Abstract
Objectives
We quantified the effect of environmental temperature on mouse energy homeostasis and body temperature.
Methods
The effect of environmental temperature (4–33 °C) on body temperature, energy expenditure, physical activity, and food intake in various mice (chow diet, high-fat diet, Brs3-/y, lipodystrophic) was measured using continuous monitoring.
Results
Body temperature depended most on circadian phase and physical activity, but also on environmental temperature. The amounts of energy expenditure due to basal metabolic rate (calculated via a novel method), thermic effect of food, physical activity, and cold-induced thermogenesis were determined as a function of environmental temperature. The measured resting defended body temperature matched that calculated from the energy expenditure using Fourier's law of heat conduction. Mice defended a higher body temperature during physical activity. The cost of the warmer body temperature during the active phase is 4–16% of total daily energy expenditure. Parameters measured in diet-induced obese and Brs3-/y mice were similar to controls. The high post-mortem heat conductance demonstrates that most insulation in mice is via physiological mechanisms.
Conclusions
At 22 °C, cold-induced thermogenesis is ∼120% of basal metabolic rate. The higher body temperature during physical activity is due to a higher set point, not simply increased heat generation during exercise. Most insulation in mice is via physiological mechanisms, with little from fur or fat. Our analysis suggests that the definition of the upper limit of the thermoneutral zone should be re-considered. Measuring body temperature informs interpretation of energy expenditure data and improves the predictiveness and utility of the mouse to model human energy homeostasis.
Keywords: Thermoneutrality, Basal metabolic rate, Cold-induced thermogenesis, Body temperature, Energy expenditure, Heat conductance
Abbreviations: Ta, environmental temperature; Tb, core body temperature; dTb, defended body temperature; EE, energy expenditure; TEE, total energy expenditure; PAEE, physical activity energy expenditure; TEF, thermic effect of food; BMR, basal metabolic rate; CIT, cold-induced thermogenesis; RQ, respiratory quotient; LCT, lower critical temperature; HFD, high-fat diet
1. Introduction
The mouse is a mainstay of obesity research, useful for obtaining mechanistic understanding of clinical observations and for guiding clinical investigation, while allowing experiments not possible in humans. For example, identification of brain regions, neurotransmitters, and connectivity has been determined predominantly in rodents [1] and the mouse is often used to identify pharmaceutical targets [2] and to test candidate drugs. It is crucial to know when human and mouse biology are similar in order to increase the predictive value of mouse models.
Humans and mice differ in mass by ∼3000-fold. Since body surface area scales to the 2/3 power of mass and surface area is a determinant of heat exchange, thermal biology can be different between mice and humans. Inter-species metabolic rate is proposed to scale to the ¾ power of body mass and mass-specific metabolic rate therefore to the −¼ power of body mass, meaning the mouse has a ∼7-fold higher mass-specific metabolic rate than a human [3], [4], [5]. Adult humans live in or near their thermoneutral zone, so body heat is generated predominantly as a byproduct of metabolic processes with a small contribution from adaptive thermogenesis. Upon cold challenge, large mammals maintain an unchanged core body temperature (Tb), while reducing their surface temperature, increasing the size of a substantial thermal ‘shell’ and further insulating the core.
Small mammals, such as mice, have disproportionately greater heat loss, and thus their physiology is oriented more towards heat generation than heat dissipation [6]. Mice are typically studied below their thermoneutral zone [7], [8]. Small mammals have a minimal thermal shell and their Tb, while also tightly regulated, varies much more than in large mammals [9]. In general, as a mammal's body size becomes smaller, the animal will exhibit more variability in Tb [10], greater rates of change in Tb, increased sensitivity to a cool environment (for example in the increase in metabolic rate), a warmer lower bound of the thermoneutral zone, and use of Tb reduction for energy conservation.
The importance of Tb in energy homeostasis has been recognized since at least 1867 (attributed to Sander-Ezn by Ref. [11]) and is acknowledged in reviews [12], [13], [14] but is underappreciated in most studies of mouse energy homeostasis. Previously Tb has been invoked to explain phenotypes, but has not been quantitatively integrated into the analysis. Here we examine the effect of environmental temperature (Ta) on energy homeostasis in mice with continuous measurement of Tb, energy expenditure, physical activity, and food intake. Integration of the Tb data indeed provides new insights into the costs of circadian Tb variation, physical activity, and adaptation to the cold.
2. Experimental methods
2.1. Animals
Mice were singly housed at 21–22 °C with a 12:12-h dark–light cycle (lights on at 0600 h) in a clean, conventional facility with water and food provided ad libitum. Experiments were approved by the NIDDK Institutional Animal Care and Use Committee. Two groups of six mice were studied in each of three experiments (Table S1). Experiment 1 studied 16–20-week old male chow-fed littermate C57BL/6J and Brs3-/y [15] mice. Experiment 2 used 18–19-week old male C57BL/6J mice fed chow (NIH-07, 15 kcal % from fat, Harlan) or a high-fat diet (D12492, 60 kcal % from fat, Research Diets) for the prior 4 weeks. Experiment 3 analyzed 15-week old female chow-fed FVB/N and A-ZIP/F-1 [16] mice.
2.2. Body temperature measurement
G2 E-Mitter transponders (Starr Life Sciences, Oakmont, PA) were implanted intraperitoneally under anesthesia (isoflurane or ketamine 100 mg/kg ip and xylazine 10 mg/kg ip) with flunixin analgesia (2.2 mg/kg sc at operation and daily for two days). Mice were studied at least one week after surgery. Signals were acquired using ER4000 Energizer/Receivers and the manufacturer's software. Body weights are reported after subtraction of the weight of implanted E-Mitters. Body composition was measured by time domain Echo MRI 3-in-1 (Echo Medical Systems, Houston, TX) immediately after euthanasia and removal of the E-Mitter.
2.3. Indirect calorimetry
The indirect calorimetry system (CLAMS using Vital View version 5.0, Columbus Instruments, Columbus, OH) was housed in a temperature-controlled enclosure and used to measure O2 consumption, CO2 production, activity (infrared beam break; one beam break is one count), food intake, and Tb (telemetry) from 12 chambers (2.5 L volume, constant flow rate of 0.5 L/min, sampling flow of 0.4 L/min, without bedding or nesting), each sampled every 13 min. Mice were acclimated to the chambers for 3 days at 22 °C, followed in order by one day each at 22 °C, 26 °C, 30 °C, 33 °C, 28 °C, 24 °C, 18 °C, 12 °C, and 4 °C, with the chamber temperature changed at 1200. Data during Ta transition and cage maintenance (1200–1300) were excluded. Food and water were provided ad libitum at all times. Protein oxidation was not measured and the RQ was not corrected for protein oxidation. Each experiment yielded ∼960 time points per mouse.
2.4. Analysis of energy expenditure components
Each analysis was performed individually for each mouse, at each Ta and each of the light/dark phases (18 data sets per mouse). The total energy expenditure (TEE) was fit by second order regression to the physical activity. Taking the Y-axis intercept as the TEE in the absence of physical activity, the energy expenditure of physical activity (PAEE) was defined as: PAEE = TEE − Y-intercept. Very similar PAEEs were obtained using linear, 2nd, or 4th order regression. The 2nd order regression was used since the fit was slightly better than using linear regression; linear regression is suitable if only the Y-intercept is of interest.
Thermic effect of food (TEF, also known as specific dynamic action) was calculated from the diet manufacturer's data using the consensus thermic effects of fat (2.5%), carbohydrate (7.5%), and protein (25%) [17]. Note that this method of calculating TEF specifically avoids incorporating changes in energy expenditure due to neurobehavioral adaptations to the fed or fasted state. The TEF for chow diet (7022 NIH-07, Harlan; 3.1 metabolizeable kcal/g, 15% calories from fat, 56% calories from carbohydrate, 29% calories from protein) is 11.8% or 0.367 kcal/g. The NIH-07 food quotient was calculated as 0.909 using values of 0.71 for fat, 1.00 for carbohydrate, and 0.835 for protein [17]. Similarly, the TEF for the high fat diet (D12492, Research Diets; 5.24 metabolizeable kcal/g, 60% calories from fat, 20% calories from carbohydrate, 20% calories from protein) is 8.0% or 0.419 kcal/g, with a food quotient of 0.793. In humans, TEF peaks sooner after smaller meals than large ones [18]. Since the time course of TEF in mice is not known, TEF was calculated as the average over each light/dark phase, assuming no time delay.
The basal metabolic rate (BMR) is calculated at thermoneutrality (33 °C) during the light phase. Under these conditions, BMR = TEE–PAEE–TEF. Cold-induced thermogenesis (CIT) was defined as CIT = TEE–PAEE–TEF–BMR.
2.5. Heat conductance after death
The heat conductance in kcal/h/gradient °C (where gradient °C = Tb − Ta) was calculated in mice with implanted E-mitters each minute from 6 to 35 min after death as = Cpsystem*(ΔTb/Δt)/(meanTb − meanTa), where Δt is the 1-min time interval (t2 − t1), ΔTb is the Tb change during the interval (Tb2 − Tb1), meanTb is the mean Tb of the interval (Tb1 + Tb2)/2, and meanTa is the mean Ta of the interval (Ta1 + Ta2)/2 and was constant at 21.6 °C. Thus, the rate of heat loss can also be written: Cpsystem*(Tb2 − Tb1)/(t2 − t1)/([Tb1 + Tb2]/2 − [Ta1 + Ta2]/2). Accounting for the implanted E-mitters, the total heat capacity of the mouse plus E-mitter system, Cpsystem, is Cpmouse*(Mtotal − MEmitter) + (CpEmitter * MEmitter) cal/°C, where MEmitter is the mass of the E-mitter, 1.111 ± 0.008 g (mean ± SEM, n = 21), Mtotal is the mass of the mouse + MEmitter, and CpEmitter is 0.160 ± 0.015 cal/g/°C (n = 7). The heat capacity of a mouse alone, Cpmouse, was taken as 0.9100 − 0.5231 * mf cal/g/°C, where mf is the fat fraction [19]. Since the implanted E-mitter precludes mf measurement by magnetic resonance spectroscopy, mfs were taken from historical data of mice matched for body weight, sex, and genotype. Compared to using Cpsystem = Cpmouse, incorporating CpEmitter into Cpsystem contributed a correction of ≤4%.
2.6. Statistical analysis
Data points represent mean ± SEM of 5–6 mice, unless indicated otherwise. Standard least squares mixed model analysis with mouse as a random effect was performed using JMP 11.0.0 (SAS Institute Inc, Cary, NC).
3. Results
3.1. Determinants of body temperature
We varied Ta from 4 to 33 °C while continuously measuring Tb, TEE, respiratory quotient (RQ), locomotor activity, and food intake in singly housed mice initially acclimatized to 22 °C (Figure S1). Tb was higher in the dark phase, with physical activity, and at 30–33 °C (light phase only), and lower at 4–12 °C (Figure 1A,B). The mild Tb reduction at 4–12 °C was not the large decrease seen in torpor [9]. A statistical analysis demonstrated that Tb variance is explained most by circadian phase and physical activity and less by environmental temperature, with smaller but significant interactions between these factors (Figure 1B; Table S2).
Figure 1.
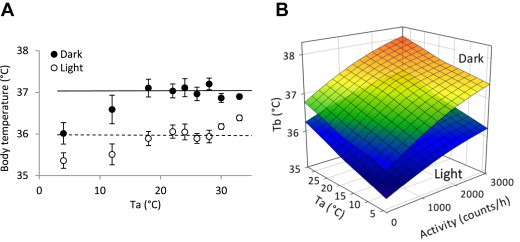
Tb is regulated by mainly by circadian phase and physical activity. A. Effect of circadian phase and Ta. Mean of the 18–28 °C data (the range where Tb is constant) during the dark (solid line) and light (dotted line) phases are indicated. Data are mean ± SEM, n = 11, chow-fed male C57BL/6J mice. B. Effect of circadian phase, activity, and Ta. The surfaces are calculated from the statistical model, detailed in Table S2.
3.2. Partitioning of total energy expenditure
TEE was measured by indirect calorimetry and partitioned into four components: physical activity energy expenditure, thermic effect of food, basal metabolic rate, and cold-induced thermogenesis. Due to the circadian Tb differences, light and dark phase data were analyzed separately. The merged total daily expenditure results are summarized in Figure 2A,B.
Figure 2.
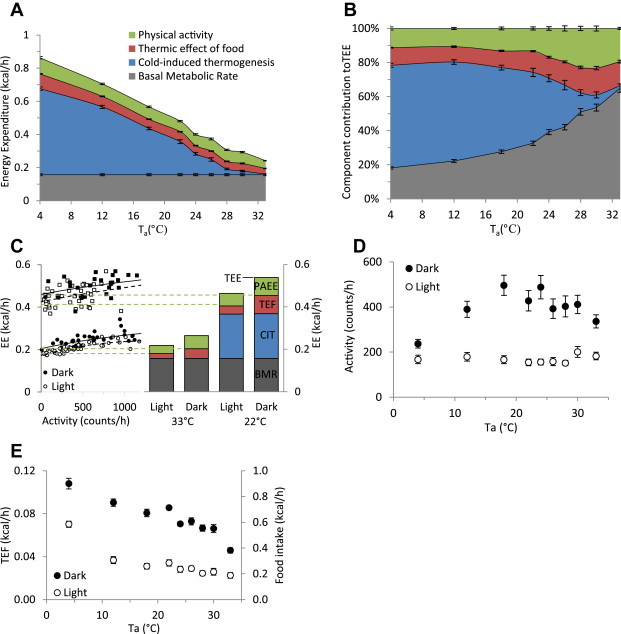
Effect of environmental temperature and light–dark phase on physical activity and food intake. A,B. Components of energy expenditure as a function of environmental temperature. PAEE (green), TEF (red), CIT (blue), and BMR (black) are mean of daily average ± SEM of 11 chow-fed C57BL/6J mice, presented in kcal/h (A) or as a fraction of total daily energy expenditure (B). C. Example of calculation of PAEE in one mouse. PAEE (green) is the TEE minus the EE at rest (no activity), determined by quadratic regression of EE vs activity. TEF (red), CIT (blue), and BMR (black) are defined in the methods and results. Squares are 22 °C; circles are 33 °C. D. Physical activity during the dark and light phases. E. Food intake during the dark and light phases. Data in A,B,D,E are mean ± SEM, pooling 11 chow-fed male C57BL/6J mice from two independent experiments.
PAEE, the energy cost of activity, was calculated as the difference between the EE measured at rest and the TEE (Figure 2C) [20]. The level of light phase physical activity was similar at all environmental temperatures (Figure 2D). Dark phase activity was slightly reduced at 33 °C and more so at 4 °C; in the 12–30 °C range it averaged 72% of the total daily activity.
TEF is the energetic cost of handling and storing ingested food [17], calculated as 12% of the measured caloric intake of the chow diet (see 2.4). Food intake, and thus TEF, increased at cooler Tas (Figure 2E).
BMR is the energy expended in a thermoneutral environment, at physical rest, in the postabsorptive state, and in the absence of other metabolic demands such as growth, pregnancy, or lactation [21]. The BMR can be problematic to measure, since fasting mice adopt behavioral and physiologic changes (locomotion, mild Tb reduction, torpor) that alter EE. We defined BMR as TEE–PAEE–TEF at thermoneutrality (33 °C) during the light phase in the ad libitum fed state. This adjusts measurements taken in the fed state to the fasting level while avoiding the confounding behavioral and physiologic changes of actually fasting the mice.
CIT is the energy expended to maintain Tb and occurs only in an environment below thermoneutrality. CIT was calculated as: CIT = TEE–PAEE–TEF–BMR.
3.3. Cost of physical activity
Multiple regression of TEE vs light phase physical activity and vs Ta shows that PAEE increases with increasing activity at all Tas, with apparently greater cost at 4 °C (Figure 3A). The energy cost of activity was greater in the light phase than in the dark phase (Figure 3B). In general, the energy cost per unit of activity was higher when the resting Tb was lower. The PAEE is 11%, 13%, and 23% of TEE at 4 °C, 22 °C, and 30 °C, respectively.
Figure 3.
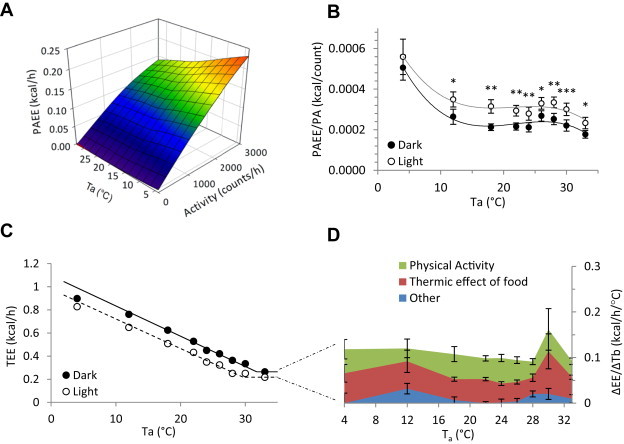
Energy cost of physical activity and of the dark phase. A. Energy cost of physical activity at various environmental temperatures during the light phase. PAEE = 0.0470 − (0.000973 * Ta) + (0.0000381 * activity); SE are 0.00272, 0.000111, and 0.000000730, respectively, adjusted R2 = 0.57. B. Mean light and dark phase apparent PAEE normalized for activity, illustrating the consistently higher normalized cost during the light phase and at Ta = 4 °C (*, p < 0.05; **, p < 0.01; ***, p < 0.001). C. Light and dark phase TEE. D. Cost of maintaining the warmer dark phase Tb. The Y axis is the difference in TEE of the dark and light phases, divided by the differences in mean Tb of the dark and light phases. The EE is attributed to PAEE (green), TEF (red), and residual (blue). Data are mean ± SEM, n = 11, chow-fed male C57BL/6J mice (in C the error bars fall within the symbols).
3.4. Energy cost of the active circadian phase
Energy must be expended to maintain the warmer Tb during the active circadian period (i.e., dark phase for mice, Figure 3C). We calculated this cost to be 0.109 ± 0.007 kcal/h/°C of Tb (in chow-fed C57BL/6J mice, weighing 26.9 ± 0.5 g). The cost of the higher active phase Tb is 4.4%, 11%, and 16% of TEE at 4 °C, 22 °C, and 30 °C, respectively. The extra heat is derived chiefly from the increased dark phase locomotion (PAEE) and food intake (TEF) (Figure 3D).
3.5. Heat conductance and the lower critical temperature
The thermoneutral zone is the Ta range over which EE is at a minimum and Tb is constant [7], [21]. The lower boundary of the thermoneutral zone is the lower critical temperature (LCT), the point at which heat conservation mechanisms (e.g., vasoconstriction, minimization of evaporative heat loss) are maximally recruited. Below the LCT, energy-expending mechanisms (CIT) are activated to maintain Tb, and the EE vs Ta line indicates the heat required to maintain Tb [22], [23]. Figure 4A illustrates the dependence of EE on Ta (only the 18–28 °C data are used for the regression, since Tb is reduced at cooler Tas, see Figure 1A). The LCT is 29.1–29.6 °C in the light phase (Table 1). From Fourier's law of heat conduction, the extrapolated X-intercept is the point where net heat transfer is zero and the Ta equals the apparent defended Tb (dTb). The slope in this graph is the heat conductance, which mathematically is identical to 1/insulation. The slope (conductance) was similar when calculated from TEE, TEE-PAEE, or TEE-PAEE-TEF (Table 1). However, the dTbs differ, being highest for TEE, then TEE-PAEE, and the lowest for TEE-PAEE-TEF (38.1 ± 0.3, 36.2 ± 0.3, and 35.4 ± 0.2 °C, respectively; light phase data). The dTbs calculated from the TEE-PAEE and TEE-PAEE-TEF agree remarkably well with the measured Tbs (Table 1), an internal check of the data [24]. The dTbs calculated from the TEE are ∼2 °C higher (Table 1), suggesting that the Tb set point is increased in the physically active state.
Figure 4.
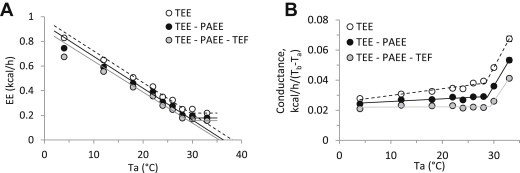
Heat conductance and energy expenditure. A. Energy expenditure as a function of environmental temperature during the light phase. TEE is total energy expenditure (open symbol, dashed line), TEE-PAEE is resting energy expenditure (black symbol, black line), and TEE-PAEE-TEF is basal energy expenditure (grey symbol, grey line). Regression lines were calculated using the 18–28 °C Ta range. The X intercept is the defended body temperature, dTb. The LCT is the Ta at which the regression line meets the thermoneutral metabolic rate. B. Effect of environmental temperature on heat conductance, calculated as EE/(Tb − Ta) during light phase. The LCT is the point above which the conductance increases. Data are mean ± SEM, n = 11, chow-fed male C57BL/6J mice (some error bars are within the symbols).
Table 1.
Parameters calculated from the dependence of energy expenditure on environmental temperature.
| Units | C57BL/6J chow |
C57BL/6J HFD |
C57BL/6 Brs3-/y |
FVB/N |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Dark | Light | Dark | Light | Dark | Light | Dark | Light | ||
| Tb (mean, Ta 18–28 °C) | °C | 37.08 ± 0.17 | 35.96 ± 0.15 | 36.91 ± 0.05 | 35.99 ± 0.06 | 37.09 ± 0.08 | 35.81 ± 0.13 | 37.03 ± 0.11 | 36.70 ± 0.10 |
| dTb (TEE vs Ta) | °C | 41.9 ± 0.4 | 38.1 ± 0.3 | 42.2 ± 1.0 | 39.2 ± 0.4 | 42.2 ± 0.6 | 39.0 ± 0.4 | 45.8 ± 0.8 | 43.1 ± 0.8 |
| dTb (TEE–PAEE vs Ta) | °C | 38.6 ± 0.6 | 36.2 ± 0.3 | 38.1 ± 0.3 | 36.9 ± 0.4 | 41.0 ± 0.7 | 36.3 ± 0.4 | 42.7 ± 3.9 | 40.9 ± 1.7 |
| dTb (TEE–PAEE–TEF vs Ta) | °C | 36.3 ± 0.5 | 35.4 ± 0.1 | 36.6 ± 0.4 | 36.2 ± 0.3 | 37.4 ± 0.4 | 35.1 ± 0.4 | 41.1 ± 3.7 | 40.2 ± 1.8 |
| LCT (TEE vs Ta) | °C | 31.7 ± 0.2 | 29.6 ± 0.2 | 31.4 ± 0.4 | 30.7 ± 0.1 | 30.9 ± 0.2 | 29.7 ± 0.2 | 31.2 ± 0.4 | 30.8 ± 0.6 |
| LCT (conductance vs Ta) TEE | °C | 28.4 ± 0.1 | 29.0 ± 0.2 | 28.5 ± 0.2 | 28.0 ± 0.2 | 27.8 ± 0.2 | 28.5 ± 0.1 | 29.0 ± 0.2 | 28.3 ± 0.8 |
| LCT (TEE–PAEE vs Ta) | °C | 30.3 ± 0.3 | 29.2 ± 0.2 | 30.7 ± 0.2 | 30.5 ± 0.2 | 30.7 ± 0.3 | 28.4 ± 0.1 | 30.3 ± 0.5 | 30.1 ± 0.3 |
| LCT (conductance vs Ta) TEE–PAEE | °C | 28.8 ± 0.1 | 29.2 ± 0.1 | 28.9 ± 0.2 | 29.2 ± 0.1 | 27.8 ± 0.3 | 28.6 ± 0.1 | 28.9 ± 0.4 | 29.0 ± 0.4 |
| LCT (TEE-PAEE-TEF vs Ta) | °C | 29.5 ± 0.3 | 29.1 ± 0.3 | 30.1 ± 0.2 | 30.5 ± 0.1 | 29.4 ± 0.2 | 28.2 ± 0.1 | 30.2 ± 0.4 | 30.5 ± 0.3 |
| LCT (conductance vs Ta) TEE–PAEE–TEF | °C | 29.4 ± 0.3 | 29.5 ± 0.1 | 29.7 ± 0.1 | 29.8 ± 0.2 | 28.4 ± 0.3 | 28.9 ± 0.1 | 29.2 ± 0.7 | 29.0 ± 0.6 |
| slope (TEE vs Ta) | kcal/h/°C | −0.0260 ± 0.0005 | −0.0254 ± 0.0007 | −0.0316 ± 0.0012 | −0.0310 ± 0.0004 | −0.0268 ± 0.0009 | −0.0256 ± 0.0009 | −0.0264 ± 0.0011 | −0.0270 ± 0.0004 |
| slope (TEE–PAEE vs Ta) | kcal/h/°C | −0.0254 ± 0.0009 | −0.0255 ± 0.0006 | −0.0336 ± 0.0008 | −0.0327 ± 0.0008 | −0.0236 ± 0.0009 | −0.0263 ± 0.0010 | −0.0274 ± 0.0040 | −0.0272 ± 0.0028 |
| slope (TEE–PAEE–TEF vs Ta) | kcal/h/°C | −0.0240 ± 0.0008 | −0.0248 ± 0.0005 | −0.0333 ± 0.0010 | −0.0327 ± 0.0006 | −0.0238 ± 0.0009 | −0.0260 ± 0.0011 | −0.0250 ± 0.0038 | −0.0241 ± 0.0025 |
Tb, is the measured mean body temperature, using all environmental temperatures from 18 to 28 °C. dTb is the calculated defended Tb, the X-intercept of the energy expenditure vs Ta graph. LCT is the lower critical temperature, the lower bound of the thermoneutral zone. The LCT is reported as calculated from the energy expenditure vs Ta graph and from the conductance vs Ta graph. Slope is the conductance, the slope of the energy expenditure vs Ta graph. All parameters were calculated for each mouse and then averaged. Data are mean ± SEM, n = 6/group (n = 11 for the C57BL/6J chow group).
The measured Tb can be used to calculate the conductance (=EE/(Tb − Ta) [25]), and this method is applicable even when the Tb changes, such as at Ta = 4–12 °C. The plot of the heat conductance vs Ta is flat at cool Tas, then abruptly increases with increasing Ta, with the transition point Ta being the LCT (Figure 4B). This method of determining the LCT is less variable and less dependent on the BMR measurement and source of the EE data (i.e., TEE vs TEE-PAEE vs TEE-PAEE-TEF) (Table 1).
3.6. Energy balance
There was no net change in body weight over the 9-day experiment (0.05 ± 0.20 g). Consistent with the unchanged weights, total food intake was not significantly different from energy expenditure (−1.1 ± 2.5 kcal; this is −1.6 ± 2.5% of the 9-day caloric intake). However, daily food intake differed from daily TEE (Figure S2A), suggesting that body weight changed at intermediate points. Food intake was greater than TEE during the active dark phase at Tas of 21–33 °C and was below TEE during the light phase from 4–26 °C. Daily food intake was 15–26% below TEE at 4–18 °C and was 2–27% greater than TEE at 22–33 °C.
Changes in RQ tracked well with the net energy changes (Figure S2B). At warm Tas, the RQ was greater than the food quotient, consistent with net lipogenesis and weight gain. At cool Tas in the light phase, the RQ was below the food quotient, indicating net fatty acid oxidation, consistent with weight loss. In a subsequent experiment we measured body weight daily and the expected weight gain at warm Tas and loss at low Tas was observed (not shown).
3.7. Application to mutant mice
We next applied this analysis to mutant mice. Brs3-/y mice are mildly hypometabolic and hyperphagic and develop obesity [15], [26]. The Brs3-/y mice had the expected increased fat mass, while activity was similar to controls (Figure S3A–C). Interestingly, the light phase Tb was reduced (in agreement with [27]) at 18–26 °C, but was comparable to controls at 4–12 °C (Figure S3D). Compared to wild type mice, the Brs3-/y mutants tended to eat less and burn more fat at 4–12 °C (Figure S3E,F). The EE vs Ta plot and heat loss were remarkably similar to wild type mice (Figure S3G,H).
We also studied chow-fed fatless (lipodystrophic) female A-ZIP/F-1 mice on the FVB/N genetic background [16]. The A-ZIP/F-1 mice have virtually no fat and an increased lean mass (Figure S4A). The female wild type FVB/N mice, compared to male wild type C57BL/6J mice, were more active and had a higher Tb at 30–33 °C (Figure S4B,C,E vs Figure S5A–C), possibly due to the different sexes or higher sympathetic tone [28]. Tb varied more in the A-ZIP/F-1 mice, with higher Tbs at warm Tas and lower Tbs at cold Tas (Figure S4E). The Tb reduction and loss of light–dark variation in activity and food intake in the A-ZIP/F-1 mice (Figure S4D) presumably result from the animals' efforts to ensure an energy supply despite greatly reduced caloric stores [29]. Unlike the C57BL/6J mice, wild type FVB/N mice did not have a low RQ or reduced food intake at 12 °C. At the warmer temperatures, the wild type FVB/N mice had an increased RQ and food intake and gained weight (Figure S4F,G; Table S1). The LCT of wild type FVB/N mice is similar to C57BL/6J mice; the dTb may be higher than in C57BL/6J mice (Figure S4H,I; Table 1). The variability of the A-ZIP/F-1 Tb and energy expenditure data precluded calculation of LCT and dTb.
3.8. Effect of a high-fat diet
To investigate the effect of body composition, we studied mice on a high-fat diet (HFD). The HFD mice had a 7-fold greater fat mass but similar fat-free mass as chow-fed controls (Figure 5A). HFD mice also had slightly less locomotor activity than control mice, but similar PAEE, presumably due to the higher cost of moving the heavier body mass (Figure S5A,B). Tb was similar in HFD and control mice; notably the increased adiposity did not protect Tb at lower Tas (Figure S5C). At warmer Tas, both control and HFD mice were in positive energy balance, with an RQ greater than the food quotient, indicating net lipogenesis. At cooler temperatures both groups were in net negative energy balance (food intake less than TEE and RQ less than food quotient), with the HFD group having a more negative balance (Figure S5D,E). At the end of the experiment, body weight was unchanged in the chow-fed mice, while the HFD mice lost weight (−5.95 ± 0.51 g, p < 0.001). The EE vs Ta graph showed an apparent difference in slope, which disappeared when EE was expressed relative to BMR (Figure 5B–D). The heat conductance correlates best with body weight (not shown); the greater range of fat masses causes fat mass to correlate with body weight (and conductance) better than lean mass does (Figure 5E). In contrast, the BMR is much more dependent on the lean mass (or body weight) than the fat mass (Figure 5F).
Figure 5.
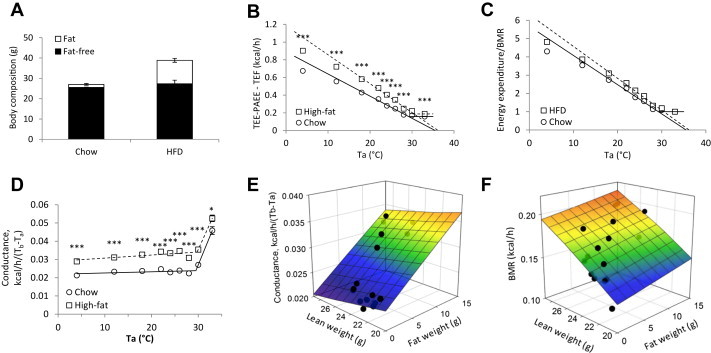
Effect of environmental temperature and light–dark phase on body temperature and energy homeostasis. A. Body composition. B. Energy expenditure as a function of environmental temperature during the light phase. Regression lines were calculated using the 18–28 °C Ta range. C. Energy expenditure as a function of environmental temperature during the light phase. Data as in B, but normalized with BMR set to 1. D. Effect of environmental temperature on the conductance, calculated as EE/(Tb − Ta) during light phase. E. Dependence of the heat conductance on lean and fat weight. Conductance = 0.0304 + (0.000884 * Fat) − (0.000339 * Lean); SE are 0.0114, 0.000191, and 0.000501, respectively, adjusted R2 = 0.71. F. Dependence of BMR on lean and fat weight. BMR = −0.00626 + (0.00113 * fat) + (0.00694 * lean); SE are 0.0682, 0.00114, and 0.00299, respectively, adjusted R2 = 0.58. In B-D, chow-fed data are circles and solid lines; HFD data are squares and dashed lines. Data are mean ± SEM of 6 chow-fed and 5 high-fat-fed male C57BL/6J mice (*, p < 0.05; ***, p < 0.001).
3.9. Determinants of heat conductance/insulation
The small effect of fat mass on heat conduction prompted the question of what controls insulation in the mouse. We measured heat loss after death, since after a few minutes, there is little heat production or biological regulation of heat loss [30]. After death, the conductance was 0.104 kcal/h/mouse/°C (Figure 6A). This is ∼450% greater than in living mice, whereas removal of the fur produced only a 40–70% increase [31](Figure 6B). Thus in mice insulation is predominantly provided by physiological rather than structural (e.g., fur, adiposity) mechanisms.
Figure 6.
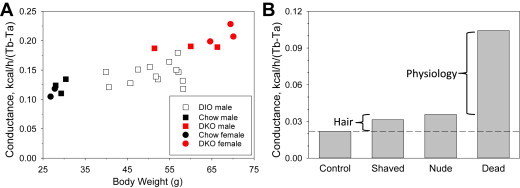
Contributors to insulation in mice. A. Heat conductance after death. Mice were euthanized by cervical dislocation, Tb was monitored by E-mitter, and the mean heat conductance from 6 to 35 min after death was calculated for each mouse. To obtain a range of body weights, male and female chow-fed C57BL/6J, male and female Mc3r−/−;Mc4r−/− (DKO, raw data from Ref. [71]), and male high fat diet-fed C57BL/6J mice were studied, as indicated. B. Heat conductance summary. Heat conductance during life (Control and dashed reference line, from Figure 5D), after death (Dead, from A, using a body weight of 29 g), and of shaved (Shaved) and nude (Nude) living mice, both approximated based on data in Ref. [31]. The contribution of fur is the difference between the nude/shaved and control groups. The non-mechanical (“physiology’) component of insulation is the difference between the dead and nude/shaved groups.
4. Discussion
4.1. Effect of environmental temperature on energy expenditure
We examined the dependence on environmental temperature of the four main components of energy expenditure: basal metabolic rate, thermic effect of food, physical activity, and cold-induced thermogenesis (Figure 2A,B). We are unaware of similar integrated analyses. At thermoneutrality these components comprise approximately 60%, 12%, 25%, and 0%, respectively, of total energy expenditure, which is similar to the percentages in humans with a low activity level. However, at a vivarium Ta (22 °C), CIT increases to ∼120% of BMR in mouse, while it is ≤ 5% of BMR in humans [32], [33]. At 4 °C, these components are approximately 20%, 12%, 10%, and 60%, respectively, of TEE.
BMR can be difficult to measure in mice due to confounding effects of physical activity, ongoing adaptation to the postabsorptive state, and variation in Tb. BMR is typically measured by selecting an interval of minimum EE during a short fast [13] (but see Ref. [34]). The method introduced here has the advantages of using a larger interval for sampling, avoiding the dynamic changes caused by fasting, and omitting the need to pick one interval at the extreme of a distribution, which can introduce variation. Using our approach, BMR is ∼60% of TEE at thermoneutrality, ∼30% at vivarium temperatures, and only ∼20% at 4 °C.
Cold-induced energy expenditure is the most variable component of TEE, ranging from none at thermoneutrality, to ∼40% at vivarium temperatures and ∼60% at 4 °C. These percentages slightly underestimate the caloric demand of the cold, since the energy cost of physical activity is higher in the cold, the increased food intake increases the TEF, and the lower Tb may reduce the BMR. A major source of heat for CIT is brown/beige/brite adipose tissue [35], [36]. Since mice lacking Ucp1 (the thermal engine in brown/beige/brite adipose tissue) are able to defend Tb, non-adipose tissues, such as muscle, likely also contribute heat [37], [38], [39]. Small mammals must be able to cope with the cold – otherwise they would be confined to tropical niches. Indeed, after adaptation, C57BL/6J mice can live and breed indefinitely at −3 °C [40]. The food intake response in lean mice promptly matched or nearly matched the energy expenditure at each of the Ta conditions. This rapid coupling of intake and expenditure is similarly an important feature of energy homeostasis. The huge role for CIT in mice means that an obesity drug that increases energy expenditure may be more effective in mice at thermoneutrality than at typical vivarium temperatures, where a compensatory reduction in CIT can offset the drug-induced increase [41], [42], [43]. This suggests that treating mice at or near thermoneutrality might better predict drug efficacy in humans.
4.2. Physical activity increases the body temperature set point
Physical activity increased the measured Tb. The Tb increase could be a byproduct of muscle metabolism, since muscle physical work is only ∼20% efficient [44]. We are not aware of prior comparisons of active and resting dTb in mice. Most previously reported dTbs are high, in the 40–42 °C range [45], [46], [47], [48], presumably because they include physical activity. An exception is the dTb of 37 °C when resting conditions were specifically used [49]. The increased Tb and dTb suggest that the Tb set point increases with activity (use of ‘set point’ is not intended to address the mechanism by which Tb is regulated [50]). A warmer Tb set point would aid heat dissipation by reducing the need for evaporative heat loss and by increasing the Tb-Ta gradient, the driving force for non-evaporative heat loss [51]. An increased Tb also improves muscle performance, a reason why athletes ‘warm up’ [52]. The observed Tb (particularly below thermoneutrality) and dTb increases with activity support the improved muscle performance hypothesis over the postulated role in heat dissipation.
The increased energy cost per unit of activity at 4 °C is likely due to increased heat loss from the less compact body position and disruption of the unstirred air layer. The higher energy cost per unit of activity at lower Tbs may also include heat generation, such as by brown/beige/brite adipose tissue, to reach the higher Tb set point.
The apparent PAEE ranged from ∼20% of TEE at thermoneutrality to ∼10% at 4 °C. Others have reported PAEE values of 5% and 27% of TEE [53], [54]. A recent paper noted that the cost of physical activity was not independent of other contributors to energy expenditure, particularly below thermoneutrality [54]. Here we show how the confounding can be minimized and the cost of physical activity be measured at cooler Tas. We have not corrected the PAEE values for the non-activity thermogenesis required to reach the higher Tb set point, so the PAEE percentage at 4 °C is likely a slight overestimate. These results suggest that measuring the true energy cost of physical activity in the mouse might best be done during the active (dark) phase, at a Ta within the thermoneutral zone near the LCT, where the animal can increase Tb passively and yet have sufficient heat dissipation reserve available to avoid overheating.
4.3. Circadian variation in body temperature and energy expenditure
In our experiments, the mice at 4 °C did not enter torpor [9], but did reduce Tb slightly. It is advantageous for a mouse to use hypothermia for energy conservation. The Tb reduction at 4 °C may not be an inability to defend a higher Tb, since the circadian variation is preserved.
Circadian variation in Tb occurs in all mammals due to regulation of the Tb set point [10]. One could define a separate category for the energy expenditure needed to achieve the warmer active phase. We chose not to do so since the extra heat comes from increased dark phase locomotion (PAEE) and food intake (TEF). However, if these were insufficient, the additional heat generation would be attributed to CIT in our accounting. The warmer Tb of the active phase costs 4%–16% of daily TEE. While the biological rationale for circadian variation in Tb is unclear, its evolutionary conservation despite the significant energetic cost suggests that a fundamental benefit is achieved by circadian thermal cycling.
4.4. Thermoneutral zone definition
The upper end of the thermoneutral zone is often defined by an increase in energy expenditure (e.g., due to increased activity from looking for a cooler location) or as the start of evaporative heat loss [8], [13], [36], [55]. There are also alternative thermoneutral zone definitions, including the thermal region of active vasomotor thermoregulation [56]. In the FVB mice, hyperthermia occurred without an increase in resting energy expenditure (Figure S4E,H), which is inconsistent with using an increase in energy expenditure as the definition. Indeed, when resting energy expenditure was measured, it did not increase, even at Ta > dTb [49], where Tb and evaporative heat loss were likely increased. These considerations suggest that the upper limit of the thermoneutral zone should not be defined by an increase in resting energy expenditure and might better be defined by an increase in Tb.
4.5. Limitations and variables affecting measurement
Metabolic rate and related parameters are notoriously dependent on the state of the mouse and measurement details, including Ta, Tb, activity (level, type, and measurement method), circadian phase, food status, body weight, body composition, acclimatization, stress, enrichment, humidity, air circulation, single housing, and bedding [12], [13]. We acclimated the mice to the chambers for three days and then exposed them to each Ta for one day. The measurements were not at steady state with weight gain at warm temperatures and weight loss at cool temperatures, particularly in mice with higher adiposity. Increasing the time at each Ta would introduce different confounders, since brown/beige/brite adipose tissue adapts to the cold [35] and adiposity increases at thermoneutrality [57], [58]. TEF was assumed to be constant, however it is possible that TEF is modulated by gut microbiota, energy demand, or other factors. We also did not measure the energy content of the feces. We measured BMR at thermoneutrality and made the simplifying assumption that it is constant at lower Tas. However, if one postulates a Q10 (van't Hoff coefficient) of 2.5, a typical value for biological systems [59], a 1 °C Tb reduction would lower the BMR by 9%. A significant technical advance would be a calorimetry system with a high sampling frequency and small time constant [60], allowing finer analysis of the effects of physical activity and TEF on energy expenditure. There is much discussion of how to compare the BMR of mice with different body weights and composition [12], [13]. The dependence of PAEE, CIT, and TEF on body weight and composition may be similarly complex. Despite the acknowledged limitations of indirect calorimetry [54], [61], reliable, reproducible observations are possible and do yield biological insights.
4.6. Insulation in mice
The insulation/conductance is a function of Ta, Tb, insulator thickness, and surface area [62]. In mice, lack of fur causes a 40–70% increase in light phase metabolic rate [31]. However, mouse fur is only 0.13 cm thick and has a thermal conductivity of 0.0000995 cal/s/cm/°C [63], meaning that it is a relatively poor insulator [64]. For perspective, in humans a constant temperature is not reached until a tissue depth of 2.5–7 cm [65]. Thus our observation that changing adiposity from 6% to 30% did not alter the heat conductivity is reasonable – in mice even a large increase in adiposity does not contribute significantly to insulation. This implies that ‘insulation’ function in the mouse includes a large physiological contribution, such as by minimization of perfusion near the body surface and recovery of heat by counter current circulation.
The remarkable linearity of the EE vs Ta slope (when Tb is constant) might seem surprising, given the multiple mechanisms contributing to heat loss (conduction, radiation, convection, and evaporation). However, the first three mechanisms have an almost linear dependence on Tb-Ta [66] and evaporative heat loss depends greatly on air circulation and humidity, but is minimized at cool Tas (only 7% of heat loss at 15 °C [67]).
4.7. Application to mutant mice
Having established a system to study the interactions of Ta, Tb, activity, and metabolic rate, we applied it to mutant mice, but were disappointed by the results. In A-ZIP/F-1 mice, Tb varied greatly, likely due to the severity of the adipose deficiency. The Tb variation invalidates some of the assumptions in the analyses of energy expenditure components, thus demonstrating the utility of monitoring Tb during studies of energy expenditure. In contrast, the Brs3-/y mice, except for a slightly reduced Tb, had a phenotype remarkably similar to control mice. The likely explanation is that Brs3-/y mice have intrinsically normal anatomic/physiologic abilities to alter Tb and EE, but use them to defend a slightly different Tb set point. Only a few other investigations have studied mutant mice in analogous ways [48], [49], [68], [69], [70]. Dwarf mice, weighing 40% of controls, had slightly increased LCT and reduced dTb [69].
4.8. Conclusions
Mice can regulate Tb within a relatively wide range, so measuring Tb improves the analysis and interpretation of energy expenditure data. The large fraction of energy expenditure used to defend Tb in the mouse contrasts with human thermal biology. Thus, mouse energy expenditure phenotyping is hugely affected by Ta. This exaggerated thermal biology in mice is useful; it can be harnessed to study physiology that is subtle in humans. Understanding the species differences also guides use of the mouse to model human energy homeostasis, for example by studying obesity drugs at thermoneutrality to improve predictiveness.
Acknowledgments
We thank Jan Nedergaard, Barbara Cannon, Alexxai Kravitz, Kong Chen, Kevin Hall, Aaron Cypess, and Clifton Bogardus for insightful discussions and Ruifeng Teng for technical assistance. This research was supported by the Intramural Research Program (DK075062, DK075063, DK075064) of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH. The visit of G. Abreu-Vieira to NIH was supported within a grant from the Swedish Research Council to Jan Nedergaard and from institutional funds from the Department of Molecular Biosciences, Wenner-Gren Institute, Stockholm University.
Footnotes
Supplementary data related to this article can be found at doi:10.1016/j.molmet.2015.03.001.
Contributor Information
Gustavo Abreu-Vieira, Email: gustavo.vieira@su.se.
Cuiying Xiao, Email: cuiyingx@niddk.nih.gov.
Oksana Gavrilova, Email: OksanaG@BDG10.NIDDK.nih.gov.
Marc L. Reitman, Email: marc.reitman@nih.gov.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Williams K.W., Elmquist J.K. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nature Neuroscience. 2012;15(10):1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brommage R. High-throughput screening of mouse knockout lines identifies true lean and obese phenotypes. Obesity. 2008;16(10):2362–2367. doi: 10.1038/oby.2008.361. [DOI] [PubMed] [Google Scholar]
- 3.Kleiber M. The fire of life. Robert E. Krieger Publishing Company; Huntington, New York: 1975. Body size and metabolic rate; pp. 179–222. [Google Scholar]
- 4.West G.B., Brown J.H., Enquist B.J. A general model for the origin of allometric scaling laws in biology. Science. 1997;276(5309):122–126. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt-Nielsen K. Scaling: why is animal size so important? Cambridge Univeristy Press; Cambridge: 1984. Metabolic rate and body size; pp. 56–74. [Google Scholar]
- 6.Gordon C.J. Cambridge University Press; New York: 1993. Temperature regulation in laboratory rodents. 276. [Google Scholar]
- 7.Cannon B., Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. Journal of Experimental Biology. 2011;214(Pt 2):242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 8.Gordon C.J. Thermal physiology of laboratory mice: defining thermoneutrality. Journal of Thermal Biology. 2012;37:654–685. [Google Scholar]
- 9.Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annual Review of Physiology. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- 10.Refinetti R. The circadian rhythm of body temperature. Frontiers in Bioscience. 2010;15:564–594. doi: 10.2741/3634. [DOI] [PubMed] [Google Scholar]
- 11.Rubner M. Academic Press; New York: 1982. The laws of energy consumption in nutrition. Nutrition Foundations' Reprints. 371. [Google Scholar]
- 12.Tschop M.H. A guide to analysis of mouse energy metabolism. Nature Methods. 2012;9:57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speakman J.R. Measuring energy metabolism in the mouse - theoretical, practical, and analytical considerations. Frontiers in Physiology. 2013;4:34. doi: 10.3389/fphys.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landsberg L. Core temperature: a forgotten variable in energy expenditure and obesity? Obesity Reviews. 2012;13(Suppl. 2):97–104. doi: 10.1111/j.1467-789X.2012.01040.x. [DOI] [PubMed] [Google Scholar]
- 15.Ladenheim E.E. Factors contributing to obesity in bombesin receptor subtype-3-deficient mice. Endocrinology. 2008;149(3):971–978. doi: 10.1210/en.2007-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moitra J. Life without white fat: a transgenic mouse. Genes & Development. 1998;12(20):3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Food and Nutrition Board . Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. The National Academies Press; Washington, DC: 2005. Energy; pp. 107–264. [Google Scholar]
- 18.D'Alessio D.A. Thermic effect of food in lean and obese men. Journal of Clinical Investigation. 1988;81(6):1781–1789. doi: 10.1172/JCI113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faber P., Garby L. Fat content affects heat capacity: a study in mice. Acta Physiologica Scandinavica. 1995;153(2):185–187. doi: 10.1111/j.1748-1716.1995.tb09850.x. [DOI] [PubMed] [Google Scholar]
- 20.Ravussin E. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. Journal of Clinical Investigation. 1986;78(6):1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiber M. The fire of life. Robert E. Krieger Publishing Company; Huntington, New York: 1975. Animal temperature regulation; pp. 150–178. [Google Scholar]
- 22.Kleiber M., Dougherty J.E. The influence of environmental temperature on the utilization of food energy in baby chicks. Journal of General Physiology. 1934;17(5):701–726. doi: 10.1085/jgp.17.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholander P.F. Heat regulation in some arctic and tropical mammals and birds. Biological Bulletin. 1950;99(2):237–258. doi: 10.2307/1538741. [DOI] [PubMed] [Google Scholar]
- 24.McNab B.K. On estimating thermal conductance in endotherms. Physiological Zoology. 1980;53(2):145–156. [Google Scholar]
- 25.Mount L.E. Metabolic rate and thermal insulation in albino and hairless mice. Journal of Physiology. 1971;217(2):315–326. doi: 10.1113/jphysiol.1971.sp009573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohki-Hamazaki H. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature. 1997;390(6656):165–169. doi: 10.1038/36568. [DOI] [PubMed] [Google Scholar]
- 27.Lateef D.M. Regulation of body temperature and brown adipose tissue thermogenesis by bombesin receptor subtype-3. American Journal of Physiology. Endocrinology and Metabolism. 2014;306(6):E681–E687. doi: 10.1152/ajpendo.00615.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shusterman V. Strain-specific patterns of autonomic nervous system activity and heart failure susceptibility in mice. American Journal of Physiology. Heart and Circulatory Physiology. 2002;282(6):H2076–H2083. doi: 10.1152/ajpheart.00917.2001. [DOI] [PubMed] [Google Scholar]
- 29.Gavrilova O. Torpor in mice is induced by both leptin-dependent and -independent mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(25):14623–14628. doi: 10.1073/pnas.96.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hart J.S. Calorimetric determination of average body temperature of small mammals and its variation with environmental conditions. Canadian Journal of Zoology. 1951;29:224–233. [Google Scholar]
- 31.Hirata M. Genetic defect in phospholipase Cdelta1 protects mice from obesity by regulating thermogenesis and adipogenesis. Diabetes. 2011;60(7):1926–1937. doi: 10.2337/db10-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celi F.S. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. European Journal of Endocrinology. 2010;163(6):863–872. doi: 10.1530/EJE-10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wijers S.L. Beta-adrenergic receptor blockade does not inhibit cold-induced thermogenesis in humans: possible involvement of brown adipose tissue. Journal of Clinical Endocrinology & Metabolism. 2011;96(4):E598–E605. doi: 10.1210/jc.2010-1957. [DOI] [PubMed] [Google Scholar]
- 34.van Klinken J.B., van den Berg S.A., van Dijk K.W. Practical aspects of estimating energy components in rodents. Frontiers in Physiology. 2013;4:94. doi: 10.3389/fphys.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 36.Nedergaard J., Cannon B. The browning of white adipose tissue: some burning issues. Cell Metabolism. 2014;20(3):396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Golozoubova V. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB Journal. 2001;15(11):2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- 38.Ukropec J. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice. Journal of Biological Chemistry. 2006;281(42):31894–31908. doi: 10.1074/jbc.M606114200. [DOI] [PubMed] [Google Scholar]
- 39.Bal N.C. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nature Medicine. 2012;18(10):1575–1579. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnett S.A. Adaptation of mice to cold. Biological Reviews of the Cambridge Philosophical Society. 1965;40:5–51. doi: 10.1111/j.1469-185x.1965.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 41.Shemano I., Nickerson M. Effect of ambient temperature on thermal responses to drugs. Canadian Journal of Biochemistry and Physiology. 1958;36(12):1243–1249. [PubMed] [Google Scholar]
- 42.Lodhi I.J., Semenkovich C.F. Why we should put clothes on mice. Cell Metabolism. 2009;9(2):111–112. doi: 10.1016/j.cmet.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Goldgof M. The chemical uncoupler 2,4-dinitrophenol (DNP) protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. Journal of Biological Chemistry. 2014;289(28):19341–19350. doi: 10.1074/jbc.M114.568204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaesser G.A., Brooks G.A. Muscular efficiency during steady-rate exercise: effects of speed and work rate. Journal of Applied Physiology. 1975;38(6):1132–1139. doi: 10.1152/jappl.1975.38.6.1132. [DOI] [PubMed] [Google Scholar]
- 45.Pertwee R.G., Tavendale R. Effects of delta9-tetrahydrocannabinol on the rates of oxygen consumption of mice. British Journal of Pharmacology. 1977;60(4):559–568. doi: 10.1111/j.1476-5381.1977.tb07535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trayhurn P., James W.P. Thermoregulation and non-shivering thermogenesis in the genetically obese (ob/ob) mouse. Pflügers Archiv. 1978;373(2):189–193. doi: 10.1007/BF00584859. [DOI] [PubMed] [Google Scholar]
- 47.Alberts P., Johansson B.G., McArthur R.A. 2006. Characterization of energy expenditure in rodents by indirect calorimetry. Current protocols in neuroscience/editorial board, Jacqueline N. Crawley... [et al.] [Chapter 9]: p. Unit9 23D. [DOI] [PubMed] [Google Scholar]
- 48.Hogberg H. Temperature dependence of O2 consumption; opposite effects of leptin and etomoxir on respiratory quotient in mice. Obesity. 2006;14(4):673–682. doi: 10.1038/oby.2006.76. [DOI] [PubMed] [Google Scholar]
- 49.Golozoubova V. Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Molecular Endocrinology. 2004;18(2):384–401. doi: 10.1210/me.2003-0267. [DOI] [PubMed] [Google Scholar]
- 50.Romanovsky A.A. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2007;292(1):R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt-Nielsen K. How animals work. Cambridge University Press; Cambridge: 1972. Exercise, energy, and evaporation; pp. 51–67. [Google Scholar]
- 52.Bennett A.F. Thermal dependence of muscle function. American Journal of Physiology. 1984;247(2 Pt 2):R217–R229. doi: 10.1152/ajpregu.1984.247.2.R217. [DOI] [PubMed] [Google Scholar]
- 53.Dauncey M.J., Brown D. Role of activity-induced thermogenesis in twenty-four hour energy expenditure of lean and genetically obese (ob/ob) mice. Quarterly Journal of Experimental Physiology. 1987;72(4):549–559. doi: 10.1113/expphysiol.1987.sp003096. [DOI] [PubMed] [Google Scholar]
- 54.Virtue S., Even P., Vidal-Puig A. Below thermoneutrality, changes in activity do not drive changes in total daily energy expenditure between groups of mice. Cell Metabolism. 2012;16(5):665–671. doi: 10.1016/j.cmet.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.IUPS Glossary of terms for thermal physiology. Japanese Journal of Physiology. 2001;51(2):245–280. [Google Scholar]
- 56.Romanovsky A.A., Ivanov A.I., Shimansky Y.P. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. Journal of Applied Physiology. 2002;92(6):2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- 57.Rippe C. Effect of high-fat diet, surrounding temperature, and enterostatin on uncoupling protein gene expression. American Journal of Physiology. Endocrinology and Metabolism. 2000;279(2):E293–E300. doi: 10.1152/ajpendo.2000.279.2.E293. [DOI] [PubMed] [Google Scholar]
- 58.Castillo M. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes. 2011;60(4):1082–1089. doi: 10.2337/db10-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blaxter K. Energy metabolism in animals and man. Cambridge University Press; Cambridge: 1989. The minimal metabolism; pp. 120–146. [Google Scholar]
- 60.Lighton J.R., Halsey L.G. Flow-through respirometry applied to chamber systems: pros and cons, hints and tips. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2011;158(3):265–275. doi: 10.1016/j.cbpa.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 61.Burnett C.M., Grobe J.L. Dietary effects on resting metabolic rate in C57BL/6 mice are differentially detected by indirect (O2/CO2 respirometry) and direct calorimetry. Molecular Metabolism. 2014;3(4):460–464. doi: 10.1016/j.molmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scholander P.F. Body insulation of some arctic and tropical mammals and birds. Biological Bulletin. 1950;99(2):225–236. doi: 10.2307/1538740. [DOI] [PubMed] [Google Scholar]
- 63.Dawson N.J., Webster E.D. The insulative value of mouse fur. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences. 1967;52(2):168–173. doi: 10.1113/expphysiol.1967.sp001899. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt-Nielsen K. Animal physiology: adaptation and environment. Cambridge University Press; Cambridge: 1990. Temperature regulation; pp. 240–295. [Google Scholar]
- 65.Burton A.C. Human calorimetry II. The average temperature of the tissues of the body. Journal of Nutrition. 1935;9(3):261–279. [Google Scholar]
- 66.Blaxter K. Energy metabolism in animals and man. Cambridge University Press; Cambridge: 1989. Energy exchanges by radiation, convection, conduction and evaporation; pp. 86–119. [Google Scholar]
- 67.Hosek B., Chlumecky J. Metabolic reaction and heat loss in hairless and normal mice during short-term adaptation to heat and cold. Pfluegers Archiv fuer die Gesamte Physiologie des Menschen und der Tiere. 1967;296(3):248–255. doi: 10.1007/BF00363765. [DOI] [PubMed] [Google Scholar]
- 68.Klaus S. Physiology of transgenic mice with brown fat ablation: obesity is due to lowered body temperature. American Journal of Physiology. 1998;274(2 Pt 2):R287–R293. doi: 10.1152/ajpregu.1998.274.2.R287. [DOI] [PubMed] [Google Scholar]
- 69.Meyer C.W. Gene or size: metabolic rate and body temperature in obese growth hormone-deficient dwarf mice. Obesity Research. 2004;12(9):1509–1518. doi: 10.1038/oby.2004.188. [DOI] [PubMed] [Google Scholar]
- 70.Klaus S. Expression of uncoupling protein 1 in skeletal muscle decreases muscle energy efficiency and affects thermoregulation and substrate oxidation. Physiol Genomics. 2005;21(2):193–200. doi: 10.1152/physiolgenomics.00299.2004. [DOI] [PubMed] [Google Scholar]
- 71.Lute B. Biphasic effect of melanocortin agonists on metabolic rate and body temperature. Cell Metabolism. 2014;20(2):333–345. doi: 10.1016/j.cmet.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


