Significance
One major goal of systems neuroscience over the past decades has been to understand how the activity of sensory neurons mediates perception in simple decision-making tasks. It has remained fundamentally unclear whether and how the activity of primary sensory afferents limits behavior and contributes to decisions. We have tackled these issues by recording, for the first time to our knowledge, from otolith afferents during a heading discrimination task. We show otolith afferents have sensitivity comparable to central neurons, with some approaching behavior. However, they do not exhibit significant choice-related activity, indicating that downstream transformations of vestibular signals are necessary for vestibular activity to become predictive of the animal’s choices. These findings have important implications for understanding how choice-related activity arises in sensory pathways.
Keywords: neuronal threshold, choice probability, psychophysical threshold, otolith afferent, heading discrimination
Abstract
How activity of sensory neurons leads to perceptual decisions remains a challenge to understand. Correlations between choices and single neuron firing rates have been found early in vestibular processing, in the brainstem and cerebellum. To investigate the origins of choice-related activity, we have recorded from otolith afferent fibers while animals performed a fine heading discrimination task. We find that afferent fibers have similar discrimination thresholds as central cells, and the most sensitive fibers have thresholds that are only twofold or threefold greater than perceptual thresholds. Unlike brainstem and cerebellar nuclei neurons, spike counts from afferent fibers do not exhibit trial-by-trial correlations with perceptual decisions. This finding may reflect the fact that otolith afferent responses are poorly suited for driving heading perception because they fail to discriminate self-motion from changes in orientation relative to gravity. Alternatively, if choice probabilities reflect top-down inference signals, they are not relayed to the vestibular periphery.
The neural basis of perception holds a long-standing fascination for neuroscientists. How do the properties of single neurons and populations of neurons relate to, and account for, sensory perception? In all sensory systems, information about the world is first translated into neural activity by peripheral receptor neurons, and then transformed by multiple stages of subcortical and cortical processing into a perceptual decision. How and where does perception emerge from multiple neural representations that appear to be at least partially redundant? These questions have been addressed often in sensory and multisensory cortex (e.g., in refs. 1–3 for vestibular perception), but much less is known about how the activity of sensory afferents relates to perceptual sensitivity and perceptual decisions.
One way to assess a potential role of sensory neurons in a perceptual task is to compare neuronal and perceptual sensitivity, measured simultaneously in the same subject (4). Although this comparison has been done many times for cortical neurons (1, 5–7), little is known about how the sensitivity of peripheral afferents compares with behavior apart from microneurography studies of tactile afferents in humans (8, 9). To our knowledge, the present study provides the first direct comparison of afferent neuronal sensitivity and perceptual sensitivity, measured simultaneously in experimental animals. Results of such comparisons have important implications for understanding how population encoding and decoding may constrain and shape the information that guides behavior.
Another way that neuroscientists have explored the functional links between sensory neurons and perception is by measuring the trial-by-trial correlations between neural activity and perceptual decisions, which are typically quantified as “choice probabilities” (CPs) (10). The “bottom-up” explanation of CPs is that trial-to-trial variability in the activity of sensory neurons drives variability in decisions; in contrast, the “top-down” explanation is that higher level signals related to decisions or featural attention are fed back to modulate the responses of sensory neurons (reviewed in ref. 11). To our knowledge, CPs have never been measured for primary afferents in any sensory system. Such measurements are potentially of interest because they may help clarify whether trial-to-trial response variability at the sensory periphery can be correlated with decisions, and whether top-down signals may propagate all of the way back to afferents.
For some sensory systems and perceptual tasks, the relationships between afferent activity, behavioral sensitivity, and perceptual decisions cannot be examined because afferents do not exhibit the same forms of stimulus selectivity seen in the cortex (e.g., binocular disparity or direction tuning in primate visual neurons). In this regard, the vestibular system may provide an alternative model to probe the origin of CPs because similar basic forms of spatiotemporal directional selectivity are seen at many levels of processing, from afferents to cortex (12). Recently, we provided the first demonstration (13), to our knowledge, that subcortical neurons in the vestibular nuclei (VN) and cerebellar nuclei (CN) could exhibit robust CPs, and these effects were even larger than those effects measured in some cortical areas under identical stimulus conditions (e.g., ref. 1). Interestingly, we also found that the CPs of these subcortical neurons were correlated with the degree to which the neurons represented translation (heading) without being confounded by head orientation relative to gravity. This result suggested that CPs might emerge in the vestibular pathways after resolution of the tilt-translation ambiguity (14). If so, this logic predicts that otolith afferents, which encode the net gravitoinertial acceleration, and therefore confound tilt and translation (15), would not exhibit CPs.
We simultaneously measured heading discrimination performance while recording from otolith afferents, the primary sensory neurons that carry linear acceleration information to the brain. We addressed the following questions. First, how does the heading sensitivity of otolith afferents compare with central vestibular neurons? Are central vestibular neurons more sensitive than afferents because they have greater average modulation amplitudes than afferents (16–23), or do changes in response variability counteract these differences in response gain? Second, do otolith afferents show choice-related activity, and how do CPs of afferents compare with CPs measured in central neurons and cortical areas?
Results
Comparison Between Neuronal and Psychophysical Thresholds.
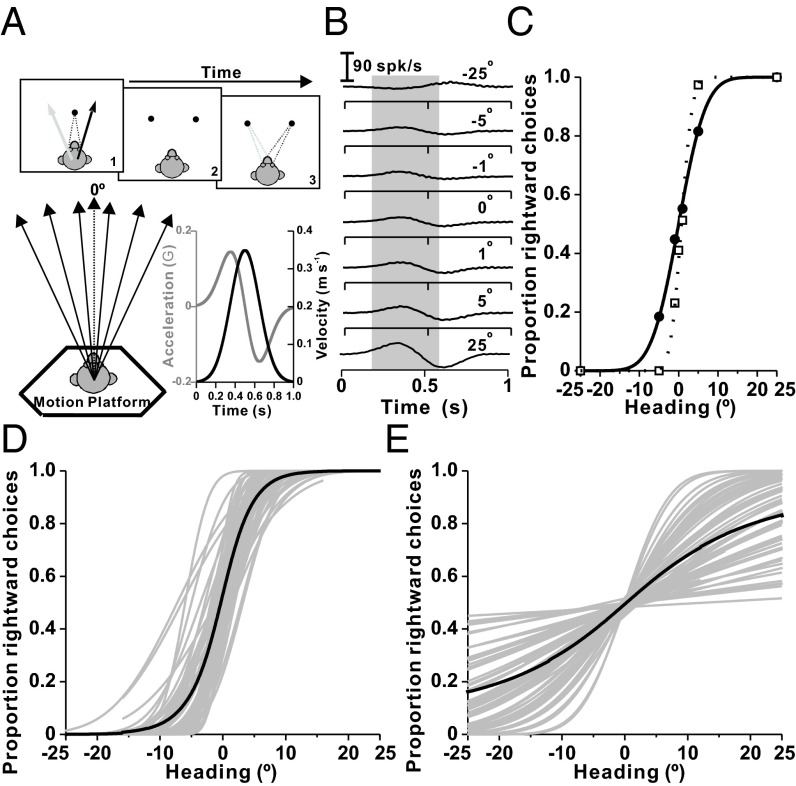
We recorded from otolith afferents during a fine heading discrimination task in which animals made saccades to indicate whether their perceived direction of translation was leftward or rightward relative to straight ahead (Fig. 1A). Receiver operating characteristic (ROC) analysis was used to construct neurometric functions that could be compared with behavioral data (Fig. 1 B and C). Psychometric (Fig. 1D) and neurometric (Fig. 1E) data were fitted with cumulative Gaussian functions to compute thresholds (additional examples and details are provided in Figs. S1 and S2).
Fig. 1.
Behavioral task, neural analysis, and summary of neuronal and psychophysical performance. (A) Layout of the heading discrimination task and illustration of the Gaussian stimulus velocity (black) and biphasic linear acceleration (gray) profiles. (B) Peri-stimulus time histograms (PSTHs) of responses corresponding to seven distinct headings (top to bottom: −25°, −5°, −1°, 0°, −1°, −5°, and −25°) for an example neuron. Responses in a 400-ms window centered on peak acceleration (gray shading) were monotonically tuned (Fig. S1D). (C) Neurometric function (● and solid curve) for the same neuron showing the proportion of “rightward” decisions for an ideal observer as a function of heading. The corresponding psychometric function fit is also shown (□). (D) Individual (gray curves) and mean (black curve) psychometric functions are shown as cumulative Gaussian fits (n = 54 sessions). (E) Individual (gray curves) and mean (black curve) neurometric functions (cumulative Gaussian fits to ROC data from n = 62 otolith afferent fibers). With the exception of eight fibers, all primary otolith afferent data were recorded while trained animals performed the discrimination task.
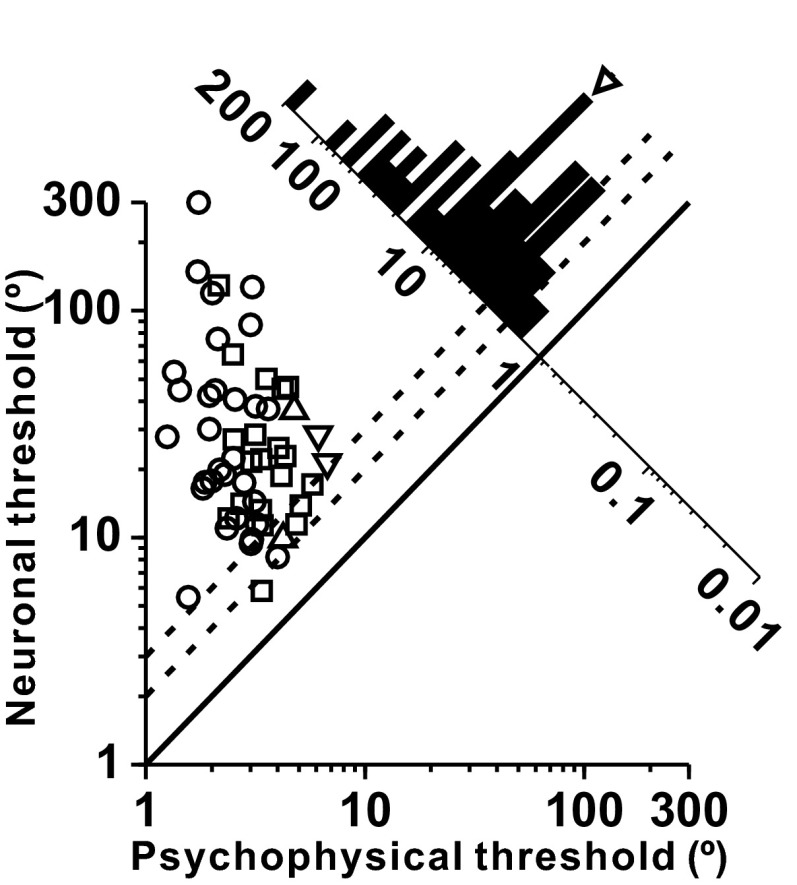
As shown in Fig. 2, which compares the simultaneously recorded neuronal and psychophysical thresholds (SI Methods), the most sensitive cells had thresholds that were twofold to threefold larger than psychophysical thresholds. Because preferred directions of cosine-tuned primary otolith afferents are distributed throughout 3D space (21, 22, 24), only a few cells with heading preferences around lateral motion axes are maximally sensitive in the heading discrimination task. As a result, because the stimulus was not tailored to the heading tuning properties of each cell, the neuronal-to-psychophysical threshold ratio averaged 9.10 ± 2.77 (geometric mean ± SD; Fig. 2, marginal distribution along the diagonal).
Fig. 2.
Comparison of psychophysical and neuronal thresholds for otolith afferents. Symbol shapes denote different animals: monkey C (n = 30, ○), monkey Y (n = 20, □), monkey O (n = 2, ▽), and monkey H (n = 2, △). The diagonal histogram shows the distribution of neuronal-to-psychophysical (N/P) threshold ratios. In this comparison, psychophysical thresholds have been divided by the square root of 2 to be directly comparable to neuronal thresholds computed using the neuron/antineuron method. The arrowhead illustrates the mean N/P ratio. Dotted lines illustrate twofold and threefold ratios.
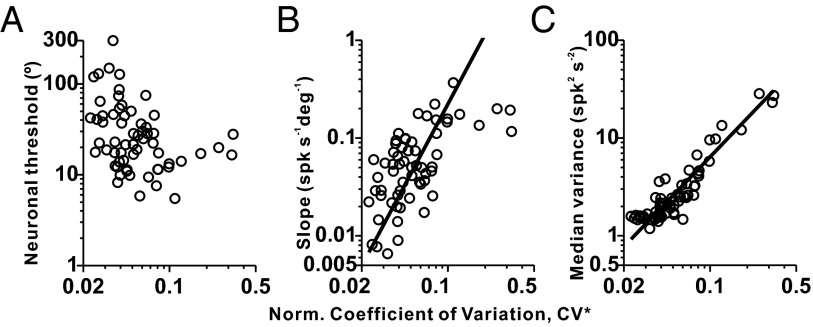
Neuronal thresholds did not depend significantly on discharge regularity, as characterized by a normalized coefficient of variation (CV*; Spearman rank correlation: P = 0.11; Fig. 3A and SI Methods), even though there was a tendency for regular afferents with CV* < 0.1 to have greater thresholds (geometric mean ± SD: 27.14 ± 2.31°, n = 56) than irregular afferents with CV* > 0.1 (geometric mean ± SD: 15.16 ± 1.65°, n = 6) (Wilcoxon rank sum test: P = 0.11). One possible reason why neuronal thresholds were relatively independent of CV* is because both tuning slope and median response variance (across all headings tested in the discrimination task) increase as a function of CV* (type II regression: P = 3.9 × 10−7, r = 0.59 in Fig. 3B; type II regression: P = 1.6 × 10−26, r = 0.92 in Fig. 3C), such that they largely counteract each other. Similar results have also been reported previously during sinusoidal stimulation in the absence of a perceptual task for both otolith (25, 26) and semicircular canal (27) afferents.
Fig. 3.
Dependence of neuronal sensitivity on discharge regularity. Scatter plots show the neuronal threshold (A), tuning curve slope (B), and median response variance (C) plotted against CV* (n = 62 afferents). Median response variance is computed across all headings tested in the discrimination task. Solid lines illustrate type II linear regression fits (shown only for significant correlations).
Comparison with Central Cell Properties.
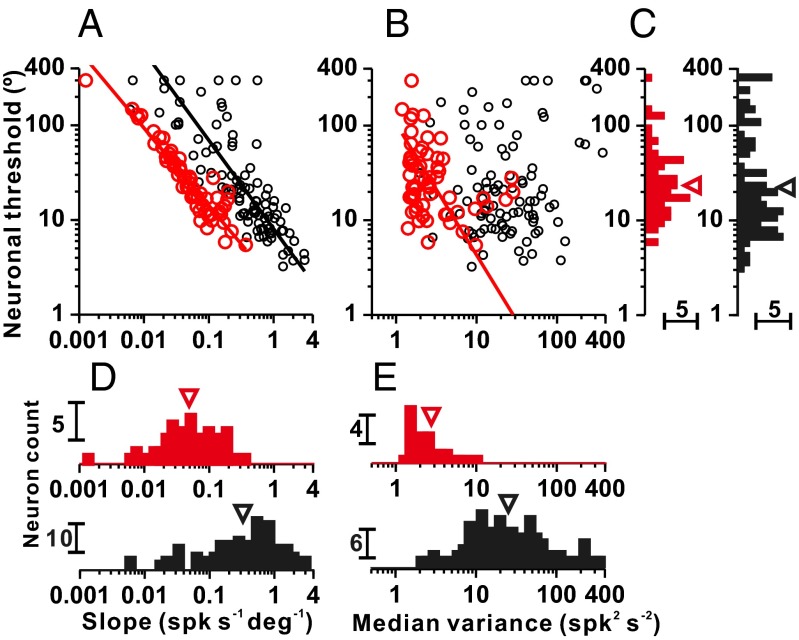
Fig. 4 compares response properties of otolith afferents with response properties of central neurons in the VN and CN. Although stimuli used in the current study had a shorter duration and greater peak acceleration than those stimuli used in the previous VN/CN study (SI Methods), behavioral thresholds of animals in the current study (using an acceleration of ± 2 cm/s2 and duration of 1 s) are within the range of thresholds measured previously with a stimulus duration of 2 s and acceleration of ± 1 cm/s2. Thus, duration and acceleration trade off to some extent, and comparison of neuronal sensitivity is probably not substantially affected by these differences. Thresholds of VN/CN neurons have previously been shown to depend strongly on tuning curve slope (type II regression: r = −0.81, P = 3.4 × 10−24) and weakly on median response variance (type II regression: r = 0.19, P = 0.07; Fig. 4 A and B, black symbols) (13). Similar relationships between neuronal threshold, tuning curve slope, and median response variance were found for our sample of otolith afferents (type II regression: r = −0.94, P = 2.7 × 10−29 for tuning slope; r = −0.33, P = 0.01 for median response variance; Fig. 4 A and B, red symbols). We also found that neuronal thresholds of otolith afferents depended on response gain for lateral accelerations (Fig. S3A). Interestingly, the geometric mean threshold for otolith afferents (25.7 ± 2.3°) was not significantly different from the threshold of central neurons in the VN/CN (23.0 ± 3.4°) (Wilcoxon rank sum test: P = 0.12; Fig. 4C), despite the much greater tuning curve slopes for central neurons (0.33 ± 3.61 spikes per s−1⋅deg−1) compared with otolith afferents (0.05 ± 2.72 spikes per s−1⋅deg−1) (Wilcoxon rank sum test: P = 9.6 × 10−16; Fig. 4D). One possible explanation for these findings is that response variance was almost 10-fold greater in VN/CN neurons (24.40 ± 2.96 spikes2⋅s−2) than in otolith afferents (2.75 ± 2.11 spikes2⋅s−2) (Wilcoxon rank sum test: P = 3.9 × 10−21; Fig. 4E). Thus, low gains and low variance in otolith afferents are transformed into higher gains and greater variability in central neurons.
Fig. 4.
Comparison between otolith afferents and central VN/CN cells. Scatter plots of neuronal threshold vs. tuning curve slope (A) and median response variance (B) for otolith afferents (red symbols, n = 64) and VN/CN neurons (black symbols, n = 97; data from ref. 13). Solid lines show type II linear regression fits (plotted only for significant correlations). Marginal distributions of neuronal threshold (C), tuning curve slope (D), and median response variance (E) are shown. Arrowheads illustrate geometric means. spk, spike.
Choice-Related Activity.
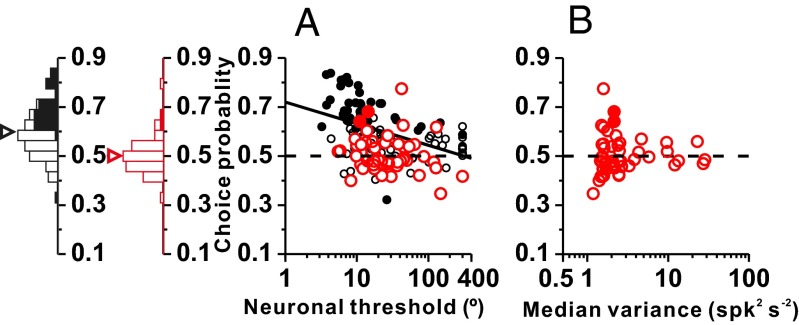
In contrast to the similar neuronal thresholds exhibited by otolith afferents and VN/CN cells, choice-related activity differed markedly at these two early stages of vestibular processing. As shown previously (13), CPs were frequently large and inversely correlated with neuronal thresholds in VN/CN (Fig. 5A, black symbols). In contrast, CPs were very weak and not significantly correlated with neuronal thresholds for the otolith afferent population (Fig. 5A, red symbols; type II regression: r = −0.16, P = 0.26). Among 54 otolith afferents for which CPs were computed, only two showed significant CPs (Fig. 5A, red filled symbols), which is approximately the proportion expected by chance. At the population level, the average CP (0.50 ± 0.07) for otolith afferents was not significantly different from chance (t test: P = 0.67), and was significantly less than the average CP for VN/CN neurons (0.60 ± 0.10; Wilcoxon rank sum test: P = 6.4 × 10−9; Fig. 5A).
Fig. 5.
Comparison of CPs between otolith afferents and VN/CN neurons. (A) Grand CPs are plotted against neuronal thresholds for otolith afferents (red, n = 54) and VN/CN neurons (black, n = 97) (VN/CN data are from ref. 13). Solid black line indicates a type II regression fit to VN/CN data (the correlation was not significant for otolith afferents). Filled symbols represent CP values significantly different from 0.5 (permutation test: P < 0.05,). Marginal histograms show distributions of CPs for VN/CN neurons (black) and otolith afferents (red). Filled and open bars indicate neurons with significant and nonsignificant CPs, respectively. Arrowheads illustrate mean values. (B) Scatter plot of CPs against response variance. Filled and open symbols indicate neurons with significant and nonsignificant CPs, respectively. Horizontal dashed lines indicate the chance level (0.5) for CP.
Several of the VN/CN neurons with the greatest CPs have neuronal thresholds that are lower than the most sensitive afferents. To ensure that the difference in CP between afferents and central neurons is not due to neuronal sensitivity, we performed two additional analyses. Using analysis of covariance, we found that the difference in CP between areas was significant when the logarithm of neuronal threshold was included as a covariate (main effect of brain region: P < 0.001; main effect of log threshold: P = 0.036; interaction: P = 0.88). We also analyzed a subset of neurons from each area with thresholds ranging from 10–40°; among this group, the median thresholds were not significantly different (Wilcoxon rank sum test: P = 0.26) but the mean CP for afferents (0.499 ± 0.064) was significantly less than the mean CP for VN/CN neurons (0.589 ± 0.097, P = 8.8 × 10−6). Thus, the difference in CP between areas cannot be attributed to differences in sensitivity. One possible explanation for the absence of significant CPs in otolith afferents is that they have very low response variance (Fig. 4E). However, we found no significant correlation between CP and median response variance among otolith afferents (Spearman rank correlation: P = 0.85; Fig. 5B). Note that even the cells with the largest variance had small CPs (Fig. 5B), suggesting that these conclusions apply to both regular and irregular afferents.
Finally, we explored how our findings for otolith afferents depend on the time window used for analysis, which was a 400-ms window centered on peak acceleration in all analyses above. We computed neuronal thresholds and CPs in 400-ms windows centered at different times relative to the stimulus onset (Fig. 6). Neuronal thresholds were smallest for times around peak acceleration (vertical dashed line) and peak deceleration, and there was no significant difference between acceleration/deceleration thresholds (Wilcoxon signed-rank test: P > 0.1; Fig. 6A). This threshold profile occurs because the dynamics of otolith afferents follow the profile of linear acceleration (20); thus, they exhibit their maximum response at either peak acceleration or deceleration. In contrast to neuronal thresholds, CPs hovered around the chance level of 0.5 at all times during the response (Fig. 6B), with only two time points having a mean CP significantly different from 0.5 (Fig. 6B, solid circles, both of which are slightly but significantly <0.5). Thus, there was no clear evidence for a correlation between otolith afferent firing and perceptual decisions at any time point during the motion stimulus. We also examined neuronal thresholds and CPs using analysis time windows of different lengths (Fig. S4). The smallest neuronal thresholds were observed with window sizes between 200 and 400 ms, whereas CPs showed no significant dependence on window length (analysis of covariance: P = 0.67).
Fig. 6.
Time dependence of neuronal thresholds (A) and CPs (B). Gray lines represent individual cells (threshold: n = 62, CP: n = 54). Open symbols (○) and the thick black line illustrate population means. Each point represents data computed in a 400-ms analysis window that is shifted by steps of 50 ms. The vertical dashed lines represent the time of peak stimulus acceleration (time point used for all other analyses). Filled symbols (●) indicate that the mean CP is significantly different from 0.5 (t test: P < 0.05).
Discussion
We have found that peripheral sensory fibers innervating the otolith organs have heading discrimination thresholds that are of similar magnitude to thresholds in the VN and CN (13), as well as to thresholds of cortical neurons (1–3). Despite having similar sensitivities, responses of otolith afferents do not correlate with perceptual decisions, whereas activity of central vestibular and cortical neurons does. These results suggest that choice-related activity first emerges in brainstem and cerebellar neurons with response properties that have been sculpted by the computations necessary to code selectively for inertial self-motion, independent of changes in orientation relative to gravity (15, 28–30).
Sensitivity of Otolith Afferents.
In our experiment, heading was varied in the stereotaxic horizontal plane, whereas the major planes of both the utricle and saccule lie elevated to the stereotaxic horizontal plane (21, 22). Specifically, the mean preferred direction of utricular afferents was measured to be 8.6° above the stereotaxic horizontal plane (22), and similar or slightly larger angles were reported in the rhesus monkey (16). Although this difference in elevation means that otolith afferent fibers were not tested under optimal conditions, the expected effect on sensitivity is quite small, on the order of a few percentage points.
Comparing neuronal and behavioral sensitivity, we find that only the most sensitive otolith afferents have discrimination thresholds that approach the psychophysical threshold of the monkey (Fig. 2). Although it might be surprising to some readers, this observation is consistent with studies that involve fine discrimination tasks in other sensory systems, where perceptual threshold may be set by the most sensitive neurons available (4). For example, similar findings have been reported for orientation discrimination in primary visual cortex (V1) (31), fine direction discrimination in the middle temporal (MT) visual area (7), and fine binocular disparity discrimination in areas V4 (32) and V1 (33). In contrast, studies that have compared neuronal and psychophysical performance in coarse discrimination or amplitude discrimination tasks have often reported that many single neurons are more sensitive than the animal (5, 6, 9, but also ref. 34).
Notably, stimuli used in the present study, as well as our previous studies of central and cortical vestibular neurons (1, 3, 13, 35), were not tailored to the tuning properties of each neuron. The most sensitive cells encountered in our sample have the largest lateral acceleration gains and heading preferences that are roughly orthogonal to the forward direction, such that they are most sensitive for discriminating headings around straight forward (Fig. S3).
Otolith afferent thresholds have been measured previously using sinusoidal stimuli (25, 26), but behavioral data were not collected simultaneously. Thus, neuronal thresholds were instead compared with human perceptual thresholds (36). However, macaque and human thresholds may not necessarily be similar. In fact, psychophysical thresholds vary greatly across subjects within both species (e.g., figure S2C of ref. 13), thus making it difficult to compare neuronal and perceptual performance that is not measured simultaneously in the same subjects. In addition, vibrations inherent to the various motion platforms used within and across laboratories may affect thresholds, thus further complicating comparisons. The present study is the first, to our knowledge, to record afferent and behavioral sensitivity simultaneously in the peripheral vestibular system.
Population Sensitivity.
An important question is how combining the responses of many sensory neurons determines the overall sensitivity of the population code. Some previous studies have attempted to determine the number of neurons (pool size) necessary to predict human perceptual thresholds from neuronal thresholds measured in macaques, assuming that neuronal population thresholds decrease as a function of the square root of pool size (26, 37). We believe that this approach has major flaws because strong assumptions must be made regarding the shared variability among neurons (correlated noise) and the efficiency of population decoding, both of which remain largely unknown. Specifically, the structure of correlated noise in vestibular afferents is very difficult to measure (but refer to ref. 27) yet may be critically important in determining the information carried by the neural population (38–44). When correlated noise is present, as is the case in the VN/CN (13, 35), population thresholds may not decrease with the square root of the number of neurons and predictions based on the square root law could be dramatically inaccurate. Moreover, for a fixed neural pool size, population thresholds can also depend substantially on whether the decoder has full knowledge of the structure of noise correlations or not (42, 45, 46). Some noise correlations can be removed by an optimal decoder, but there is no guarantee that the brain is optimal and accounts for all correlations that can be removed. Perhaps most importantly, some types of correlated noise are information-limiting and cannot be removed by any decoder (40).
These contemporary theoretical studies have important implications for our findings. Specifically, the fact that some sensory fibers are nearly as sensitive as behavior would imply either information-limiting correlations or massively suboptimal decoding to account for behavior (42, 45, 46). We consider it more likely that the total information encoded by the otolith afferent population is constrained by information-limiting correlations (40), in which case it is inappropriate to use the square root law to predict behavioral thresholds as previous studies have done (26, 37). Unfortunately, it is unlikely that measurements of correlated noise from pairs of neurons provide a good estimate of the information-limiting correlations (40). A direct test of the existence of information-limiting correlations may require simultaneous recordings from several thousands of neurons for long periods of time, which is currently not technologically feasible, especially for otolith afferents.
Choice-Related Activity.
The second major finding of the present study is that otolith afferent fibers do not exhibit choice-related activity. CPs have been reported many times for sensory neurons in cortical areas (e.g., refs. 1–3, 7, 10, 47–60). However, relatively little is known about where CPs first emerge in sensory processing streams. According to a bottom-up explanation of choice-related activity, CPs may appear at stages of processing where sensory signals are represented in a format appropriate to drive behavior. In contrast, if CPs are primarily driven by top-down signals, as suggested by recent studies (11, 48, 54, 55, 61, 62), they could appear at any stage of processing that receives decision-related feedback. In the vestibular system, targets of feedback could potentially include the primary afferents through their efferent innervation (63).
Until recently, all reports of significant CPs had come from studies of cortical neurons and it was unclear whether subcortical cells, closer to the sensory periphery, exhibited CPs. Indeed, two recent studies in the somatosensory system reported no choice-related activity in the thalamus (64, 65). Taking advantage of the fact that the same basic forms of directional selectivity are seen at many levels of vestibular processing (12), we showed that subcortical neurons in the VN and CN exhibit robust CPs that are greater than CPs seen in the cortex (dorsal medial superior temporal area) under identical conditions (13). Importantly, significant CPs were found only for VN/CN neurons that represent translation (i.e., heading) without being confounded by head orientation relative to gravity (13). From these data, we suggested that CPs may emerge in the vestibular pathways when signals are represented in a format appropriate to the task (i.e., translation signals for heading discrimination). Because otolith afferents only encode the net gravitoinertial acceleration (15), our present results showing no CPs in otolith afferents greatly strengthen this interpretation. Note that signals from otolith afferents could be used to perform our task because head orientation does not vary; however, we suggest that downstream representations are generally decoded for heading perception because translation and head orientation relative to gravity often covary under natural conditions.
Our results could be explained by either a bottom-up or top-down account of CPs (or both, because they are not mutually exclusive). To create a representation of translation in VN/CN, otolith afferent signals must be combined with processed signals from the semicircular canals and perhaps other sources (28). This transformation introduces new sources of response variability into the VN/CN representation, and may at least partially account for the greater response variance observed in these central neurons (Fig. 4). In a bottom-up account of CPs, response variability following these transformations would drive trial-by-trial fluctuations in perception, whereas the low variability of afferents would not covary systematically with percepts. Our findings are also compatible with a top-down origin of CPs if feedback signals carrying decision-related signals selectively target translation-coding neurons in the VN/CN. Although there is feedback to otolith afferents (66–69), our results suggest that decision-related signals do not propagate all of the way back to the afferents. In either case, our findings demonstrate clearly that a transformation of vestibular signals downstream of the primary afferents is necessary for the emergence of CPs in vestibular heading perception.
Methods
A detailed description of the experimental protocols and analyses is provided in SI Methods. Responses of otolith afferents were collected from four male rhesus monkeys (Macaca mulatta) during a fine heading discrimination task around psychophysical threshold. The motion trajectory (13 cm of displacement) was 1 s in duration and followed a Gaussian velocity profile (peak velocity of 41 cm/s), with a corresponding biphasic linear acceleration profile (±0.2 G = ±1.96 ms−2). All procedures were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine, and were in accordance with National Institutes of Health guidelines. Using ROC analysis, neural activity was used to compute neuronal thresholds and CPs (5, 10).
Supplementary Material
Acknowledgments
Experiments were supported by NIH Grant R01 DC04260. X.-j.Y. was supported by the Fundamental Research Funds for Central Universities 2015QN81004. G.C.D. was supported by NIH Grant R01 EY016178.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507402112/-/DCSupplemental.
References
- 1.Gu Y, DeAngelis GC, Angelaki DE. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci. 2007;10(8):1038–1047. doi: 10.1038/nn1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu Y, Angelaki DE, DeAngelis GC. Neural correlates of multisensory cue integration in macaque MSTd. Nat Neurosci. 2008;11(10):1201–1210. doi: 10.1038/nn.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen A, DeAngelis GC, Angelaki DE. Functional specializations of the ventral intraparietal area for multisensory heading discrimination. J Neurosci. 2013;33(8):3567–3581. doi: 10.1523/JNEUROSCI.4522-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker AJ, Newsome WT. Sense and the single neuron: Probing the physiology of perception. Annu Rev Neurosci. 1998;21:227–277. doi: 10.1146/annurev.neuro.21.1.227. [DOI] [PubMed] [Google Scholar]
- 5.Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: A comparison of neuronal and psychophysical performance. J Neurosci. 1992;12(12):4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uka T, DeAngelis GC. Contribution of middle temporal area to coarse depth discrimination: Comparison of neuronal and psychophysical sensitivity. J Neurosci. 2003;23(8):3515–3530. doi: 10.1523/JNEUROSCI.23-08-03515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purushothaman G, Bradley DC. Neural population code for fine perceptual decisions in area MT. Nat Neurosci. 2005;8(1):99–106. doi: 10.1038/nn1373. [DOI] [PubMed] [Google Scholar]
- 8.Johansson RS, Vallbo AB. Detection of tactile stimuli. Thresholds of afferent units related to psychophysical thresholds in the human hand. J Physiol. 1979;297(0):405–422. doi: 10.1113/jphysiol.1979.sp013048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arabzadeh E, et al. Single tactile afferents outperform human subjects in a vibrotactile intensity discrimination task. J Neurophysiol. 2014;112(10):2382–2387. doi: 10.1152/jn.00482.2014. [DOI] [PubMed] [Google Scholar]
- 10.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13(1):87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 11.Nienborg H, Cohen MR, Cumming BG. Decision-related activity in sensory neurons: Correlations among neurons and with behavior. Annu Rev Neurosci. 2012;35:463–483. doi: 10.1146/annurev-neuro-062111-150403. [DOI] [PubMed] [Google Scholar]
- 12.Chen A, DeAngelis GC, Angelaki DE. A comparison of vestibular spatiotemporal tuning in macaque parietoinsular vestibular cortex, ventral intraparietal area, and medial superior temporal area. J Neurosci. 2011;31(8):3082–3094. doi: 10.1523/JNEUROSCI.4476-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Gu Y, DeAngelis GC, Angelaki DE. Choice-related activity and correlated noise in subcortical vestibular neurons. Nat Neurosci. 2013;16(1):89–97. doi: 10.1038/nn.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angelaki DE, Yakusheva TA. How vestibular neurons solve the tilt/translation ambiguity. Comparison of brainstem, cerebellum, and thalamus. Ann N Y Acad Sci. 2009;1164:19–28. doi: 10.1111/j.1749-6632.2009.03939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature. 2004;430(6999):560–564. doi: 10.1038/nature02754. [DOI] [PubMed] [Google Scholar]
- 16.Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: Primary afferent and central vestibular neuron responses. J Neurophysiol. 2000;84(4):2113–2132. doi: 10.1152/jn.2000.84.4.2113. [DOI] [PubMed] [Google Scholar]
- 17.Chen-Huang C, Peterson BW. Frequency-dependent spatiotemporal tuning properties of non-eye movement related vestibular neurons to three-dimensional translations in squirrel monkeys. J Neurophysiol. 2010;103(6):3219–3237. doi: 10.1152/jn.00904.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carriot J, Brooks JX, Cullen KE. Multimodal integration of self-motion cues in the vestibular system: Active versus passive translations. J Neurosci. 2013;33(50):19555–19566. doi: 10.1523/JNEUROSCI.3051-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickman JD, Angelaki DE. Vestibular convergence patterns in vestibular nuclei neurons of alert primates. J Neurophysiol. 2002;88(6):3518–3533. doi: 10.1152/jn.00518.2002. [DOI] [PubMed] [Google Scholar]
- 20.Fernández C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol. 1976;39(5):996–1008. doi: 10.1152/jn.1976.39.5.996. [DOI] [PubMed] [Google Scholar]
- 21.Fernández C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force-response relations. J Neurophysiol. 1976;39(5):985–995. doi: 10.1152/jn.1976.39.5.985. [DOI] [PubMed] [Google Scholar]
- 22.Fernández C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol. 1976;39(5):970–984. doi: 10.1152/jn.1976.39.5.970. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W, Tang BF, Newlands SD, King WM. Responses of monkey vestibular-only neurons to translation and angular rotation. J Neurophysiol. 2006;96(6):2915–2930. doi: 10.1152/jn.00013.2006. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez C, Goldberg JM, Abend WK. Response to static tilts of peripheral neurons innervating otolith organs of the squirrel monkey. J Neurophysiol. 1972;35(6):978–987. doi: 10.1152/jn.1972.35.6.978. [DOI] [PubMed] [Google Scholar]
- 25.Yu XJ, Dickman JD, Angelaki DE. Detection thresholds of macaque otolith afferents. J Neurosci. 2012;32(24):8306–8316. doi: 10.1523/JNEUROSCI.1067-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamali M, Carriot J, Chacron MJ, Cullen KE. Strong correlations between sensitivity and variability give rise to constant discrimination thresholds across the otolith afferent population. J Neurosci. 2013;33(27):11302–11313. doi: 10.1523/JNEUROSCI.0459-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu XJ, Thomassen JS, Dickman JD, Newlands SD, Angelaki DE. Long-term deficits in motion detection thresholds and spike count variability after unilateral vestibular lesion. J Neurophysiol. 2014;112(4):870–889. doi: 10.1152/jn.00280.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurens J, Angelaki DE. The functional significance of velocity storage and its dependence on gravity. Exp Brain Res. 2011;210(3-4):407–422. doi: 10.1007/s00221-011-2568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurens J, Meng H, Angelaki DE. Neural representation of orientation relative to gravity in the macaque cerebellum. Neuron. 2013;80(6):1508–1518. doi: 10.1016/j.neuron.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurens J, Meng H, Angelaki DE. Computation of linear acceleration through an internal model in the macaque cerebellum. Nat Neurosci. 2013;16(11):1701–1708. doi: 10.1038/nn.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogels R. Population coding of stimulus orientation by striate cortical cells. Biol Cybern. 1990;64(1):25–31. doi: 10.1007/BF00203627. [DOI] [PubMed] [Google Scholar]
- 32.Shiozaki HM, Tanabe S, Doi T, Fujita I. Neural activity in cortical area V4 underlies fine disparity discrimination. J Neurosci. 2012;32(11):3830–3841. doi: 10.1523/JNEUROSCI.5083-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prince SJ, Pointon AD, Cumming BG, Parker AJ. The precision of single neuron responses in cortical area V1 during stereoscopic depth judgments. J Neurosci. 2000;20(9):3387–3400. doi: 10.1523/JNEUROSCI.20-09-03387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen MR, Newsome WT. Estimates of the contribution of single neurons to perception depend on timescale and noise correlation. J Neurosci. 2009;29(20):6635–6648. doi: 10.1523/JNEUROSCI.5179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Dickman JD, Newlands SD, DeAngelis GC, Angelaki DE. Reduced choice-related activity and correlated noise accompany perceptual deficits following unilateral vestibular lesion. Proc Natl Acad Sci USA. 2013;110(44):17999–18004. doi: 10.1073/pnas.1310416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacNeilage PR, Banks MS, DeAngelis GC, Angelaki DE. Vestibular heading discrimination and sensitivity to linear acceleration in head and world coordinates. J Neurosci. 2010;30(27):9084–9094. doi: 10.1523/JNEUROSCI.1304-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massot C, Chacron MJ, Cullen KE. Information transmission and detection thresholds in the vestibular nuclei: Single neurons vs. population encoding. J Neurophysiol. 2011;105(4):1798–1814. doi: 10.1152/jn.00910.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbott LF, Dayan P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 1999;11(1):91–101. doi: 10.1162/089976699300016827. [DOI] [PubMed] [Google Scholar]
- 39.Averbeck BB, Lee D. Effects of noise correlations on information encoding and decoding. J Neurophysiol. 2006;95(6):3633–3644. doi: 10.1152/jn.00919.2005. [DOI] [PubMed] [Google Scholar]
- 40.Moreno-Bote R, et al. Information-limiting correlations. Nat Neurosci. 2014;17(10):1410–1417. doi: 10.1038/nn.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sompolinsky H, Yoon H, Kang K, Shamir M. Population coding in neuronal systems with correlated noise. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64(5 Pt 1):051904. doi: 10.1103/PhysRevE.64.051904. [DOI] [PubMed] [Google Scholar]
- 42.Haefner RM, Gerwinn S, Macke JH, Bethge M. Inferring decoding strategies from choice probabilities in the presence of correlated variability. Nat Neurosci. 2013;16(2):235–242. doi: 10.1038/nn.3309. [DOI] [PubMed] [Google Scholar]
- 43.Shadlen MN, Britten KH, Newsome WT, Movshon JA. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J Neurosci. 1996;16(4):1486–1510. doi: 10.1523/JNEUROSCI.16-04-01486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ecker AS, Berens P, Tolias AS, Bethge M. The effect of noise correlations in populations of diversely tuned neurons. J Neurosci. 2011;31(40):14272–14283. doi: 10.1523/JNEUROSCI.2539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitkow X, Liu S, Angelaki DE, DeAngelis GC, Pouget A. 2015 How can single sensory neurons predict behavior? Neuron, in press. [Google Scholar]
- 46.Beck J, Moreno R, Latham P, Pouget A. Noise correlations in population codes with finite information. COSYNE Abstr. 2012;T-12 [Google Scholar]
- 47.Dodd JV, Krug K, Cumming BG, Parker AJ. Perceptually bistable three-dimensional figures evoke high choice probabilities in cortical area MT. J Neurosci. 2001;21(13):4809–4821. doi: 10.1523/JNEUROSCI.21-13-04809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krug K, Cumming BG, Parker AJ. Comparing perceptual signals of single V5/MT neurons in two binocular depth tasks. J Neurophysiol. 2004;92(3):1586–1596. doi: 10.1152/jn.00851.2003. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Newsome WT. Correlation between speed perception and neural activity in the middle temporal visual area. J Neurosci. 2005;25(3):711–722. doi: 10.1523/JNEUROSCI.4034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uka T, Tanabe S, Watanabe M, Fujita I. Neural correlates of fine depth discrimination in monkey inferior temporal cortex. J Neurosci. 2005;25(46):10796–10802. doi: 10.1523/JNEUROSCI.1637-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uka T, DeAngelis GC. Contribution of area MT to stereoscopic depth perception: Choice-related response modulations reflect task strategy. Neuron. 2004;42(2):297–310. doi: 10.1016/s0896-6273(04)00186-2. [DOI] [PubMed] [Google Scholar]
- 52.Celebrini S, Newsome WT. Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. J Neurosci. 1994;14(7):4109–4124. doi: 10.1523/JNEUROSCI.14-07-04109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nienborg H, Cumming BG. Macaque V2 neurons, but not V1 neurons, show choice-related activity. J Neurosci. 2006;26(37):9567–9578. doi: 10.1523/JNEUROSCI.2256-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nienborg H, Cumming BG. Decision-related activity in sensory neurons reflects more than a neuron’s causal effect. Nature. 2009;459(7243):89–92. doi: 10.1038/nature07821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nienborg H, Cumming BG. Psychophysically measured task strategy for disparity discrimination is reflected in V2 neurons. Nat Neurosci. 2007;10(12):1608–1614. doi: 10.1038/nn1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thiele A, Distler C, Hoffmann KP. Decision-related activity in the macaque dorsal visual pathway. Eur J Neurosci. 1999;11(6):2044–2058. doi: 10.1046/j.1460-9568.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 57.Cook EP, Maunsell JH. Dynamics of neuronal responses in macaque MT and VIP during motion detection. Nat Neurosci. 2002;5(10):985–994. doi: 10.1038/nn924. [DOI] [PubMed] [Google Scholar]
- 58.Cook EP, Maunsell JH. Attentional modulation of behavioral performance and neuronal responses in middle temporal and ventral intraparietal areas of macaque monkey. J Neurosci. 2002;22(5):1994–2004. doi: 10.1523/JNEUROSCI.22-05-01994.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leopold DA, Logothetis NK. Activity changes in early visual cortex reflect monkeys’ percepts during binocular rivalry. Nature. 1996;379(6565):549–553. doi: 10.1038/379549a0. [DOI] [PubMed] [Google Scholar]
- 60.Bradley DC, Chang GC, Andersen RA. Encoding of three-dimensional structure-from-motion by primate area MT neurons. Nature. 1998;392(6677):714–717. doi: 10.1038/33688. [DOI] [PubMed] [Google Scholar]
- 61.Cohen MR, Newsome WT. Context-dependent changes in functional circuitry in visual area MT. Neuron. 2008;60(1):162–173. doi: 10.1016/j.neuron.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nienborg H, Cumming B. Correlations between the activity of sensory neurons and behavior: how much do they tell us about a neuron’s causality? Curr Opin Neurobiol. 2010;20(3):376–381. doi: 10.1016/j.conb.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res. 2000;130(3):277–297. doi: 10.1007/s002210050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vázquez Y, Zainos A, Alvarez M, Salinas E, Romo R. Neural coding and perceptual detection in the primate somatosensory thalamus. Proc Natl Acad Sci USA. 2012;109(37):15006–15011. doi: 10.1073/pnas.1212535109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camarillo L, Luna R, Nácher V, Romo R. Coding perceptual discrimination in the somatosensory thalamus. Proc Natl Acad Sci USA. 2012;109(51):21093–21098. doi: 10.1073/pnas.1219636110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindeman HH. Studies on the morphology of the sensory regions of the vestibular apparatus with 45 figures. Ergeb Anat Entwicklungsgesch. 1969;42(1):1–113. [PubMed] [Google Scholar]
- 67.Klinke R, Galley N. Efferent innervation of vestibular and auditory receptors. Physiol Rev. 1974;54(2):316–357. doi: 10.1152/physrev.1974.54.2.316. [DOI] [PubMed] [Google Scholar]
- 68.Sans A, Highstein SM. New ultrastructural features in the vestibular labyrinth of the toadfish, Opsanus tau. Brain Res. 1984;308(1):191–195. doi: 10.1016/0006-8993(84)90936-3. [DOI] [PubMed] [Google Scholar]
- 69.Lysakowski A, Goldberg JM. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J Comp Neurol. 1997;389(3):419–443. doi: 10.1002/(sici)1096-9861(19971222)389:3<419::aid-cne5>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.