 96-well plates and transfected at 40% confluency with siRNA duplex (20 nM) using lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s protocol. Cells transfected with Stealth RNAi negative control duplex (Invitrogen) were used as controls for direct comparison. After 24 h of transfection, cells were treated with DMSO or quercetin for the indicated times, after which they were processed for Western blotting or MTT assay.
Caspase-3/7 activity
Activation of caspase-3/7 was quantified with the ApoTox-Glo™ Triplex Assay kit according to the manufacturer’s protocol (Promega). Cells were seeded onto 96-well microtiter plates and 24 h later transfected with an Nrf2-specific siRNA duplex before quercetin was added for up to 48 h. Cells were incubated with substrate containing Caspase-Glo® 3/7 assay buffer for 30 min. Caspase-3/7 activities were calculated after detection of luminescence using a GloMax-Multi Microplate Multi-mode Reader (Promega, USA). The results were expressed as percentages, based on the ratios of the luminescence of the treated cells to that of the controls (100%).
Apoptosis assay
The apoptotic cell distribution was determined using Muse™ Annexin V & Dead Cell kit according manufacturer’s protocol (Merck Milipore, Germany). Briefly, cells were collected by centrifugation, mixed with the Muse™ Annexin V and Dead Cell reagent, and analyzed using a Muse Cell Analyzer (Merck Milipore).
Cell cycle analysis
Trypsinized cells were pelleted by centrifugation, fixed in 70% ice-cold ethanol overnight at −20°C, and incubated with Muse™ Cell Cycle reagent (Merck Milipore). DNA contents were analyzed using a MACSQuant Analyzer (Miltenyi Biotec GmbH, Germany) according to fluorescence of propidium iodide (PI).
Measurement of intracellular ROS levels
Intracellular ROS levels were measured using DCF-DA. Briefly, cells were loaded with serum-free medium containing 10 μM of DCF-DA for 30 min at 37°C in the dark. Next, the cells were washed twice with PBS, trypsinised, resuspended, and then immediately analyzed using a MACSQuant Analyzer (Miltenyi Biotec GmbH). A 530 nm bandpass filter was used to detect DCF-DA fluorescence, and each determination was based on the mean fluorescence intensity of 10,000 cells.
Statistical analysis
Statistical comparisons were performed using one-way analysis of variance (ANOVA) followed by a Tukey’s post hoc correlation for multiple comparisons using SPSS version 17.0 (SPSS, Inc., USA). Data were expressed as the mean ± SEM. Significant differences were considered with values of p < 0.05.
RESULTS
Quercetin induces upregulation of Nrf2 expression at both mRNA and protein levels
As an initial approach in determining effective doses for the treatment of MM cells with quercetin, a dose- and time-response study was carried out using the MTT assay. The results revealed a concentration-dependent decrease in cell viability due to treatment with quercetin. At concentrations ≥ 20 μM, quercetin significantly decreased cell viability of both MSTO-211H and H2452 cells (Fig. 1A). Western blot analysis showed that an increase in the Nrf2 level was first observed at 2 h incubation with 20 μM quercetin and remained upregulated at longer incubation (Fig. 1B). However, the treatment of cells with quercetin at toxic doses ≥ 60 μM did not influence on the Nrf2 levels compared to untreated controls (Fig. 1C). Based on this observation, 40 μM was regarded as a subtoxic dose at which quercetin caused mild cytotoxicity in MM cells. Concentrations below that were then selected for further study to examine the efficacy of quercetin as an activator of Nrf2. Besides the total levels of Nrf2, the Nrf2-regulated gene product HO-1 was dose-dependently increased in cultures treated with ≤30 μM quercetin (Fig. 2A). To determine whether the upregulation of Nrf2 protein is due to gene expression and if it involves transcriptional activation of its downstream target genes, the levels of Nrf2 and HO-1 transcripts were analyzed by RT-PCR. As shown in Fig. 2B, treatment with quercetin increased the mRNA levels of Nrf2 and its transcription target HO-1 in both types of cells, consistent with the results obtained for the proteins. To evaluate whether quercetin has an effect on Nrf2 stability, the level of Nrf2 polyubiquitination was investigated by immunoprecipitation assay using the anti-Nrf2 antibody followed by Western blotting with anti-ubiquitin antibody, and vice versa. As shown in Fig. 2C, immunoprecipitation revealed approximately 100 kDa band, which remained unchanged among cells cultured with or without quercetin. Next, Nrf2 protein turnover was analyzed via cycloheximide (CHX) chase experiments. As shown in Fig. 2D, the level of Nrf2 protein rapidly declined over 160 min of treatment with CHX alone, or in combination with quercetin. For the decay curve, the half-lives of endogenous Nrf2 were approximately 20.1 min in MSTO-211H and 27.4 min in H2452 cells, and treatment with quercetin caused no delay compared to the cells treated with CHX alone. Collectively, the data suggest that that the quercetin-induced upregulation of Nrf2 in MM cells was accomplished largely at the transcriptional level rather than by the prolongation of protein stability.
Fig. 1.
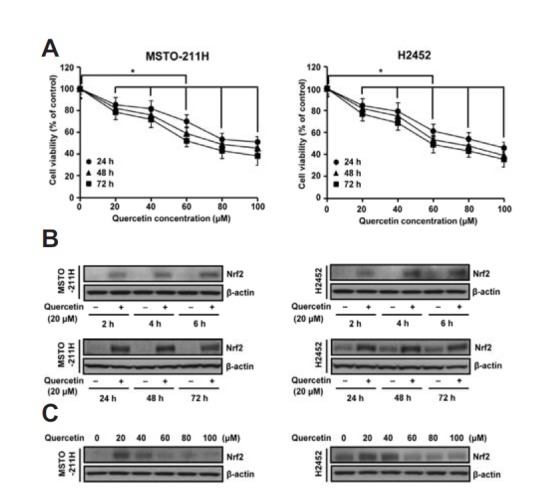
Effects of quercetin treatment on cell viability and Nrf2 levels in MM cells. (A) Cells were treated with various concentrations (0–100 μM) of quercetin for the indicated times. The percentage of viable cells was then determined by MTT assay. (B and C) Cells were treated with quercetin (20 μM) for the short times (2 h, 4 h and 6 h) and the long times (24 h, 48 h, and 72 h) (b), or quercetin (0–100 μM) for 48 h (c). Cell lysates were then analyzed by Western blotting with anti-Nrf2 antibody. Error bars represent the mean ± S.D. for three independent experiments. *P < 0.05 for respective control cells.
Fig. 2.
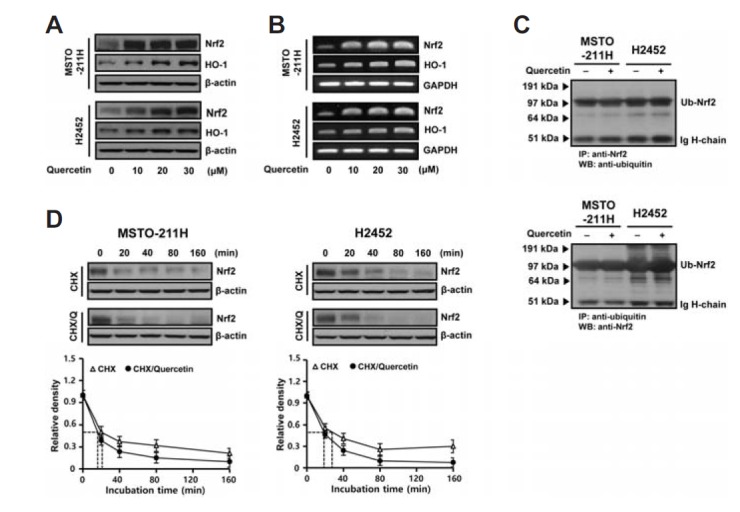
Effects of quercetin treatment on Nrf2 expression in MM cells. Cells were incubated with the indicated concentrations of quercetin for 48 h before the extraction of cell lysates and total RNA for Western blot (A) and RT-PCR (B) analyses, respectively. (C) Cells were treated with quercetin (20 μM) for 48 h before immunoprecipitation of Nrf2 or ubiquitin from cell lysate (500 μg), after which immunoprecipitates were analyzed by Western blotting with the anti-ubiquitin or anti-Nrf2 antibody, respectively. (D) Cells were pretreated with 0.1 μM CHX for 2 h and followed by treatment with or without 20 μM quercetin for varying intervals as indicated. Immunodetection was carried out by using antibodies against Nrf2 and β-actin. Normalized intensity of Nrf2 versus β-actin was presented as the mean value from two independent experiments. Q, quercetin; Ub, ubiquitnated; IP, immunoprecipitation; WB, Western blotting; CHX, cycloheximide.
Quercetin enhances transactivation of Nrf2
Increase in the nuclear localization of Nrf2 protein is usually considered as a marker of Nrf2 activation in response to stressors. As shown in Fig. 3A, quercetin treatment increased the nuclear accumulation of Nrf2 protein at both early (3 h) and late time points (48 h), whereas signals of the cytoplasmic Nrf2 band were only faintly detected in both types of cells. Next, the specificity of Nrf2 DNA binding was assessed in nuclear extracts using an anti-human Nrf2 antibody. As shown in Fig. 3B, quercetin treatment increased the binding activity of Nrf2 toward oligonucleotides containing the ARE consensus binding site (5′-GTCACAGTGACTCAGCAGAATCTG-3′), which correlated with the results of transient transfection of cells with a firefly luciferase reporter gene driven by the upstream Nrf2 responsive element (Nrf2RE-luc) (Fig. 3C). To further determine a possible role of the elevated Nrf2 protein induced by quercetin, the effects of inhibiting Nrf2 expression using an siRNA-based technique were evaluated. Transfection with Nrf2-targeted siRNA repressed not only the transcript levels of Nrf2 and Nrf2-regulated gene HO-1 but also their upregulated levels by quercetin (Fig. 3D). Similar patterns were observed in Western blot analysis of Nrf2 protein (Fig. 3E). Moreover, the siRNA-mediated inhibition of Nrf2 led to a significant decrease in the quercetin-induced binding of Nrf2 to ARE, as compared to those of control siRNA (Fig. 3F). Based on these results, the nuclear increase in Nrf2 was confirmed to be functional.
Fig. 3.
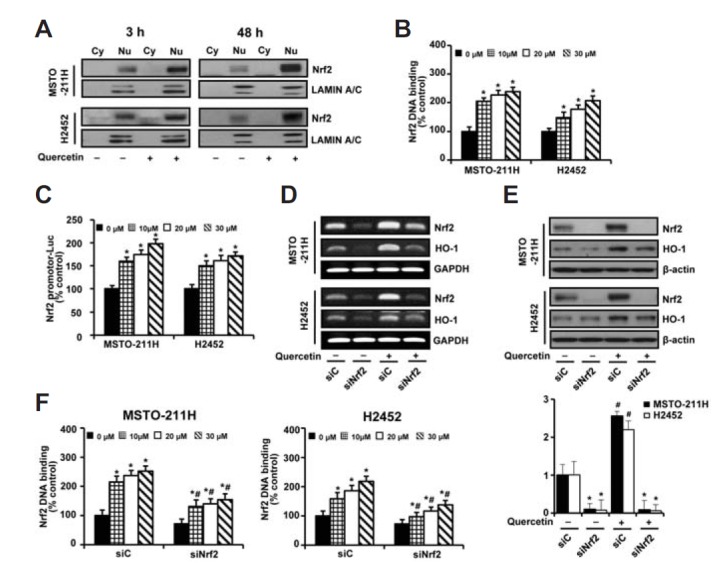
Functional activity of Nrf2 in MM cells. (A) Cells were treated with quercetin (20 μM) of quercetin for 3 h and 48 h. Nuclear and cytoplasmic extracts were analyzed by Western blotting with antibodies against Nrf2 and lamin A/C. (B) Cells were treated with various concentrations (0–30 μM) of quercetin for 48 h. Nuclear extracts were processed by the DNA binding assay of Nrf2. (C) Cells were transfected with a firefly luciferase reporter gene, driven by the upstream Nrf2 response element (Nrf2RE-luc), for 24 h prior to incubation with various concentrations (0–30 μM) of quercetin for another 48 h. Afterwards, the luciferase activity in the cell lysates was determined. (D–F) Cells were transfected with 10 nM Nrf2-targeting siRNA (siNrf2) or Stealth RNAi control (siC) for 24 h. Cells were incubated with quercetin (20 μM) for 48 h prior to RT-PCR (d) and Western blot (e) analyses. Nuclear extracts were processed by the DNA binding assay of Nrf2 (f). Error bars represent the mean ± S.D. for three independent experiments. *P < 0.05 for respective control cells. #P < 0.05 for siNrf2-transfected cells vs. respective siC-transfected cells.
Nrf2 silencing increases apoptosis dependently of caspase activation
To determine the importance of Nrf2 upregulation on cell viability and apoptosis, cells were transfected with Nrf2-targeting siRNA (siNrf2), and then the viability of MM cells and their sensitivity to quercetin was examined. The degree of inhibition of cell viability was similar in both cell types. As shown in Fig 4A, treatment of cells with siNrf2 alone or in combination with quercetin (20 μM) decreased cell viability, respectively, to approximately 85.9% or 70.4% in MSTO-211H cells and 88% or 73.8% in H2452 cells, compared with those of control siRNA alone. However, the degree of inhibition by quercetin (20 μM) treatment during Nrf2 knockdown (13.4% in MSTO-211H cells and 12% in H2452 cells) were similar with those of siNrf2 alone (14% and 11%, respectively), compared to respective control siRNA groups.
Fig. 4.
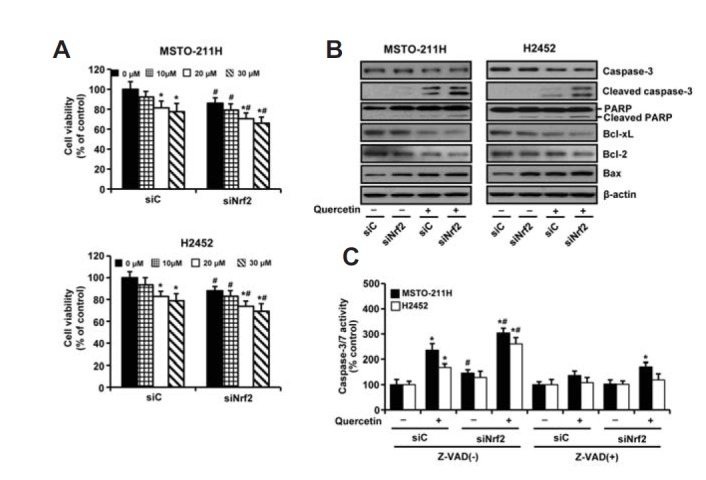
Effects of Nrf2 knockdown on querectin-induced cytotoxicity in MM cells. Cells were transfected with 10 nM of Nrf2-targeting siRNA (siNrf2) or Stealth RNAi control (siC) for 24 h. (A–B) Cells were then incubated with the indicated concentrations of quercetin for 48 h, after which cell viability and the levels of proapoptotic and antiapoptotic proteins were measured by MTT assay (a) and Western blot analysis (b), respectively. (C) Cells were treated with quercetin (20 μM) for 48 h in the presence or absence of Z-VAD (10 μM) prior to the measurement of caspase-3/7 activity. The blot was then stripped and reprobed with an anti-β-actin antibody as a loading control. Error bars represent the mean ± S.D. for three independent experiments. *P < 0.05 for respective control cells. #P < 0.05 for siNrf2-transfected cells vs. respective siC-transfected cells.
To determine whether the reduction of cell viability during Nrf2 knockdown was related to apoptosis, we measured the levels of the regulatory proteins associated with apoptosis. An increase in the level of proapoptotic Bax, enhanced cleavage of caspase-3 and PARP proteins, and a decrease in the level of antiapoptotic Bcl-2 and Bcl-xL by Western blotting indicated that inhibition of cell viability was, at least in part, due to the induction of apoptosis, which was most pronounced in the combined treatment of siNrf2 and quercetin (Fig 4B). Consistently, these responses were accompanied with an increase in the caspase-3/7 activity, which could be suppressed by the pan-caspase inhibitor Z-VAD (Fig. 4C). These observations were similar in both cell types.
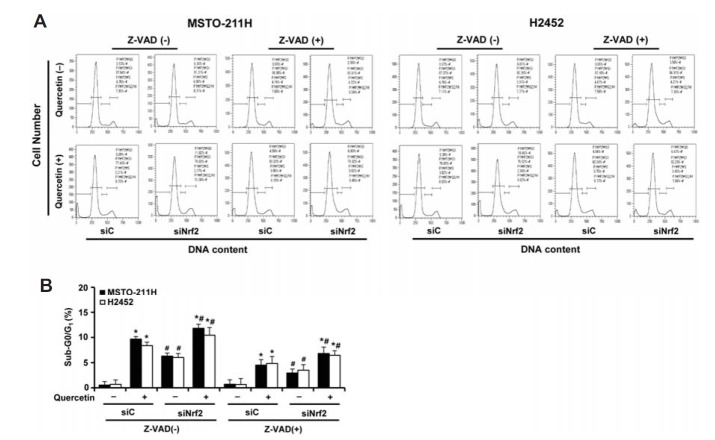
When only the attached cells were analyzed by flow cytometry, the occurrence of a sub-G0/G1 peak, indicative of apoptosis, was detected in cells treated with quercetin (20 μM) alone, siNrf2 alone or in combination with quercetin, respectively, to approximately 9.2%, 5.8% or 11.3% in MSTO-211H cells and 7.9%, 5.3% or 11.3% in H2452 cells, respectively, after correcting for baseline apoptosis in that of control siRNA (Fig. 5). These observations could be suppressed by the pan-caspase inhibitor Z-VAD and were similar in both cell types.
Fig. 5.
Effects of Nrf2 knockdown and pan-caspase inhibitor on cell distribution at sub-G0/G1, G1, S, and G2/M phases in quercetin-treated MM cells. Cells were transfected with 10 nM Nrf2-targeting siRNA (siNrf2) or Stealth RNAi control (siC) for 24 h, after which cells were incubated with quercetin (20 μM) for 48 h in the presence or absence of Z-VAD (10 μM). (A) Cells were then analyzed using a flow cytometry after staining with propidium iodide (20 μg/ml). The percentages of cell population in sub-G0/G1 phase were calculated from three independent experiments. Representative results were obtained from one of three independent experiments. (B) The quantitative data are shown as mean ± S.D. *P < 0.05 for Z-VAD-treated cells vs. respective control cells. #P < 0.05 for siNrf2-transfected cells vs. respective siC-transfected cells.
In annexin V binding assay, treatment of cells with quercetin alone, siNrf2 alone or in combination with quercetin (20 μM) significantly elevated the percentage of apoptotic propensities, respectively, to 18.68%, 14.06% or 28.58% in MSTO-211H cells and 17.54%, 13.42% or 25.54% in H2452 cells, compared with those of control siRNA alone (0.74% and 1%, respectively) (Fig. 6). These observations were significantly restored, but not completely, by the presence of Z-VAD, which were similar in both cell types. Collectively, these data indicate that Nrf2 silencing increases apoptosis in a caspase-dependent manner, further supporting the antiapoptotic role of Nrf2 protein in MM cells.
Fig. 6.
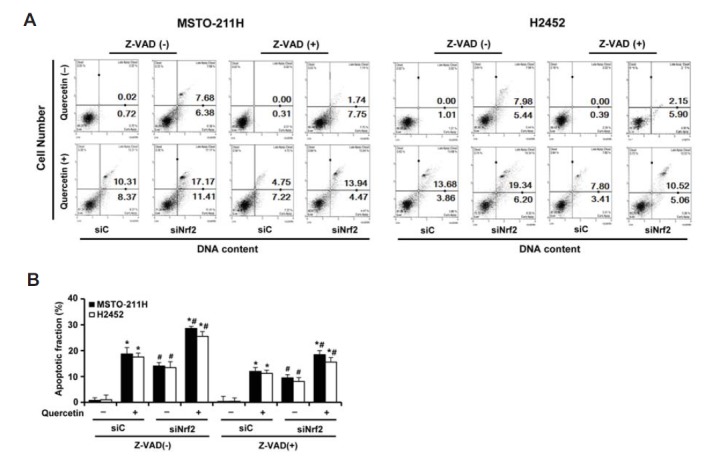
Effects of Nrf2 knockdown and pan-caspase inhibitor on quercetin-induced apoptosis in MM cells. Cells were transfected with 10 nM Nrf2-targeting siRNA (siNrf2) or Stealth RNAi control (siC) for 24 h, after which cells were incubated with quercetin (20 μM) for 48 h in the presence or absence of Z-VAD (10 μM). (A) The percentage of apoptotic cells after annexin V binding was analyzed by a Muse cell analyzer. (B) The quantitative data are shown as mean ± S.D. for three independent experiments. *P < 0.05 for Z-VAD-treated cells vs. respective control cells. #P < 0.05 for siNrf2-transfected cells vs. respective siC-transfected cells.
Nrf2 silencing sensitizes MM cells to cisplatin
To examine the specific role of Nrf2 in the control of drug resistance, MSTO-211H and H2452 cells were transfected with Nrf2-targeted siRNA for 24 h, followed by treatment with cisplatin. As shown in Fig. 7A, exposure of cells to increasing concentrations of cisplatin for 72 h inhibited cell viability with an average IC50 of 76.1 μM in MSTO-211H, whereas H2452 cells were much more resistant to the same concentrations of cisplatin. However, Nrf2 knockdown led to a significant decrease in cell survival, as compared with cells treated with cisplatin after transfection with control siRNA. These results suggest that Nrf2 activation plays an important role in enhancing cell survival against cisplatin-mediated cytotoxicity in MM cells. Next, the antioxidative role of Nrf2 in the generation of ROS by cisplatin treatment was examined using a flow cytometry. As indicated in the representative histogram, an increase in the ROS levels was indicated as a shift of cellular DCF fluorescence to the right. As shown in Fig. 7B, treatment with 80 μM cisplatin alone for 24 h increased ROS production from approximately 0.14% to 34% in MSTO-211H cells and from 0.4% to 9% in H2452 cells, and they were augmented following Nrf2 knockdown to 45.9% and 33.7%, respectively. Collectively, these data indicate that decreased Nrf2 activity further increases the amount of cellular ROS generated by cisplatin, thus strengthening its effectiveness to kill the MM cells.
Fig. 7.
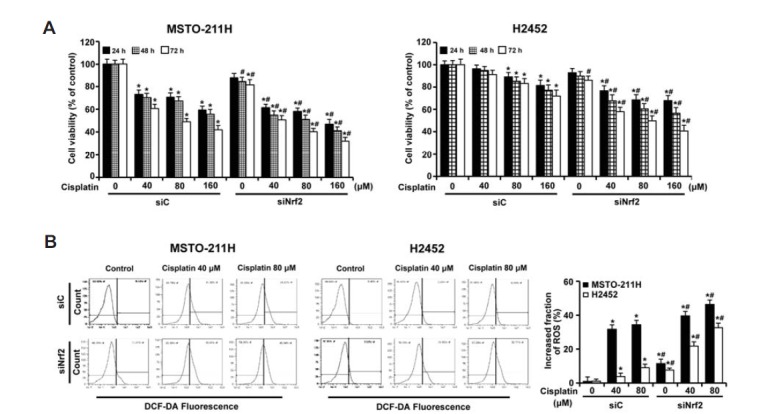
Effects of Nrf2 knockdown on cisplatin-induced cytotoxicity in MM cells. Cells were transfected with 10 nM Nrf2-targeting siRNA (siNrf2) or Stealth RNAi control (siC) for 24 h. (A) Cells were incubated with the indicated concentrations of cisplatin for the indicated times, after which cell viability was measured by MTT assay. Error bars represent the mean ± SEM for three independent experiments. *P < 0.05 for respective control cells. #P < 0.05 for siNrf2-transfected cells vs. respective siC-transfected cells. (B) Cells were incubated with cisplatin (0, 40, and 80 μM) for 24 h, after which the levels of cellular ROS were measured using a flow cytometry after staining with DCF-DA (10 μM). A shift of DCF fluorescence to the right indicates an increase of ROS. The quantitative data in the ROS levels were presented as the mean value from three independent experiments. The quantitative data are shown as mean ± S.D. *P < 0.05 for cisplatin-treated cells vs. respective control cells. #P < 0.05 for siNrf2-transfected cells vs. respective siC-transfected cells.
DISCUSSION
In contrast to the growth-inhibiting and apoptosis-inducing effects of quercetin in various cancer cell types, it is also known to protect cells against oxidative damage by inducing the expression of antioxidant proteins involved in scavenging ROS. The apparent opposite double action of quercetin has been shown to be presumably dependent on the concentration and duration of exposure; nonetheless, the mechanism(s) of these multiple effects have not yet been clearly determined. Among the different molecules produced intracellularly, the transcription factor Nrf2 plays a vital role in the prevention of cell dysfunction in response to oxidative stress and in protection against exposure to toxins and carcinogens through the ARE-mediated expression of a battery of cytoprotective genes (Lee et al., 2014). We present here the first evidence that the quercetin-induced activation of Nrf2 may provide an advantage for MM cell survival. At toxic doses ≥ 60 μM leading to cytotoxicity, quercetin did not influence on the Nrf2 levels, however, quercetin at subtoxic doses elicited evident accumulation of Nrf2 in the nucleus, along with upregulation of Nrf2 on both the mRNA and protein levels, In turn, this led to the increased affinity of Nrf2 for binding to its consensus sequence, and, consequently, enhanced expression of the ARE-driven reporter genes. These responses could be antagonized by Nrf2 knockdown using an siRNA-based technique.
An increase in the nuclear localization of Nrf2 protein, enhancement in its DNA binding and reporter gene activities, as well as corresponding up-regulation of a well-known transcriptional target of Nrf2 protein, HO-1, were prominent in quercetin-treated MM cells. The overall response indicated that the quercetin-induced expression of Nrf2 protein was transcriptionally active in both MM cell lines. Increased production of Nrf2 protein or its increased stability can contribute to the increased activity of Nrf2 as a transcriptional factor. We previously observed that MSTO-211H cells have the potential to regulate the levels of Nrf2 at multiple steps, including at the levels of transcription, protein synthesis, and posttranslation (Lee et al., 2012). In the present study, quercetin treatment induced the concomitant increase of total cellular amounts of Nrf2 protein with its nuclear accumulation, whereas untreated cells had low levels of Nrf2 protein. Moreover, no difference on the ubiquitination of Nrf2 was noticed among cells cultured with or without quercetin. Thrower et al. reported that the binding of four or more ubiquitin monomers to substrate serves as a signal for efficient targeting to the proteasome (Thrower et al., 2000). The size (∼100 kDa) of ubiquitinated Nrf2 molecule was consistent with the addition of four ubiquitin moieties to Nrf2 (67 kDa). According to the decay curve, quercetin treatment caused no apparent delay in the half-life of endogenous Nrf2 protein. In particular, it is also important to note that the expression of Nrf2 mRNA correlated with the expression of the encoded protein. In this regard, the stability of the Nrf2 protein was unlikely to be relevant to the quercetin-induced upregulation. Therefore, the nuclear accumulation of Nrf2 at later time points after quercetin treatment mainly appears to be due to an increase in its de novo synthesis, although quercetin-induced activation of the existing Nrf2 may lead to an initial elevation of Nrf2 in the nucleus.
Nrf2 activation plays a potential role in tumor cell growth and survival, whereas its depletion contributes to activation of the intrinsic apoptotic pathway (Ji et al., 2013). Recently, the potential of Nrf2 to induce Bcl-2 protein has been demonstrated to be associated with decreased apoptosis, cancer cell survival, and drug resistance (Niture and Jaiswal, 2013). Consistent with these observations, gene silencing of Nrf2 with siRNA induced spontaneous apoptosis of MM cells, which was accompanied by downregulation of the antiapoptotic protein Bcl-2 and upregulation of the proapoptotic protein Bax. Activation of procaspases plays an important role in the classical apoptosis pathway. Of these, the cleavage of caspase-3 and its substrate PARP serve as a biochemical hallmark of apoptosis. In this study, quercetin treatment significantly increased the caspase-3/7 activity, along with the resultant cleavage of caspase-3 and PARP, the appearance of a sub-G0/G1 peak in DNA flow cytometric assay, and the increased percentage of apoptotic propensities in the annexin V binding assay. In addition, the observed apoptosis was effectively reversed when the cells were pretreated with the pan-caspase inhibitor Z-VAD. These findings indicate that the apoptosis-inducing effect of quercetin proceeded dependently of caspase activation. Although the inhibition of percentage viability by quercetin (20 μM) treatment during Nrf2 knockdown was similar with that of siNrf2 alone, compared to respective control siRNA group, Nrf2 knockdown augmented the apoptosis caused by quercetin. This finding suggests that quercetin-induced upregulation of Nrf2 expression could contribute to inhibiting apoptosis rather than promoting MM cell proliferation. Thus, the apoptosis-inducing effects incurred by quercetin treatment may be counteracted as a result of activation of the Nrf2/ARE pathway. These data could be contradictory regarding quercetin’s ability to induce MM cell death, which apparently would be Nrf2-independent. Given the complexity of physiologic functions of quercetin, it is likely that quercetin regulates various molecular pathways at a different dose-response reaction in mediating this cytotoxic effect. The effect of quercetin like a double-edged sword may be due to its unique characteristic, in that it behaves as an antioxidant and/or pro-oxidant depending on the concentration and duration of exposure. Moreover, quercetin regulates the expression of numerous genes and mediates the effects through its direct targets and their crosstalks with other pathways in cells depending on cell type and cell context. Further studies are needed to address the precise mode of the multiple action of quercetin for inducing cell death through apoptotic signals or favoring cell protection via Nrf2 induction in MM cells.
The cytoprotective roles of Nrf2 may confer protection against carcinogensis; however, new emerging data has revealed the deleterious effects of the Nrf2 signal, such as advantages for growth and resistance to chemotherapy in cancer cells (Lau et al., 2008). Nrf2 can protect cancer cells from the oxidative damage induced by chemotherapeutic drugs and irradiation (Zhang et al., 2010). Merikallio et al reported that strong expression of Nrf2 was associated with poor survival in lung tumors (Merikallio et al., 2012). Yang et al. suggested the role of Nrf2 as a potential marker for the prediction of chemoresistance and tumor progression in patients with advanced-stage non-small-cell lung cancer (Yang et al., 2011). Given this significant role of Nrf2 in the resistance of MM cells against apoptosis, the inhibition of Nrf2 may provide an effective tool to overcome resistance and sensitize MM cells to anticancer therapy. It has been shown that cisplatin, one of the standard drugs in chemotherapy for MM patients, causes significant oxidative stress via the generation of ROS as one of its anticancer mechanisms (Chung et al., 2014). This is consistent with the data of our study. The suppression of Nrf2 production with Nrf2-specific siRNA in combination with cisplatin treatment proved to be much more effective in potentiating cytotoxicity and enhancing the levels of ROS than treatment of cisplatin alone, particularly in H2452 cells showing resistance to cisplatin. This finding indicates that the toxic effect of cisplatin is associated with ROS production, and suggests that Nrf2 activation may contribute to a reduced response to conventional chemotherapy in MM cells. In this regard, it seems that overproduction of Nrf2 protein has a beneficial effect for the protection of normal cells from harmful stimuli; however, its presence in cancer patients may be an untoward situation that favors the survival of cancer cells, potentially making tumor cells more refractory to treatment by undermining the free radical mechanism of existing chemotherapy. Previous studies have shown that an association exists between the coordinated overexpression of Nrf2-regulated proteins and resistance to chemotherapeutic drugs. As an example, the overexpression of MRP and gamma-glutamylcysteine synthetase has been reported to be correlated with doxorubicin resistance in MM cells (Ogretmen et al., 1998), and increases in HO-1 and NQO1 have been associated with resistance to cisplatin in ovarian carcinoma (Bao et al., 2014). It has been recently reported that acquired resistance of human breast cancer MCF-7/DOX cells to doxorubicin was linked to increases in the endogenous expression of Nrf2 and Nrf2-regulated genes (Zhong et al., 2012). They found that treatment with wogonin, one of active flavonoids isolated from the root of Scutellaria baicalensis Georgi, diminished the amount of total and nuclear Nrf2 and restored sensitivity to doxorubicin. These observations support a critical role of Nrf2 in overcoming the acquisition of drug resistance.
In conclusion, our data demonstrated a significance of Nrf2 targeting as a promising strategy for inducing oxidative stress and MM cell killing, and this strategy represents a potential efficient way to overcome resistance to some chemotherapeutic drugs and enhance the efficacy of these compounds in MM. Nrf2 may be a determining factor for the sensitivity of some tumors to various chemotherapeutic agents. Therefore, the development of an evidence-based guidance on antioxidant supplementation in cancer therapy through further assessment for their efficacy and safety are worth to be carried out in relevant animal models or human study, in order to obtain an optimal therapeutic output.
Acknowledgments
This research was supported by the Soonchunhyang University Research Fund (No. 20130608) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. NRF-2012R1A1A4A01014255).
REFERENCES
- Bao LJ, Jaramillo MC, Zhang ZB, Zheng YX, Yao M, Zhang DD, Yi XF. Nrf2 induces cisplatin resistance through activation of autophagy in ovarian carcinoma. Int. J. Clin. Exp. Pathol. 2014;7:1502–1513. [PMC free article] [PubMed] [Google Scholar]
- Borska S, Chmielewska M, Wysocka T, Drag-Zalesinska M, Zabel M, Dziegiel P. In vitro effect of quercetin on human gastric carcinoma: targeting cancer cells death and MDR. Food Chem. Toxicol. 2012;50:3375–3383. doi: 10.1016/j.fct.2012.06.035. [DOI] [PubMed] [Google Scholar]
- Ceccarelli J, Delfino L, Zappia E, Castellani P, Borghi M, Ferrini S. The redox state of the lung cancer microenvironment depends on the levels of thioredoxin expressed by tumor cells and affects tumor progression and response to prooxidants. Int. J. Cancer. 2008;123:1770–1778. doi: 10.1002/ijc.23709. [DOI] [PubMed] [Google Scholar]
- Chung TW, Choi HJ, Kim SJ, Kwak CH, Song KH, Jin UH, Chang YC, Chang HW, Lee YC, Ha KT, et al. The ganglioside GM3 is associated with cisplatin-induced apoptosis in human colon cancer cells. PLoS One. 2014;9:e92786. doi: 10.1371/journal.pone.0092786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraresi R, Troiano L, Roat E, Lugli E, Nemes E, Nasi M, Pinti M, Fernandez MI, Cooper EL, Cossarizza A. Essential requirement of reduced glutathione (GSH) for the anti-oxidant effect of the flavonoid quercetin. Free Radic. Res. 2005;39:1249–1258. doi: 10.1080/10715760500306935. [DOI] [PubMed] [Google Scholar]
- Granado-Serrano A, Martin M, Bravo L, Goya L, Ramos S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-Kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2) J. Nutr. 2006;136:2715–2721. doi: 10.1093/jn/136.11.2715. [DOI] [PubMed] [Google Scholar]
- Jiang T, Chen N, Zhao F, Wang XJ, Kong B, Zheng W, Zhang DD. High levels of Nrf2 determine chemoresistance in type II endometrial cancer. Cancer Res. 2010;70:5486–5496. doi: 10.1158/0008-5472.CAN-10-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Xiao S, Khan MA, Xue M. Defective anti-oxidant systems in cervical cancer. Tumour Biol. 2013;34:2003–2009. doi: 10.1007/s13277-013-0804-1. [DOI] [PubMed] [Google Scholar]
- Ji XJ, Chen SH, Zhu L, Pan H, Zhou Y, Li W, You WC, Gao CC, Zhu JH, Jiang K, et al. Knockdown of NFE2-related factor 2 inhibits the proliferation and growth of U251MG human glioma cells in a mouse xenograft model. Oncol. Rep. 2013;30:157–164. doi: 10.3892/or.2013.2476. [DOI] [PubMed] [Google Scholar]
- Khanduja KL, Gandhi RK, Pathania V, Syal N. Prevention of N-nitrosodiethylamine-induced lung tumorigenesis by ellagic acid and quercetin in mice. Food Chem. Toxicol. 1999;37:313–318. doi: 10.1016/s0278-6915(99)00021-6. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Miyoshi Y, Taguchi T, Tamaki Y, Nakamura H, Yodoi J, Kato K, Noguchi S. High thioredoxin expression is associated with resistance to docetaxel in primary breast cancer. Clin. Cancer Res. 2005;11:8425–8430. doi: 10.1158/1078-0432.CCR-05-0449. [DOI] [PubMed] [Google Scholar]
- Kim YR, Oh JE, Kim MS, Kang MR, Park SW, Han JY, Eom HS, Yoo NJ, Lee SH. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J. Pathol. 2011;220:446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol. Res; 2008. pp. 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Jeong HY, Kim YB, Lee YJ, Won SY, Shim JH, Cho MK, Nam HS, Lee SH. Reactive oxygen species and PI3K/Akt signaling play key roles in the induction of Nrf2-driven heme oxygenase-1 expression in sulforaphane-treated human mesothelioma MSTO-211H cells. Food Chem. Toxicol. 2012;50:116–123. doi: 10.1016/j.fct.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Lee HS, Lee GS, Kim SH, Kim HK, Suk DH, Lee DS. Anti-oxidizing effect of the dichloromethane and hexane fractions from Orostachys japonicus in LPS-stimulated RAW 264.7 cells via upregulation of Nrf2 expression and activation of MAPK signaling pathway. BMB Rep. 2014;47:98–103. doi: 10.5483/BMBRep.2014.47.2.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Sun C, Zhou B, Xing H, Ma D, Chen G, Weng D. Low concentration of quercetin antagonizes the cytotoxic effects of anti-neoplastic drugs in ovarian cancer. PLoS One. 2014;9:e100314. doi: 10.1371/journal.pone.0100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikallio H, Pääkkö P, Kinnula VL, Harju T. Nuclear factor erythroid-derived 2-like 2 (Nrf2) and DJ1 are prognostic factors in lung cancer. Hum. Pathol. 2012;43:577–584. doi: 10.1016/j.humpath.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Niture SK, Jaiswal AK. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radic. Biol. Med. 2013;57:119–131. doi: 10.1016/j.freeradbiomed.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley TD, Oberley LW. Antioxidant enzyme levels in cancer. Histol. Histopathol. 1997;12:525–535. [PubMed] [Google Scholar]
- Ogretmen B, Bahadori HR, McCauley MD, Boylan A, Green MR, Safam AR. Co-ordinated over-expression of the MRP and gamma-glutamylcysteine synthetase genes, but not MDR1, correlates with doxorubicin resistance in human malignant mesothelioma cell lines. Int. J. Cancer. 1998;75:757–761. doi: 10.1002/(sici)1097-0215(19980302)75:5<757::aid-ijc15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- Qu LY, Gao P, Wang HY, Wang XJ, Tang XW. Nrf2 down-regulated cell line H460-N5 with Keap1 overexpression increased sensitivity to anti-cancer drugs. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010;39:6–10. doi: 10.3785/j.issn.1008-9292.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Robaszkiewicz A, Balcerczyk A, Bartosz G. Anti-oxidative and prooxidative effects of quercetin on A549 cells. Cell Biol. Int. 2007;31:1245–1250. doi: 10.1016/j.cellbi.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Samuel T, Fadlalla K, Mosley L, Katkoori V, Turner T, Manne U. Dual-mode interaction between quercetin and DNA-damaging drugs in cancer cells. Anticancer Res. 2012;32:61–71. [PMC free article] [PubMed] [Google Scholar]
- Saw CL, Guo Y, Yang AY, Paredes-Gonzalez X, Ramirez C, Pung D, Kong AN. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014;72:303–311. doi: 10.1016/j.fct.2014.07.038. [DOI] [PubMed] [Google Scholar]
- Sharma M, Rajappa M, Kumar G, Sharma A. Oxidant-antioxidant status in Indian patients with carcinoma of posterior one-third of tongue. Cancer Biomark. 2009;5:253–260. doi: 10.3233/CBM-2009-0110. [DOI] [PubMed] [Google Scholar]
- Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, Hirohashi S. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135:1358–1368. doi: 10.1053/j.gastro.2008.06.082. [DOI] [PubMed] [Google Scholar]
- Shim GS, Manandhar S, Shin DH, Kim TH, Kwak MK. Acquisition of doxorubicin resistance in ovarian carcinoma cells accompanies activation of the NRF2 pathway. Free Radic. Biol. Med. 2009;47:1619–1631. doi: 10.1016/j.freeradbiomed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Slocum SL, Kensler TW. Nrf2: control of sensitivity to carcinogens. Arch. Toxicol. 2011;85:273–284. doi: 10.1007/s00204-011-0675-4. [DOI] [PubMed] [Google Scholar]
- Stacy DR, Ely K, Massion PP, Yarbrough WG, Hallahan DE, Sekhar KR. Increased expression of nuclear factor E2 p45-related factor 2 (NRF2) in head and neck squamous cell carcinomas. Head Neck. 2006;28:813–818. doi: 10.1002/hed.20430. [DOI] [PubMed] [Google Scholar]
- Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang W, Zhang Y, Zhao J, Lin E, Gao J, He J. The role of NF-E2-related factor 2 in predicting chemoresistance and prognosis in advanced non-small-cell lung cancer. Clin. Lung Cancer. 2011;12:166–171. doi: 10.1016/j.cllc.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, Bodas M, Wu H, Bova SG, Biswal S. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol. Cancer Ther. 2010;9:336–346. doi: 10.1158/1535-7163.MCT-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Zhang F, Sun Z, Zhou W, Li ZY, You QD, Guo QL, Hu R. Drug resistance associates with activation of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by down-regulating Nrf2-mediated cellular defense response. Mol. Carcinog. 2012;52:824–834. doi: 10.1002/mc.21921. [DOI] [PubMed] [Google Scholar]