Abstract
Interleukin-34 (IL-34) is a cytokine consisting of a 39kD homodimer, shown to be a ligand for both the Macrophage Colony Stimulating Factor (M-CSF/CSF-1) receptor and the Receptor-like protein tyrosine phosphatase-zeta (RPTP-ƺ). IL-34 has been shown to promote monocyte viability and proliferation as well as the differentiation of bone marrow cells into macrophage progenitors. Published work on IL-34 involves its effects on normal hematopoietic and osteoclast progenitors. However, it is not known whether IL-34 has biologic effects in cancer, including leukemia. Here we report that the biological effects of IL-34 include induction of differential expression of Interleukins-1α and -1β as well as induction of differentiation of U937, HL-60 and THP-1 leukemia cell lines demonstrating monocyte-like characteristics. The ability of IL-34 to induce monocytic-like differentiation is supported by strong morphological and functional evidence. Cell surface markers of myeloid lineage, CD64 and CD86, remain constant while the levels of CD11b and CD71 decline with IL-34 treatment. IL-34 also induced increases in CD14 and CD68 expression, further supporting maturation toward monocytic character. IL-34-induced differentiated U937 and THP-1 cell lines exhibited biological functions such as endocytosis and respiratory burst activities. Collectively, we conclude that while IL-34 does not induce cell growth or proliferation, it is able to induce differentiation of leukemia cell lines from monoblastic precursor cells towards monocyte- and macrophage-like cells, mediated through the JAK/STAT and PI3K/Akt pathways. To our knowledge, this is the first report that IL-34 induces differentiation in human leukemic cells, let alone any cancer model.
Keywords: Interleukin-34, IL-34, differentiation, JAK/STAT, PI3K/Akt, leukemia
Introduction
Interleukin-34 (IL-34) is a cytokine identified by Lin et. al. in 2008 [1] as a 39kDA homodimer. It is comprised of 241 amino acids and employs the c-FMS receptor and Receptor-like protein tyrosine phosphatase-zeta (RPTP-ƺ) [1-3]. Since then, a myriad of research and clinical studies have characterized the biological effects as well as associated diseases in which IL-34 is suspected to play significant roles. IL-34 is most abundantly expressed in the spleen. It is also found in the heart, brain, liver, kidney, thymus, testes, ovary, small intestine, prostate, and colon [1,2,4,5], which may provide insight into its potential importance in immunity. IL-34 has also been associated with a number of inflammatory diseases including rheumatoid arthritis [5], equine encephalitis [6], and Sjogren’s syndrome [7]. Hence, it has been implicated as a pro-inflammatory cytokine [5,8-10].
While IL-34 shares a receptor and some functional redundancy with M-CSF, Felix et al. has shown that the IL-34 ligand interacts with the c-FMS receptor differently than M-CSF, which may influence some of the independent physiological effects of IL-34 [1,4,8,11].
Thus far the biologic effects of IL-34 on normal hematopoietic cells and osteoclast progenitors have been well characterized. However, it is unknown whether IL-34 has biologic effects in leukemia cells. Because of the known effects of IL-34 on monocytes and macrophages as reported by Lin et. al others [1,2,4,5], IL-34 is an attractive ligand to evaluate in U937, HL-60 and THP-1 cell lines, all of which are models of acute myeloid leukemia. This prompted us to investigate its effects on cell proliferation and differentiation. Here we report that IL-34 elicits differentiation of U937, HL-60 and THP-1 cell lines to morphological and functional characteristics typically seen in monocytes while inducing Interleukin-1 expression. We also provide evidence that these changes may be mediated through the JAK/STAT and PI3K/Akt signaling pathways. To our knowledge, this is the first report demonstrating the biological effects of IL-34 in human leukemia cells, suggesting that IL-34 may have effects on other cancer cells.
Materials and methods
Chemicals and reagents
Recombinant human Interleukin-34 (rhIL-34) of 70 kDa was purchased from R&D Systems (Minneapolis, MN, USA). Trypan blue, MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetra-zoliumbromide), dimethyl sulfoxide (DMSO), Dihydrorhodamine 123, Giemsa stain and protease inhibitors (leupeptin and aproteinin) were purchased from Sigma Chemicals (St. Louis, MO). CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) kit was purchased from Promega Corporation (Madison, WI). Customized IL-1α/IL-1β ELISA Assay kit was purchased from Qiagen (Valencia, CA). FITC- Dextran was purchased from Molecular Probes/Invitrogen (Carlsbad, CA). Phorbol 12-myristate 13-acetate (PMA) was purchased from Abcam Biochemicals (Cambridge, MA). Specific antibodies to CD11b-PE, CD14-FITC, CD64, CD71-FITC, control IG1 and FITC were purchased from eBioscience (San Diego, CA). CD68-FITC and CD86-Alexa Fluor antibodies were also purchased from BioLegend (San Diego, CA). Bayer-18 was purchased from B-Bridge International (Cupertino, CA). LY29-4002 was purchased from SelleckChem (Houston, TX). C-FMS/CSF-1R and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-RPTP-ƺ was purchased from BD Transduction Laboratories (San Jose, CA).
Culture of human myeloid leukemia U937, HL-60 and THP-1 cells
The human acute myeloid leukemia U937 cell line was obtained from ATCC and the THP-1 cells were a gift from Dr. Chandravanu Dash originally obtained from ATCC. The HL-60 cells were a gift from Dr. Ann Richmond, originally obtained from ATCC. The cell lines were maintained at 37°C in a 95% air and 5% CO2 incubator and were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FBS (HyClone), 100 units/ml penicillin and 50 units/ml streptomycin (GIBCO) unless otherwise noted.
Trypan blue exclusion assay for cell viability
For Trypan Blue staining, 200 ul of U937 cells were incubated for three minutes with an equal volume of 0.4% (w/v) Trypan Blue solution prepared in deionized water. Cells were then counted in a dual chambered hemacytometer on a light microscope. Viable and non-viable cell counts were used to calculate percent viability. The cell viability for all experiments was 95% or better.
Western blot analysis
Fresh or previously frozen cell pellet from untreated or IL-34 treated U937 and THP-1 cells were thawed and lysed on ice in RIPA buffer containing 50 mM Tris-HCl (pH 7.4), 1% Nonidet P-40, 0.1% sodium deoxycholate, 150 mM NaCl and 1 mM EDTA. To inhibit endogenous proteases and phosphatases, the RIPA buffer was supplemented with P8340, a cocktail of protease inhibitor (10 uM leupeptin) and phosphatase inhibitors (20 mM sodium fluoride, 50 mM β-glycerophosphate, and 1 mM sodium orthovanadate), respectively. Throughout the lysing process the lysates were maintained on ice with intermittent agitation by pipetting. The total lysates were centrifuged at 13,300 rpm for 15 minutes at 4°C to eliminate debris. The clear lysate supernatants were assayed for protein level by the Bradford Protein Assay (Bio-Rad). Next, 20 ug of cell lysate proteins were separated by 4-12% SDS–PAGE (Invitrogen) followed by transfer onto nitrocellulose membranes (Southern Scientific), unless otherwise noted. The efficiency of the transfer was verified by Ponceau staining and destained by extensive washing. To enhance specificity of our western blotting, non-specific binding on the membrane was blocked with 5% BSA (Sigma) in Tris-Buffered Saline containing Tween-20 (TBST). Blots were probed with the c-FMS/CSF-1R or Receptor-like protein tyrosine phosphatase-zeta primary antibody by overnight incubation at 4°C with rocking. Following 4 washes (7 minutes each) with TBST buffer with agitation, the membranes were incubated with HRP-conjugated secondary antibody for 2 hours at room temperature. Finally, the membranes were washed 4 × (7 minutes each) with agitation and protein bands detected using chemiluminescence. The membranes were exposed to film and developed or the bands were analyzed by the Gel Logic docking system and band intensity quantified using Image J (NIH).
Cellular growth and proliferation determination
U937 (1 × 104 in 100 µl) per well were either untreated or treated with 10 ng/ml or 50 ng/ml of IL-34 for up to 48 hours. DMSO was used as the vehicle control.
For the MTT assay, three hours before each time point, 20 µl of MTT solution (5 mg/ml in PBS) was added into each well and incubated at 37°C. The media was removed and 100 µl of DMSO was added into each well. The plate was gently rotated on an orbital shaker for 10 min to completely dissolve the precipitation. The absorbance was detected at 570 nm with a microplate reader. All experiments were repeated three times.
For the MTS assay, the CellTiter 96® AQueous One Solution Cell Proliferation Assay kit was used according to the manufacturer’s instruction. Three hours before each time point, 10 µl of the MTS reagent was added into each well and incubated at 37°C. The absorbance was detected at 490 nm with a microplate reader. All experiments were repeated three times.
Evaluation of IL-1α and IL-1β induction by ELISA assay
Triplicate experiments in six-well plates were set up in which each well contained U937 cells (2 × 106) in 2 ml of serum free media. The wells were either untreated or treated with 50 ng/ml of IL-34 and incubated in the incubator for 6, 12, 24, 48, or 144 hours, respectively. At the end of each time point, the cells in each well were harvested into a 2 ml Eppendorf tube and placed on ice for 10 minutes. Following 2 minutes centrifugation at 4°C at 13, 300 rpm the supernatants were carefully transferred into new tubes. The supernatants were either immediately used for ELISA analysis (according to manufacturer’s instructions) of Interleukin-1α and Interleukin-1β or stored at -20°C for 24 hours and then moved to -80°C for later evaluation by ELISA.
Morphological analysis for evidence of differentiation
U937, HL-60 and THP-1 cell lines (5 × 103) were suspended in 1 ml serum free media in six-well plates. The wells were either untreated or treated with either 10 ng/ml or 50 ng/ml of IL-34 in triplicate experiments and incubated for 6, 24, 48, or 96 hours, respectively. At the end of each time point, cells were harvested and loaded into a Cytopro sample chamber for the Cytocentrifuge (Wescor). The samples with accompanying slides were centrifuged for 10 minutes at 700 rpm. Following centrifugation, the slides were fixed in ice-cold 100% methanol for 5 minutes and allowed to dry at room temperature. The dried cell spots were stained with Giemsa Stain (Sigma) at a 1:20 dilution in deionized water. After staining, slides were washed with deionized water and the stained cells were examined on an Olympus CK2 microscope (Olympus) with a Nikon 40 Ph3DL (Nikon) objective for distinct staining. Pictures of cells were taken using the Olympus CK2 microscope with a DCM200 digital camera and the images captured using the Scopephoto program.
Flow cytometry
FACS analysis of the cell surface expression of monocyte, macrophage, myeloid maturity and myeloid lineage markers, CD11b, CD14, CD64, CD68, CD71 and CD86 was performed in both untreated and IL-34 treated U937, HL-60 and THP-1 cell lines. For this analysis U937, HL-60 and THP-1 cell lines were either untreated or treated with IL-34 (50 ng/ml) for up to 96 hours in serum-free RPMI 1640 supplemented with 1% BSA. As a positive control for differentiation, U937 cells were treated with PMA (50 ng/ml) for 48 hours under same conditions. After incubation, the cells were pelleted, washed and labeled with conjugated antibodies at optimal concentrations as determined by prior titration studies. The expression of the various cell surface markers was analyzed with a Guava easyCyte flow cytometer (Millipore) and analyzed by FlowJo 10 software (Tree Star, Ashland, OR).
Endocytosis determination assay
In order to assess whether the IL-34 treated cells exhibit endocytic functions compared to untreated cells, U937 and THP-1 cell lines were either untreated or treated with IL-34 (50 ng/ml) for 24 hours. At the end of the time point the cells were incubated with 1 mg/ml FITC-dextran for 60 min at 37°C. Cellular uptake of FITC-dextran was monitored by flow cytometry. A negative control was performed in parallel by incubating cells with FITC-dextran at 4°C. Flow cytometric data was analyzed as indicated above.
Respiratory burst activity detection
Respiratory burst activity detection was performed using the reactive oxygen intermediate-sensitive probe dihydrorhodamine 123. For this assay, U937 and THP-1 cell lines were either untreated or treated with IL-34 (50 ng/ml) in serum-free RPMI medium supplemented with 1% BSA for 24 hours. U937 cells treated with PMA (50 ng/ml) in serum free medium containing 1% BSA for 48 hours were used as positive control for differentiation. After incubation, cells were pelleted, washed and pre-stimulated for 45 min with 100 ng/ml PMA (an agent commonly used to trigger oxidative burst activity in phagocytic cells) to trigger oxidative burst. After washing, cells were incubated with 5 ug/ml dihydrorhodamine 123 for 45 min at 37°C. Rhodamine 123 fluorescence in intact cells is formed by the action of reactive oxygen intermediates such as hydrogen peroxide, was then measured by flow cytometry. Data were expressed as fluorescence arbitrary units, or percentage fluorescent cells, as appropriate for comparison.
Evaluation of JAK/STAT and PI3K/Akt signaling
Triplicate experiments in six-well plates were set up in which each well contained U937 cells (5 × 103) in 2 ml of serum free media. The wells were either untreated or pretreated for at least one hour with 2.5 uM LY294002 or 100 nM Bayer-18 prior to be treated with 50 ng/ml of IL-34 and incubated as previously described for up to 96 hours. The cells were then harvested and prepared for slides as previously described for differentiated cell count analysis and observation of morphological changes.
Statistical analysis
Data was expressed as means ± SEM or 95% confidence interval (95% CI) and was analyzed with GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA) and comparisons were made by Student’s t test (two-tailed), one-way analysis of variance or two-way analysis of variance, as appropriate. A probability of p < 0.05 indicated statistical significance.
Results
U937 and THP-1 cell lines express both receptors for interleukin-34
In order to examine the potential biologic effects of IL-34 on U937 and THP-1 cell lines, it was important to verify the presence of the purported receptors of IL-34, c-FMS and RPTP-ƺ. We conducted western blot analysis for the presence of the c-FMS receptor in both the U937 and THP-1 cell lines, using THP-1 as a known reference for c-FMS for comparison [12,13]. As shown in Figure 1A, the c-FMS receptor, is present in U937 cells though the c-FMS expression level is lower than in THP-1 cells. In Figure 1B, we note that as compared to THP-1 cells the U937 cells do not express the RPTP-ƺ receptor. This data also indicates that the RPTP-ƺ receptor may be inducible, as shown with THP-1 cells treated with IL-34. These results suggest that perhaps both the c-FMS receptor and RPTP-ƺ could bind to IL-34 and mediate the effects of IL-34 in the U937 and THP-1 cell lines.
Figure 1.
Detection of the c-FMS and RPTP-ƺ receptors and identification of biological effects of IL-34. Both THP-1 and U937 cells express c-FMS receptor. In order to detect c-FMS 10 ug of lysate proteins from THP-1 and 60 ug of lysate proteins from U937 cells were analyzed for c-FMS protein expression by western blotting using monoclonal antibody to c-FMS (A). THP-1 cells express RPTP-ƺ after IL-34 treatment. 20 ug of lysate proteins from both THP-1 and U937 cells were analyzed for RPTP-ƺ by western blotting using monoclonal antibody to RPTP-ƺ (B). IL-34 fails to promote cell growth and proliferation in U937 cells. Using the Trypan blue exclusion assay we assessed cell viability. We also used MTS and MTT assays for cell growth and proliferation to monitor cell proliferation in untreated cells and cells treated with IL-34 for 48 h (C). IL-34 induces differential expression of IL-1α (red) and IL-1β (blue) in U937 cells. IL-1 protein expression was assessed by ELISA using 500 ul of culture media from either untreated or cells treated with IL-34 (50 ng/ml) for different time points (D). Each experiment was performed in triplicate.
IL-34 does not promote growth and proliferation
Previous research has demonstrated that IL-34 promotes growth and proliferation in monocytes [6,14]. Therefore, we evaluated whether IL-34 has the potential to induce similar effects in U937 cells. As seen in Figure 1C, IL-34 failed to promote growth or proliferation in U937 cells during a 48 hour treatment. Likewise, U937 cell viability remained unchanged during the 48 hour treatment, suggesting that IL-34 does not induce cell death in these cells.
IL-34 induces differential expression of IL-1α and IL-1β
Next, we evaluated the biochemical and physiological effects of IL-34. In Figure 1D, we noted that treatment with 50 ng/ml of IL-34 over a 144 hour time course resulted in induction of differential expression of IL-1α and IL-1β. Clearly, there was an initial increase in IL-1β expression over a 24 hour period, which reached a maximum level by 48 hours followed by a decline. In contrast, IL-34 induced a steady increase in expression of IL-1α over the entire 144 hour time course. The data strongly suggests that IL-34 is capable of inducing differential expression of IL-1α and IL-1β. These observations are of interest because there are a myriad of implications related to the differential expression of IL-1α and IL-1β in the myeloid differentiation pathway. For example, it has been reported that transition from IL-1β to IL-1α synthesis is associated with differentiation of recruited monocytes into inflammatory macrophages [12,15]. Thus, it should be expected that an intermediate cell type is able to co-produce both forms of IL-1.
Together, as compared to untreated (control), there is a marked increase in both IL-1α and IL-1β expression over a 144 hour time course. This data raises an important possibility regarding whether IL-34 could serve as regulatory cytokine on the myeloid differentiation cascade.
IL-34 alters the morphology of U937, HL-60 and THP-1 cell lines
After noting the differential induction of IL-1α and IL-1β in response to IL-34 treatment we next examined if IL-34 induces morphological changes in U937, HL-60 and THP-1 cell lines associated with monocytic differentiation. While the untreated U937, HL-60 and THP-1 cell lines did not exhibit any morphological changes, IL-34 treated cells exhibited marked morphological changes within 6 to 96 hours. The morphological changes seen in IL-34 treated cells were clearly indicative of transition from the basic, immature monoblastic phenotype to towards a more matured phenotype. As shown in Figure 2A and 2B, untreated U937, HL-60 and THP-1 cell lines had characteristic rounded morphology with high nuclear to cytoplasmic ratio, which is characteristic of myeloid lineage progenitors and more related to U937, HL-60 and THP-1 cell lines [12,13,16-23]. In contrast, IL-34 treated cells had significantly high cytoplasmic to nuclear ratio, extensive pseudopodia-like structures as well as condensed chromatin with indented and/or kidney-shaped morphology. These morphological changes were observed at both lower and higher concentrations of IL-34 tested [24-26]. For comparison, we specifically examined THP-1 cells treated with PMA, a known inducer of differentiation in these cells. As shown in Figure 2B, similar morphological changes were observed between the IL-34 treated THP-1 and HL-60 cells and PMA-treated THP-1 cells.
Figure 2.
Interleukin-34 induces morphological changes. U937 (2 × 105) cells were either untreated or treated with different concentration of IL-34 (10 to 50 ng/ml) for up to 96 hours (A). THP-1 (2 × 105) cells were either untreated or treated with 50 ng/ml of IL-34 or 10 mM of PMA for 96 hours (B). HL-60 (2 × 105) cells were either untreated or treated with 50 ng/ml of IL-34 for 96 hours (C). For all figures the cells were concentrated by centrifugation on cytospin slides and stained with Giemsa. Morphological changes were observed as described under the Methods Sections.
IL-34 alters the expression of cell surface markers in U937, HL-60 and THP-1 cell lines
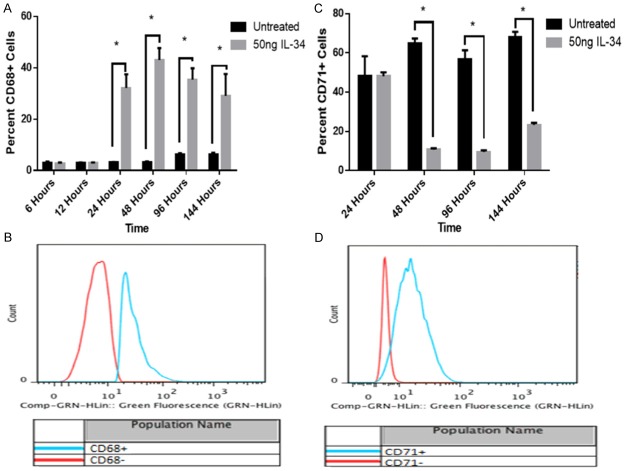
Noting that IL-34 treated U937, HL-60 and THP-1 cell lines appear morphologically differentiated into monocytes, we decided to qualify these phenotypic changes. To that effect we evaluated differentiated cells for monocyte and macrophage cell lineage cell surface markers: CD11b, CD14, CD64, CD68, CD71 and CD86, respectively. CD11b is a differentiation marker for cells of the myelo-monocytic lineage. Notably there was a marked decline in the level of CD11b within 48 to 144 hours (Figure 3A and 3B) in U937 cells. HL-60 cells showed a decline in the level of CD11b after 96 hours of IL-34 treatment as compared to untreated HL-60 cells (Figure 5C). Similar results were observed in THP-1 cells over the same time period (data not shown).
Figure 3.
IL-34 induces marked changes in CD11b and CD14 expression in U937 and THP-1 cell lines. Amongst the cell surface markers with the most significant changes are CD11b induction over 144 hour time course (A), Representative histogram of induction of CD11b between U937 cells untreated or treated with IL-34 at 24 hours (B), CD14 induction over 144 hour time course (C), Representative histogram of induction of CD14 between U937 cells untreated or treated with IL-34 at 144 hours (D). The mean ± SD for each time point are shown (n = 6), *p < 0.0001.
Figure 5.

IL-34 induces marked changes in cell surface marker expression in U937, THP-1, and HL-60 cell lines. The percentage of fluorescent positive cells for CD14, CD64 and CD71 in THP-1 cells treated with IL-34 for 96 hours (A). The percentage of fluorescent positive cells for CD11b, CD14 and CD86 in U937 cells treated with PMA as positive control (B). The percentage of fluorescent positive cells for CD11b, CD68 and CD86 in HL-60 cells treated with IL-34 for 96 hours (C). The mean ± SD for each time point are shown (n = 6), *p < 0.0001.
CD14, a binding site for LPS (lipopolysaccharide) is expressed on monocytes and macrophages [7,27,28]. At 48 hours post IL-34 treatment, there was a notable decrease in CD14 expression as compared to the untreated U937 cells (Figure 3C). However, as shown in Figure 3C and 3D, there was a rebound in CD14 expression between 96 and 144 hours after stimulation of U937 cells with IL-34. Importantly, at 144 hours post stimulation with IL-34, approximately 45% of cells expressed CD14 cell surface marker, as compared to less than 5% at the 24 hour time point. Comparatively, we note a similar trend of increase in CD14 expression in THP-1 cells, is reported later in Figure 5A. IL-34 treated THP-1 exhibited a robust increase of almost 75% in CD14 expression in a 96 hour period as compared to untreated.
CD68, also known as macrosialin, is located in the cytoplasmic granules of monocytes and macrophages [30]. In Figure 4B, we showed that at 48 hours post IL-34 treatment there was a profound increase in CD68 expression as compared to untreated U937 cells. Also, in Figure 4A, we noted significant induction of CD68 at 24 hours post IL-34 treatment, and that the increase in expression was maintained for 144 hours. After 96 hours of IL-34 treatment, HL-60 cells show a similar increase in CD68 as compared to untreated HL-60 cells (Figure 5C).
Figure 4.
IL-34 induces marked changes in CD68 and CD71 expression in U937 and THP-1 cell lines. CD68 induction over 144 hour time course (A), Representative histogram of induction of CD68 between U937 cells untreated or treated with IL-34 at 48 hours (B) and CD71 induction over 144 hour time course (C), Representative histogram of induction of CD71 between U937 cells untreated or treated with IL-34 at 48 hours (D). The mean ± SD for each time point are shown (n = 6), *p < 0.0001.
CD71, also known as OKT-9, is a cell surface marker for immature, proliferating cells similar to the monoblastic U937 and THP-1 cell lines. At 48 hours post treatment with IL-34, we observed a notable decrease in the percentage of U937 cells expressing CD71. This observation was also seen in treated THP-1 cells after 96 hours (Figure 5A). The decline in CD71 expression began early and was most noticeable at 48 hours in response to IL-34 in U937 cells. As shown in Figure 4C and 4D, the percentage of CD71+ cells in IL-34 treated U937 cells remained lower than in untreated cell at all time points.
CD64 is a marker for myeloid lineage [29]. There was no change in CD64 expression following treatment of cells with IL-34. We show that in THP-1 cells, the expression of CD64 remains high, approximately 90% whether treated or untreated (Figure 5A). Also, there was no significant change in the percentage of CD64+ cells as the percentage of CD64+ cells remained approximately 95% over the entire 144 hour time course in U937 cells (data not shown).
As a positive control for evidence of characteristic cell surface markers associated with monocytic differentiation, U937 cells were treated with 50 ng/ml of PMA for 48 hours. The cells were analyzed for markers of differentiation. The results in Figure 5B, strongly indicate that U937 cells treated with 50 ng/ml PMA showed similar trends with respect to induction of CD11b and CD14 expression. The PMA treated cells also maintained high percentage of CD86 positive characteristics as compared to IL-34 treated cells. This high percentage of CD86 positivity was also observed in HL-60 cells, both untreated and treated with IL-34.
IL-34-induced differentiated U937 and THP-1 cell lines exhibit endocytic function
In addition to characterizing the morphological changes and the expression of cell surface markers of differentiation induced by IL-34 in U937 and THP-1 cell lines we examined whether these changes translate to acquisition of biochemical functions associated with monocytic differentiation. To do so, we compared untreated and IL-34 treated U937 and THP-1 cell lines for their ability to perform endocytosis of extracellular particulates by monitoring FITC-Dextran uptake into the cells using the FITC-Dextran. Typically, undifferentiated cells are unable to endocytose foreign material effectively. However, congruent with the alteration of morphological and cell surface marker characteristics induced by IL-34 in U937 and THP-1 cell lines we expected that these differentiated cells would be capable of uptake of FITC-Dextran. As expected, IL-34-induced differentiated cells were able to take up significant amount of FITC-Dextran as compared to the untreated (undifferentiated) cells. In Figure 6A and 6B, we noted that U937 and THP-1 cell lines treated with IL-34 endocytosed FITC-Dextran far greater than the untreated cells at both 37°C and 4°C even though the extent of endocytosis was much greater at 37°C than at 4°C. These results support the observation that IL-34 induces monocytic-like characteristics in U937 and THP-1 cell lines.
Figure 6.
Induction of endocytosis and respiratory burst in U937 and THP-1 cells. U937 and THP-1 cells (1 × 105) were either untreated or treated with IL-34 (50 ng/ml) in time course experiments. Endocytosis was measured using the FITC-dextran assay and the data is reported in mean fluorescence intensity. The mean ± SD for each time point are shown (n = 3), *p < 0.0001 at 4°C and 37°C, respectively (A and B). Respiratory burst activity was assessed as described under the Methods Section (C and D). The data is reported in reactive oxygen intermediates/fluorescence arbitrary units. Percentage of fluorescent positive U937 cells under untreated conditions was compared to the fluorescent positive cells under either IL-34 or PMA treated conditions to obtain the fold induction of respiratory burst activity. The mean ± SD for each time point are shown (n = 6), *p < 0.0001, **p = 0.0002.
IL-34 induced-differentiated U937 and THP-1 cell lines are able to perform respiratory burst
As seen in Figure 6C and 6D, unlike their untreated counterparts, IL-34 treated U937 and THP-1 cells are able to perform respiratory burst activity when stimulated with PMA to initiate the production of reactive oxygen intermediates. Clearly, as compared to the untreated cells the IL-34 treated cells showed a 12-fold, and 5-fold increase in respiratory burst activity at 96 hours for U937 and THP-1 cell lines, respectively. Though the respiratory burst activity in the IL-34 treated cells declined slightly to 10 fold at 144 hours in U937 cells, it was still significantly higher than in the untreated cells.
From this data it can be inferred that IL-34 treatment promoted the transition of the U937 and THP-1 cell lines to a more differentiated phenotype with acquired respiratory burst functional activity in a manner associated with monocytic differentiation.
IL-34 differentiation in U937 and THP-1 cell lines may be mediated through the JAK/STAT and PI3K/Akt pathways
After noting both morphological and functional gains associated with treatment with IL-34 in these leukemia models, we decided to see if any of the prominent cytokine related signaling pathways could be related to these observations. Because of the presence of both receptors for IL-34, c-FMS and RPTP-ƺ, we decided to look at the JAK/STAT and PI3K/Akt pathways, both of which have been implicated in literature [8,31-34]. In Figure 7A, we observed that after pretreating cells with PI3K inhibitor (LY294002) or TYK-2 inhibitor (Bayer-18), then stimulating with IL-34 for up to 96 hours, the cells maintained rounded morphology with high nuclear to cytoplasmic ratio, and did not demonstrate a decrease or change in nuclear size and shape, nor a reduction of number of nucleoli. Next we quantified whether these treatments had any effect on the number of undifferentiated and differentiated cells over the same time course. We note that in Figure 7B, as compared to IL-34 treated cells alone, when blocking TYK-2 with Bayer-18 or PI3K with LY294002, there was a significantly higher number of undifferentiated cells as compared to differentiated cells. There was a 16.6 fold and 25 fold increase of undifferentiated cells in the presence of TYK-2 and PI3K inhibitors, respectively. The lack of ability of IL-34 to induce the same level of differentiation in these cell lines in the presence of the specific PI3K or TYK-2 inhibitor suggest that perhaps both the JAK/STAT and PI3K/Akt signaling pathways mediate the differentiation effects of IL-34.
Figure 7.
Evidence of the involvement of JAK/STAT and PI3K/Akt signaling by IL-34. U937 cells were pre-treated with LY294002 or Bayer-18 prior to IL-34 treatment for 96 hours as compared to untreated (A). Cell counts per microscopic field for U937 and THP-1 cells treated alone with IL-34 as compared to U937 cells pre-treated with LY294002 or Bayer-18 prior to IL-34 treatment (B). The mean ± SD for each point is shown (n = 5), *p < 0.0001.
Discussion
In this study, we have provided strong evidence that IL-34 induces differentiation of U937, HL-60 and THP-1 cell lines into monocyte-like cells. IL-34-induced differentiated cells were characterized by alterations in specific surface markers, as well as functionally by their ability to phagocytize foreign particles and perform respiratory burst activity.
First, we confirmed that U937 and THP-1 cell lines express the receptors for IL-34, c-FMS and RPTP-ƺ [1,8]. The presence of c-FMS in both cell lines, as compared to the lack of RPTP-ƺ in U937 cells does not negate that one or the other may be responsible for mediating the biologic effects of IL-34 reported here. To our surprise lL-34 did not stimulate cell growth and/or proliferation. Furthermore, long term treatment with IL-34 did not affect viability, suggesting that IL-34 does not cause cell death in these cells. These results are both contradictory and enlightening because previous studies reported that IL-34 induces both growth and proliferation in monocytes and osteoclast progenitors [2,8,35,36].
In contrast to its inability to stimulate proliferation, IL-34 induced differential expression of IL-1α and IL-1β. Differential expression of IL-α and IL-1β mRNA has been observed in various cell types, including human monocytes, T cells, and B cell clones [15]. IL-1α and IL-1β are produced by activated mononuclear phagocytes and other cell types and they act as mediators that increase host defense mechanisms as well as inflammatory reactions. Despite their significant structural differences IL-1α and IL-1β share many biological activities and recognize the same cell surface receptors [15,37]. It is however known that while IL-1β mediates immunomodulation in vivo, IL-1α acts as a negative regulator of the IL-1β activity via competition for their common receptor. It is possible that the differential expression of the two forms of two IL-1 may be regulated through a sequential production of IL-1β and IL-1α as cells become more monocytic-like. Understanding this, we evaluated the induction of IL-1 in U937 cells. Our results are compatible to those exhibited by recruited monocytes during their maturation to inflammatory macrophages. The molecular mechanisms by which IL-34 induces differential expression of IL-1α and IL-1β remains unknown, which would be the focus of our future studies.
Based on the IL-1 cytokine induction profile in U937 cells, we decided to examine in U937, HL-60 and THP-1 cells lines whether IL-34 would induce morphological changes associated with maturation towards monocytic-like cells. As indicated earlier IL-34 stimulates progression of U937, HL-60 and THP-1 cell lines along the myeloid differentiation pathway towards monocyte-like cells. Typically, U937, HL-60 and THP-1 cell lines remain monoblastic in character, similar to monocytes in vivo [12,13,20,28] but retains the ability to differentiate into mature macrophages in response to may inducers of differentiation [16,26,38-41]. During differentiation, cells assume irregular flat shape with increasing cytoplasmic space. Furthermore, their nuclei exhibit chromatin condensation as they become kidney-shaped in morphology [16,24-26,39,42]. Interestingly, IL-34 treated U937 cells exhibit most of these morphological characteristics within 6 to 24 hours of IL-34 treatment with more pronounced changes occurring after 48 hours. We noted that HL-60 and THP-1 cells also exhibited these same morphological characteristics within 96 hours of treatment. Furthermore, slight morphological changes occurred in cells treated with 10 ng/ml of IL-34, a significantly lower concentration compared to the dose (50 ng/ml) at which IL-34 is reported to elicit its maximal biological effects [1,2,5,7,36,43,44]. These observations suggest that secretion of relatively low amount of IL-34 from an inflammatory disease into the microenvironment could produce physiologically relevant responses in cancer cells.
IL-34-induced monocyte differentiation was validated by distinct cell-surface marker expression for myeloid lineage and monocytes/macrophages immaturity. Clearly, CD11b level initially remained elevated within the first 12 hours post treatment followed by an overall decline after 24 hours. While an initial increase in CD11b suggests maturation of myeloid precursors, an overall decrease in CD11b levels after 24 hours could signify monocyte differentiation towards more macrophage-like characteristics [45]. CD14 is a typical cell surface marker for monocytes and macrophages and it serves as a binding site for bacterial lipopolysaccharide [27,28]. Overall, the increase in CD14 expression in IL-34 treated cells correlated well with the morphological changes induced by IL-34 in the U937 and THP-1 cell lines.
The cell surface markers CD64 and CD86 are monocyte/macrophage/myeloid lineage markers. The minimal effects of IL-34 on CD64 and CD86 levels in these cells demonstrate that IL-34 does not alter the myeloid lineage of these cells [14,46-50]. Further evidence of IL-34-induced monocytic differentiation is exemplified by the decrease in CD71 (OKT-9) levels [51] with concomitant increase in CD68 expression in IL-34 treated cells. These changes are comparable to those induced by PMA, a known inducer of monocytic differentiation [25,42,52]. Taken together, the aforementioned evidence strongly supports the notion that IL-34 treated cells are re-programmed to move along the myeloid differentiation pathway away from immaturity towards more mature and functional monocytic-like cells.
The biological functionality of the IL-34 differentiated leukemia cells is amongst the most important validations of this study. In addition to induction of changes in morphology and cell surface markers, IL-34 promoted the ability of U937 and THP-1 cell lines to endocytose and perform respiratory burst, which are two distinct activities of monocytes and macrophages. Under such conditions monocytes and macrophages rapidly consume significant amount of oxygen and generate oxygen free radicals with which to degrade invading bacteria [53-55]. Our results demonstrate that in comparison to their untreated counterparts, IL-34 treated cells exhibit marked endocytosis and respiratory burst activities that are characteristics of monocytes and macrophages.
To understand the importance of downstream signaling effectors of IL-34 in mediating its differentiation effects we have observed, we decided to explore whether there was any changes to these observations in the presence of inhibitors to components of the JAK/STAT and PI3K/Akt signaling pathways. Our data suggests the involvement of both pathways in mediating the differentiation effects of IL-34 since the blockage of either of these pathways decreases the differentiation effects of IL-34. These findings, while preliminary, align with literature concerning the downstream effectors of both the c-FMS and RPTP-ƺ receptors [1,3,8,31-34,56-60].
In conclusion, we have provided compelling evidence that IL-34 induces differentiation of leukemic cells to monocyte-like cells. To our knowledge this is the first report indicating that IL-34 induces differentiation in a cancer cell line. As a follow up we had planned to examine whether IL-34 is capable of eliciting these biologic effects in primary leukemia cells. However, due to the logistics and difficulties in obtaining primary samples of leukemia it was not feasible to perform such experiments at the time. Instead, using a third leukemia cell line (HL-60), we successfully demonstrated that the differentiation effects of IL-34 is not restricted to U937 and THP-1 leukemia cell lines and that HL-60 cells also respond to IL-34 with respect to differentiation. Furthermore, this is the first report that IL-34 also induces differential expression of IL-1α and IL-1β. Though all the signaling components that contribute to the effects of IL-34 reported here have yet to be determined, our findings indicate that the activation of the JAK/STAT and PI3K/Akt signaling pathways may be crucial for the biological effects of IL-34 and is not negated by lack of presence of RPTP-ƺ in U937 cells. Because of the ability of IL-34 to induce differentiation in leukemia cells our findings suggest potential clinical relevance regarding future consideration of IL-34 as a therapy for leukemia.
Acknowledgements
Burthia Booker and Ryan Clark are supported by the RISE grant #5R25GM059994 to the School of Graduate Studies and Research. Burthia Booker is supported by the T32 pre-doctoral training grant #5T32HL007735-19 to Dr. Adunyah. Samuel Pellom is supported by the T32 pre-doctoral training grant #2T32HL007737. The work was supported with funds from the T32 training grant, vouchers from the Vanderbilt CTSA #UL1TR000445, the RCMI program #G12MD007586, U54CA091408, and S10RR0254970. Dr. Adunyah is supported by the MMC-VICC-TSU Cancer Partnership NCI U54 grant #5U54CA163069-03 and Meharry Medical College Translational Research NIMHD U54 grant #NIMHD U54 MD007593.
References
- 1.Chihara T, Suzu S, Hassan R, Chutiwitoonchai N, Hiyoshi M, Motoyoshi K, Kimura F, Okada S. IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ. 2010;17:1917–1927. doi: 10.1038/cdd.2010.60. [DOI] [PubMed] [Google Scholar]
- 2.Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 3.Nandi S, Cioce M, Yeung YG, Nieves E, Tesfa L, Lin H, Hsu AW, Halenbeck R, Cheng HY, Gokhan S, Mehler MF, Stanley ER. Receptor-type protein-tyrosine phosphatase zeta is a functional receptor for interleukin-34. J Biol Chem. 2013;288:21972–21986. doi: 10.1074/jbc.M112.442731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eda H, Zhang J, Keith RH, Michener M, Beidler DR, Monahan JB. Macrophage-colony stimulating factor and interleukin-34 induce chemokines in human whole blood. Cytokine. 2010;52:215–220. doi: 10.1016/j.cyto.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Hwang SJ, Choi B, Kang SS, Chang JH, Kim YG, Chung YH, Sohn DH, So MW, Lee CK, Robinson WH, Chang EJ. Interleukin-34 produced by human fibroblast-like synovial cells in rheumatoid arthritis supports osteoclastogenesis. Arthritis Res Ther. 2012;14:R14. doi: 10.1186/ar3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covaleda L, Fuller FJ, Payne SL. EIAV S2 enhances pro-inflammatory cytokine and chemokine response in infected macrophages. Virology. 2010;397:217–223. doi: 10.1016/j.virol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciccia F, Alessandro R, Rodolico V, Guggino G, Raimondo S, Guarnotta C, Giardina A, Sireci G, Campisi G, De Leo G, Triolo G. IL-34 is overexpressed in the inflamed salivary glands of patients with Sjogren’s syndrome and is associated with the local expansion of pro-inflammatory CD14brightCD16+ monocytes. Rheumatology (Oxford) 2013;52:1009–17. doi: 10.1093/rheumatology/kes435. [DOI] [PubMed] [Google Scholar]
- 8.Droin N, Solary E. Editorial: CSF1R, CSF-1, and IL-34, a “menage a trois” conserved across vertebrates. J Leukoc Biol. 2010;87:745–747. doi: 10.1189/jlb.1209780. [DOI] [PubMed] [Google Scholar]
- 9.Garceau V, Smith J, Paton IR, Davey M, Fares MA, Sester DP, Burt DW, Hume DA. Pivotal Advance: Avian colony-stimulating factor 1 (CSF-1), interleukin-34 (IL-34), and CSF-1 receptor genes and gene products. J Leukoc Biol. 2010;87:753–764. doi: 10.1189/jlb.0909624. [DOI] [PubMed] [Google Scholar]
- 10.Lenzo JC, Turner AL, Cook AD, Vlahos R, Anderson GP, Reynolds EC, Hamilton JA. Control of macrophage lineage populations by CSF-1 receptor and GM-CSF in homeostasis and inflammation. Immunol Cell Biol. 2012;90:429–440. doi: 10.1038/icb.2011.58. [DOI] [PubMed] [Google Scholar]
- 11.Felix J, Elegheert J, Gutsche I, Shkumatov AV, Wen Y, Bracke N, Pannecoucke E, Vandenberghe I, Devreese B, Svergun DI, Pauwels E, Vergauwen B, Savvides SN. Human IL-34 and CSF-1 Establish Structurally Similar Extracellular Assemblies with Their Common Hematopoietic Receptor. Structure. 2013;21:528–539. doi: 10.1016/j.str.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 14.Fischer DG, Pike MC, Koren HS, Snyderman R. Chemotactically responsive and nonresposive forms of a continuous human monocyte cell line. J Immunol. 1980;125:463–465. [PubMed] [Google Scholar]
- 15.Beuscher HU, Rausch UP, Otterness IG, Rollinghoff M. Transition from interleukin 1 beta (IL-1 beta) to IL-1 alpha production during maturation of inflammatory macrophages in vivo. J Exp Med. 1992;175:1793–1797. doi: 10.1084/jem.175.6.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adunyah SE, Unlap TM, Franklin CC, Kraft AS. Induction of differentiation and c-jun expression in human leukemic cells by okadaic acid, an inhibitor of protein phosphatases. J Cell Physiol. 1992;151:415–426. doi: 10.1002/jcp.1041510223. [DOI] [PubMed] [Google Scholar]
- 17.Cline MJ. The molecular basis of leukemia. N Engl J Med. 1994;330:328–336. doi: 10.1056/NEJM199402033300507. [DOI] [PubMed] [Google Scholar]
- 18.Golde DW, Cline MJ. Human preleukemia. Identification of a maturation defect in vitro. N Engl J Med. 1973;288:1083–1086. doi: 10.1056/NEJM197305242882101. [DOI] [PubMed] [Google Scholar]
- 19.Huang ZL, Failla ML, Reeves PG. Differentiation of human U937 promonocytic cells is impaired by moderate copper deficiency. Exp Biol Med (Maywood) 2001;226:222–228. [PubMed] [Google Scholar]
- 20.Schwende H, Fitzke E, Ambs P, Dieter P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J Leukoc Biol. 1996;59:555–561. [PubMed] [Google Scholar]
- 21.Birnie GD. The HL60 cell line: a model system for studying human myeloid cell differentiation. Br J Cancer Suppl. 1988;9:41–45. [PMC free article] [PubMed] [Google Scholar]
- 22.Brackman D, Lund-Johansen F, Aarskog D. Expression of leukocyte differentiation antigens during the differentiation of HL-60 cells induced by 1,25-dihydroxyvitamin D3: comparison with the maturation of normal monocytic and granulocytic bone marrow cells. J Leukoc Biol. 1995;58:547–555. doi: 10.1002/jlb.58.5.547. [DOI] [PubMed] [Google Scholar]
- 23.Brackman D, Lund-Johansen F, Aarskog D. Expression of cell surface antigens during the differentiation of HL-60 cells induced by 1,25-dihydroxyvitamin D3, retinoic acid and DMSO. Leuk Res. 1995;19:57–64. doi: 10.1016/0145-2126(94)00061-e. [DOI] [PubMed] [Google Scholar]
- 24.Dijkgraaf EM, Heusinkveld M, Tummers B, Vogelpoel LT, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR, van der Burg SH. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res. 2013;73:2480–2492. doi: 10.1158/0008-5472.CAN-12-3542. [DOI] [PubMed] [Google Scholar]
- 25.Garcia A, Serrano A, Abril E, Jimenez P, Real LM, Canton J, Garrido F, Ruiz-Cabello F. Differential effect on U937 cell differentiation by targeting transcriptional factors implicated in tissue- or stage-specific induced integrin expression. Exp Hematol. 1999;27:353–364. doi: 10.1016/s0301-472x(98)00038-1. [DOI] [PubMed] [Google Scholar]
- 26.Pagliara P, Lanubile R, Dwikat M, Abbro L, Dini L. Differentiation of monocytic U937 cells under static magnetic field exposure. Eur J Histochem. 2005;49:75–86. doi: 10.4081/930. [DOI] [PubMed] [Google Scholar]
- 27.Haziot A, Chen S, Ferrero E, Low MG, Silber R, Goyert SM. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141:547–552. [PubMed] [Google Scholar]
- 28.Simmons DL, Tan S, Tenen DG, Nicholson-Weller A, Seed B. Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood. 1989;73:284–289. [PubMed] [Google Scholar]
- 29.Tacken PJ, Batenburg JJ. Monocyte CD64 or CD89 targeting by surfactant protein D/anti-Fc receptor mediates bacterial uptake. Immunology. 2006;117:494–501. doi: 10.1111/j.1365-2567.2006.02324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–1613. [PubMed] [Google Scholar]
- 31.Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007;12:104–107. doi: 10.1016/j.ccr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Cadena DL, Gill GN. Receptor tyrosine kinases. FASEB J. 1992;6:2332–2337. doi: 10.1096/fasebj.6.6.1312047. [DOI] [PubMed] [Google Scholar]
- 33.Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 34.Feng ZJ, Gao SB, Wu Y, Xu XF, Hua X, Jin GH. Lung cancer cell migration is regulated via repressing growth factor PTN/RPTP beta/zeta signaling by menin. Oncogene. 2010;29:5416–5426. doi: 10.1038/onc.2010.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baud’huin M, Renault R, Charrier C, Riet A, Moreau A, Brion R, Gouin F, Duplomb L, Heymann D. Interleukin-34 is expressed by giant cell tumours of bone and plays a key role in RANKL-induced osteoclastogenesis. J Pathol. 2010;221:77–86. doi: 10.1002/path.2684. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, Buki K, Vaaraniemi J, Gu G, Vaananen HK. The critical role of IL-34 in osteoclastogenesis. PLoS One. 2011;6:e18689. doi: 10.1371/journal.pone.0018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valledor AF, Borras FE, Cullell-Young M, Celada A. Transcription factors that regulate monocyte/macrophage differentiation. J Leukoc Biol. 1998;63:405–417. doi: 10.1002/jlb.63.4.405. [DOI] [PubMed] [Google Scholar]
- 38.Kim SJ, Bang OS, Lee YS, Kang SS. Production of inducible nitric oxide is required for monocytic differentiation of U937 cells induced by vitamin E-succinate. J Cell Sci. 1998;111:435–441. doi: 10.1242/jcs.111.4.435. [DOI] [PubMed] [Google Scholar]
- 39.Netea MG, Lewis EC, Azam T, Joosten LA, Jaekal J, Bae SY, Dinarello CA, Kim SH. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc Natl Acad Sci U S A. 2008;105:3515–3520. doi: 10.1073/pnas.0712381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao KW, Li X, Zhao Q, Huang Y, Li D, Peng ZG, Shen WZ, Zhao J, Zhou Q, Chen Z, Sims PJ, Wiedmer T, Chen GQ. Protein kinase Cdelta mediates retinoic acid and phorbol myristate acetate-induced phospholipid scramblase 1 gene expression: its role in leukemic cell differentiation. Blood. 2004;104:3731–3738. doi: 10.1182/blood-2004-04-1630. [DOI] [PubMed] [Google Scholar]
- 41.Zuckerman SH, Surprenant YM, Tang J. Synergistic effect of granulocyte-macrophage colony-stimulating factor and 1,25-dihydroxyvitamin D3 on the differentiation of the human monocytic cell line U937. Blood. 1988;71:619–624. [PubMed] [Google Scholar]
- 42.Baek YS, Haas S, Hackstein H, Bein G, Hernandez-Santana M, Lehrach H, Sauer S, Seitz H. Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunol. 2009;10:18. doi: 10.1186/1471-2172-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foucher ED, Blanchard S, Preisser L, Garo E, Ifrah N, Guardiola P, Delneste Y, Jeannin P. IL-34 Induces the Differentiation of Human Monocytes into Immunosuppressive Macrophages. Antagonistic Effects of GM-CSF and IFNgamma. PLoS One. 2014;8:e56045. doi: 10.1371/journal.pone.0056045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z, Jin D, Wu Y, Zhang K, Hu P, Cao X, Chen Z. Increased serum interleukin-34 in patients with coronary artery disease. J Int Med Res. 2012;40:1866–1870. doi: 10.1177/030006051204000525. [DOI] [PubMed] [Google Scholar]
- 45.Park H, Shelley CS, Arnaout MA. The zinc finger transcription factor ZBP-89 is a repressor of the human beta 2-integrin CD11b gene. Blood. 2003;101:894–902. doi: 10.1182/blood-2002-03-0680. [DOI] [PubMed] [Google Scholar]
- 46.Fleischer J, Soeth E, Reiling N, Grage-Griebenow E, Flad HD, Ernst M. Differential expression and function of CD80 (B7-1) and CD86 (B7-2) on human peripheral blood monocytes. Immunology. 1996;89:592–598. doi: 10.1046/j.1365-2567.1996.d01-785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koren HS, Anderson SJ, Larrick JW. In vitro activation of a human macrophage-like cell line. Nature. 1979;279:328–331. doi: 10.1038/279328a0. [DOI] [PubMed] [Google Scholar]
- 48.Larrick JW, Fischer DG, Anderson SJ, Koren HS. Characterization of a human macrophage-like cell line stimulated in vitro: a model of macrophage functions. J Immunol. 1980;125:6–12. [PubMed] [Google Scholar]
- 49.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Wolk K, Hoflich C, Zuckermann-Becker H, Docke WD, Volk HD, Sabat R. Reduced monocyte CD86 expression in postinflammatory immunodeficiency. Crit Care Med. 2007;35:458–467. doi: 10.1097/01.CCM.0000254724.54515.2F. [DOI] [PubMed] [Google Scholar]
- 51.Salcedo TW, Fleit HB. Plasma membrane and intracellular pools of transferrin receptors decline during in vitro cultivation of U937 cells. Cell Prolif. 1991;24:383–401. doi: 10.1111/j.1365-2184.1991.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 52.Huang ZL, Failla ML. Copper deficiency suppresses effector activities of differentiated U937 cells. J Nutr. 2000;130:1536–1542. doi: 10.1093/jn/130.6.1536. [DOI] [PubMed] [Google Scholar]
- 53.van Grevenynghe J, Rion S, Le Ferrec E, Le Vee M, Amiot L, Fauchet R, Fardel O. Polycyclic aromatic hydrocarbons inhibit differentiation of human monocytes into macrophages. J Immunol. 2003;170:2374–2381. doi: 10.4049/jimmunol.170.5.2374. [DOI] [PubMed] [Google Scholar]
- 54.Wang JC, Kobie JJ, Zhang L, Cochran M, Mosmann TR, Ritchlin CT, Quataert SA. An 11-color flow cytometric assay for identifying, phenotyping, and assessing endocytic ability of peripheral blood dendritic cell subsets in a single platform. J Immunol Methods. 2009;341:106–116. doi: 10.1016/j.jim.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeller JM, Caliendo J, Lint TF, Nelson DJ. Changes in respiratory burst activity during human monocyte differentiation in suspension culture. Inflammation. 1988;12:585–595. doi: 10.1007/BF00914320. [DOI] [PubMed] [Google Scholar]
- 56.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 58.Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- 59.Xu Y, Zhou J, Carey TE, McHugh JB, Voorhees JJ, Fisher GJ. Receptor-type Protein tyrosine phosphatase beta regulates met phosphorylation and function in head and neck squamous cell carcinoma. Neoplasia. 2012;14:1015–1022. doi: 10.1593/neo.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamane F, Nishikawa Y, Matsui K, Asakura M, Iwasaki E, Watanabe K, Tanimoto H, Sano H, Fujiwara Y, Stanley ER, Kanayama N, Mabbott NA, Magari M, Ohmori H. CSF-1 receptor-mediated differentiation of a new type of monocytic cell with B cell-stimulating activity: its selective dependence on IL-34. J Leukoc Biol. 2014;95:19–31. doi: 10.1189/jlb.0613311. [DOI] [PMC free article] [PubMed] [Google Scholar]