Significance
Bird feeding is essentially a massive global supplementary feeding experiment, yet few studies have attempted to explore its ecological effects. In this study we use an in situ experimental approach to investigate the impacts of bird feeding on the structure of local bird assemblages. We present vital evidence that bird feeding contributes to the bird community patterns we observe in urban areas. In particular, the study demonstrates that common feeding practices can encourage higher densities of introduced birds, with potential negative consequences for native birds.
Keywords: avian ecology, community composition, garden birds, human interactions, wildlife feeding
Abstract
Food availability is a primary driver of avian population regulation. However, few studies have considered the effects of what is essentially a massive supplementary feeding experiment: the practice of wild bird feeding. Bird feeding has been posited as an important factor influencing the structure of bird communities, especially in urban areas, although experimental evidence to support this is almost entirely lacking. We carried out an 18-mo experimental feeding study at 23 residential properties to investigate the effects of bird feeding on local urban avian assemblages. Our feeding regime was based on predominant urban feeding practices in our region. We used monthly bird surveys to compare avian community composition, species richness, and the densities of local species at feeding and nonfeeding properties. Avian community structure diverged at feeding properties and five of the commonest garden bird species were affected by the experimental feeding regime. Introduced birds particularly benefitted, with dramatic increases observed in the abundances of house sparrow (Passer domesticus) and spotted dove (Streptopelia chinensis) in particular. We also found evidence of a negative effect on the abundance of a native insectivore, the grey warbler (Gerygone igata). Almost all of the observed changes did not persist once feeding had ceased. Our study directly demonstrates that the human pastime of bird feeding substantially contributes to the structure of avian community in urban areas, potentially altering the balance between native and introduced species.
Numerous factors influence the structure of urban bird assemblages, including habitat fragmentation, competition, and predation (1, 2). One of the most critical factors in the regulation of all animal populations is food resource availability (3–5). Urban birds have access to novel food resources derived from human activities. This provisioning may be unintentional, for example, the foraging of waste or refuse (6), or deliberate in the form of bird feeding by the public (7). The deliberate act of feeding birds is common in many parts of the world, including the United States, United Kingdom, Australia, and New Zealand (8–12). Large quantities of food, and hence energy and nutrients, are added into urban systems each year, with birds the primary target; it is estimated that in 2002 over 450 million kg of seed was fed to wild birds in the United States alone (13). For species capable of exploiting these anthropogenic food sources there may be profound effects on almost every aspect of their ecology (14, 15). Direct benefits for feeder-visiting birds may include reduced time foraging or improved body condition, which in turn may increase reproductive success or survival and lead to population level changes (16–18). A greater availability of food may artificially inflate the carrying capacity of the urban environment, resulting in higher densities of species capable of exploiting anthropogenic food resources (15, 19).
Very few studies have experimentally investigated the effects of feeding birds in the urban environment (15), although there is correlational evidence that this human pastime has a significant influence on the urban bird community (e.g., refs. 10, 20, and 21). In the context of enhancing biodiversity of our cities, bird feeding may, at first glance, be construed as a positive activity by increasing the capacity of urban areas to support birds (22). However, the reality is far more complex (17). Biodiversity may be reduced where a subset of species become dominant at feeding locales, either through competitive advantage or numerical dominance. Alternatively, there may be negative effects for the individuals exploiting supplementary food sources because of, for example, increased disease transmission (23, 24) and malnutrition (25), which may lead to reductions in overall population size.
The interpretation of these potential effects differs further depending on whether the species is native or introduced. Enhancing carrying capacity of urban areas, for example, would be unfavorable ecologically where introduced species were likely to benefit disproportionately. This is a possible scenario in New Zealand, where urban habitats are characterized by a high proportion of introduced species (26). The most popular food types provided by the bird-feeding public in New Zealand, bread and seed (11), are likely to be consumed primarily by introduced birds rather than natives, as a result of a fundamental partition in dietary guilds in urban bird assemblages. Native species persisting in urban areas are principally nectarivorous (e.g., tūī Prosthemadera novaeseelandiae), insectivorous (e.g., grey warbler Gerygone igata), or frugivourous (e.g., New Zealand pigeon Hemiphaga novaeseelandiae), compared with the typically granivorous or omnivorous introduced species (e.g., house sparrow Passer domesticus and common myna Acridotheres tristis, respectively) (27, 28). Consequently, common feeding practices in New Zealand may be supporting increased densities of introduced birds in urban areas.
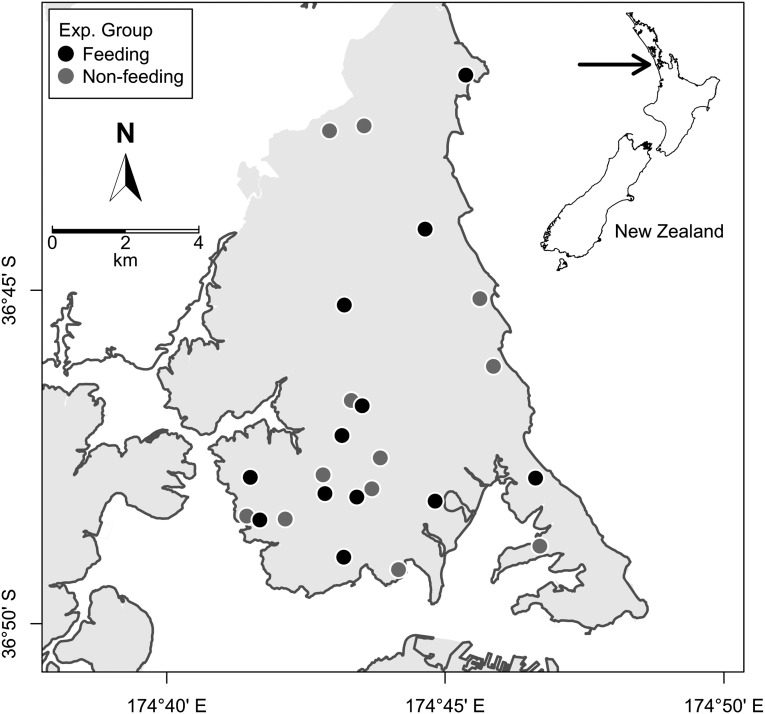
In this study we sought to test the hypothesis that supplementary feeding restructures local bird communities, by using an experimental in situ approach. We established a series of feeding stations (n = 11) in volunteers’ gardens in urban Auckland, New Zealand (Fig. 1). These were active for 18 mo, with a feeding regime designed to mimic common feeding practices of the general public (bread and seed fed daily), ascertained by a nationwide survey (11). This approach ensured that our results were relevant and applicable to the current food provisioning in urban areas. We compared the bird communities at feeding properties with those at nonfeeding properties (n = 12) before, during, and after the implementation of the feeding regime. Our main research question was: Do typical feeding practices influence the avian species assemblages observed in urban habitats? Our objectives were to determine whether feeding had an effect on avian community composition, species richness, and the densities of local species. Specifically, we were interested in how typical feeding practices affect native vs. introduced species, and what happens to local bird communities once feeding stops. Given the dietary divide between native and introduced birds in urban New Zealand, we predicted that typical grain-based feeding practices would increase densities of introduced species.
Fig. 1.
Map of northern Auckland, New Zealand, showing the location of properties participating in an experimental bird feeding study. The urban–rural boundary is also shown, with land zoned as urban shaded in gray. Reference coordinates are expressed as latitude and longitude (WGS84).
Results
Initial Observations.
Householders in the experimental feeding group reported that there were dramatic increases of birds at their properties within weeks of the feeding regime starting. The time lag for recruitment to the food varied between properties; a few householders had feeder visitors within a few days, whereas at one property avian visitors took 2 wk to arrive. Nonetheless, within approximately 4 wk all feeding stations were well established, with feeder visitors coming daily and pre-empting the provision of food. Once established, birds quickly removed the supplementary food at the stations, typically within 2 h of the food being put out.
Avian Community Composition.
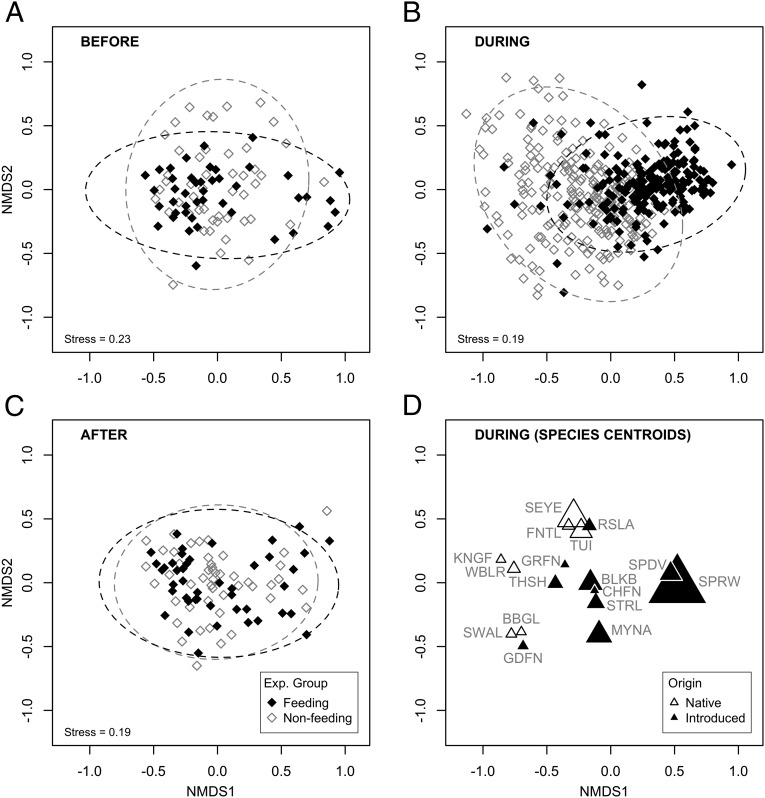
A total of 33 bird species (18,228 individuals) were recorded at the study properties, and over 597 bird surveys (10-min point counts), 16 of which were native species and 17 introduced. Twenty-seven species were recorded at feeding properties and 31 at nonfeeding properties. The house sparrow was the most commonly observed species (96.6% of surveys), followed by blackbird (Turdus merula; 91.1% of surveys), silvereye (Zosterops lateralis; 90.8% of surveys), and common myna (87.6% of surveys) (Table S1). Nonmetric multidimensional scaling (NMDS) ordination plots indicated that before the start of the feeding regime avian community composition did not differ between the two experimental treatment groups (feeding and nonfeeding properties) (Fig. 2A); this was supported by permutational multivariate analysis of variance (PERMANOVA) (Table 1) and permutational analysis of multivariate dispersions (PERMDISP; F = 0.122, df = 1, P = 0.71). The greatest amount of variation in community composition before feeding was explained by property ID and vegetation (R2 = 0.28 and 0.17, respectively). During experimental feeding, however, there was evidence of a divergence in avian communities at feeding compared with nonfeeding properties, with the feeding-group centroid shifting to the right (Fig. 2B). PERMANOVA analyses confirmed that provision of food had a significant effect on community composition (Table 1) and explained the greatest amount of variation in the data (R2 = 0.16). The effect of feeding did vary among months but the proportion of variation explained by the interaction term was comparatively small (R2 = 0.04). The proportion of variation explained by property ID and vegetation were smaller than in the “before” period (R2 = 0.13 and 0.07, respectively). PERMDISP analyses indicated that that avian communities were also significantly less variable at feeding compared with nonfeeding properties in the “feeding” period (PERMDISP; F = 35.5, df = 1, P < 0.001). Introduced species were associated with the community shift at feeding properties, most distinctly house sparrow and spotted dove (Streptopelia chinensis) (Fig. 2D). These changes did not persist when the provision of food stopped (Fig. 2C and Table 1) (PERMDISP; F = 1.03, df = 1, P = 0.34).
Fig. 2.
NMDS ordinations of avian community composition (A) before, (B) during, and (C) after the experimental feeding regime at urban study properties in northern Auckland, New Zealand, grouped by experimental treatment. The dotted ellipses denote the 95% confidence intervals for each experimental (Exp.) group. The species centroids (relationships among species as defined by their relative abundance at different sites) are also presented (D) for the “during feeding” period, scaled by percentage of total abundance (square root-transformed) for that period. Species abbreviations (for scientific names see Table S1): BBGL, southern black-backed gull; BLKB, Eurasian blackbird; CHFN, chaffinch; FNTL, New Zealand fantail; GDFN, goldfinch; GRFN, greenfinch; KNGF, New Zealand kingfisher; MYNA, common myna; RSLA, eastern rosella; SEYE, silvereye; SPDV, spotted dove; SPRW, house sparrow; STRL, common starling; SWAL, welcome swallow; THSH, song thrush; TUI, tūī; WBLR, grey warbler.
Table 1.
Summary of PERMANOVA results for the effects of feeding treatment (experimental group) on avian community structure for each experimental period: before, during, and after feeding regime implementation
| Factor | Before | During | After | |||||||||
| df | F | R2 | P | df | F | R2 | P | df | F | R2 | P | |
| Experimental group | 1 | 3.05 | 0.021 | 0.41 | 1 | 113.48 | 0.162 | <0.001 | 1 | 0.77 | 0.005 | 0.67 |
| Month no. | 3 | 1.27 | 0.027 | 0.24 | 17 | 2.72 | 0.066 | <0.001 | 3 | 0.74 | 0.015 | 0.79 |
| Vegetation | 2 | 12.10 | 0.170 | 0.41 | 2 | 24.86 | 0.071 | <0.001 | 2 | 12.70 | 0.167 | 0.67 |
| Background feeding | 2 | 2.73 | 0.038 | 0.41 | 2 | 10.90 | 0.031 | <0.001 | 2 | 4.82 | 0.064 | 0.67 |
| Property ID | 17 | 2.36 | 0.282 | 0.41 | 17 | 5.20 | 0.126 | <0.001 | 17 | 2.79 | 0.314 | 0.67 |
| Experimental group × month | 3 | 0.83 | 0.018 | 0.63 | 17 | 1.52 | 0.037 | <0.001 | 3 | 1.04 | 0.021 | 0.46 |
| Residuals | 63 | 0.443 | 356 | 0.501 | 63 | 0.415 | ||||||
| Total | 91 | 412 | 91 | |||||||||
F-values (pseudo-F) are derived from 999 permutations. Values in bold are significant at P < 0.05.
Species Richness and Abundance.
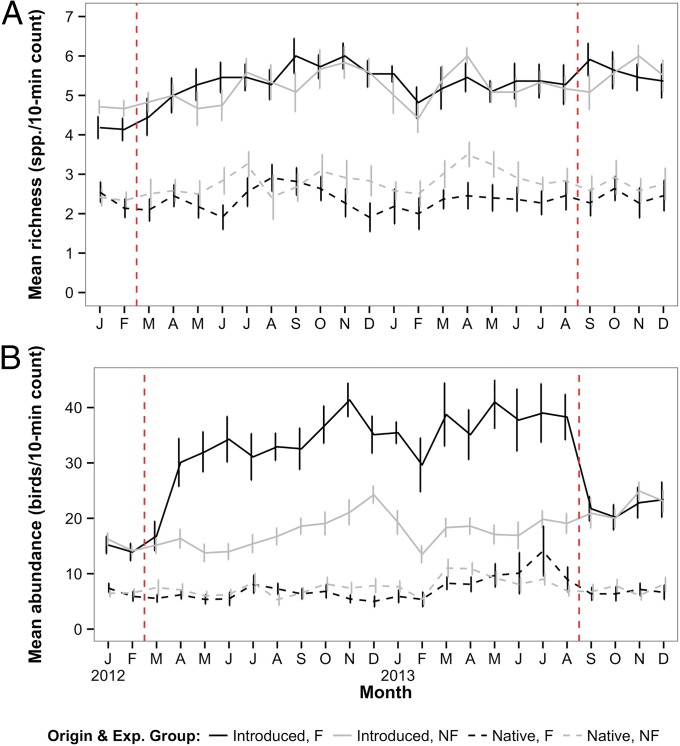
The mean number of introduced species recorded per count was well above that for native species over all properties for the duration of the study (Fig. 3A). We found no evidence of the onset of the feeding regime having an effect on species richness overall [see Table S2 for full generalized linear mixed model (GLMM) results], instead finding that feeding had differing effects on introduced and native species richness (Table 2). Introduced species richness was slightly lower at feeding compared with nonfeeding properties before the start of the feeding regime (modeled mean count = 4.25 vs. 4.96 spp.; t = −2.39, df = 567, P = 0.03). The introduced species richness of both experimental groups increased during the feeding period, but the increase was significantly greater (by 0.66 species) at feeding properties (Table 2). There was no significant change from during feeding to after feeding in either group (feeding: t = 0.15, df = 567, P = 0.88; nonfeeding: t = −0.26, df = 567, P = 0.79). Native species richness was equivalent at feeding and nonfeeding properties before the start of the feeding regime (modeled mean count = 2.50 and 2.31 spp., respectively; t = 0.65, df = 567, P = 0.52). At nonfeeding properties, native species richness increased from before to during the feeding regime (t = 2.61, df = 567, P = 0.009), yet richness at feeding properties did not change (t = −0.25, df = 567, P = 0.80); this difference in observed pattern was significant (Table 2). Native species richness did not change significantly in either group after feeding was stopped (feeding: t = −0.22, df = 567, P = 0.82; nonfeeding: t = 0.90, df = 567, P = 0.37).
Fig. 3.
Overall (A) species richness and (B) relative abundance of garden birds recorded during 10-min point counts at urban study properties in northern Auckland, New Zealand, before, during, and after the implementation of an experimental feeding regime. Experimental (Exp.) group: F, feeding properties; NF, nonfeeding properties. The vertical dotted lines indicate the start and end of the feeding regime. Error bars represent the SEM.
Table 2.
Summary of the effects of the feeding regime on community structure measures and individual species abundances at urban study properties in northern Auckland, New Zealand
| Species/measure | Evidence of feeding regime effect | Comparison of pattern between experimental periods (between feeding and nonfeeding groups) | Ratio of multiplicative factors* (F/NF) | t | P |
| Overall community structure responses | |||||
| Overall species richness | No | During/before | 0.21† | 0.58 | 0.56 |
| During/after | −0.10† | −0.28 | 0.78 | ||
| Introduced species richness | Yes | During/before | 0.66† | 2.42 | 0.015 |
| During/after | 0.09† | 0.32 | 0.75 | ||
| Native species richness | Yes | During/before | −0.45† | −2.07 | 0.039 |
| During/after | −0.19† | −0.86 | 0.39 | ||
| Overall abundance | Yes | During/before | 1.68 | 7.42 | <0.0001 |
| During/after | 1.69 | 7.80 | <0.0001 | ||
| Individual responses: Introduced species | |||||
| House sparrow (Passer domesticus) | Yes | During/before | 2.36 | 6.10 | <0.0001 |
| During/after | 2.93 | 9.13 | <0.0001 | ||
| Common myna (Acridotheres tristis) | No | During/before | 1.72† | 1.36 | 0.18 |
| During/after | 1.23† | 0.52 | 0.60 | ||
| Eurasian blackbird (Turdus merula) | No | During/before | 1.33 | 1.81 | 0.07 |
| During/after | 1.16 | 1.27 | 0.20 | ||
| Spotted dove (Streptopelia chinensis) | Yes | During/before | 6.61 | 7.52 | <0.0001 |
| During/after | 3.44 | 5.24 | <0.0001 | ||
| European starling (Sturnus vulgaris) | Yes | During/before | 1.96 | 2.04 | 0.042 |
| During/after | 1.09 | 0.35 | 0.72 | ||
| Song thrush (Turdus philomelos) | Yes | During/before | 0.80 | −0.58 | 0.56 |
| During/after | 0.58 | −1.98 | 0.049 | ||
| Eastern rosella (Platycercus eximius) | No | During/before | 0.71 | −0.66 | 0.51 |
| During/after | 0.94 | −0.17 | 0.86 | ||
| Chaffinch (Fringilla coelebs) | No | During/before | 0.23 | −1.18 | 0.24 |
| During/after | 1.01 | 0.01 | 0.99 | ||
| Individual responses: Native species | |||||
| Silvereye (Zosterops lateralis) | No | During/before | 1.17 | 0.86 | 0.39 |
| During/after | 1.10 | 0.51 | 0.61 | ||
| Tūī (Prosthemadera novaeseelandiae) | No | During/before | 0.82 | −0.93 | 0.35 |
| During/after | 1.03 | 0.14 | 0.89 | ||
| Grey warbler (Gerygone igata) | Yes | During/before | 0.43 | −2.54 | 0.011 |
| During/after | 0.90 | −0.31 | 0.76 | ||
| New Zealand fantail (Rhipidura fuliginosa) | No | During/before | 0.75 | −0.49 | 0.62 |
| During/after | 0.61 | −0.88 | 0.38 | ||
Significance tests for the relevant interaction terms (experimental group × experimental period) from GLMM results are presented, assessing whether patterns of change between experimental periods (before, during, or after the feeding regime) differ between experimental groups (feeding or nonfeeding). The ratio of multiplicative factors is the change from one period to the next in the feeding group compared with the nonfeeding group. Significant effects are highlighted in boldface. Species are listed by mean overall abundance (highest first).
A ratio of 1 indicates that the change from one period to the next was the same for both the feeding and the nonfeeding groups. A ratio of 2 indicates that change from one period to the next was two times higher in the feeding group. A ratio of 0.5 indicates that change from one period to the next was 0.5× that of the nonfeeding group.
These measures are interpreted in terms of a difference in the difference in means (period 1 – period 2; feeding – nonfeeding), rather than a ratio of multiplicative factors, as these measures were modeled using a normal distribution.
There was strong evidence of the feeding regime having an effect on overall avian abundance (Fig. 3B and Table 2). Before the feeding regime there was no significant difference in overall abundance recorded at feeding and nonfeeding properties (modeled mean count = 21.01 and 22.86, respectively; t = −0.97, df = 567, P = 0.33). Both experimental groups showed an increase in overall abundance from the before period to the during period, but the increase was significantly more at feeding properties compared with the nonfeeding properties (modeled mean count for feeding period = 40.14 vs. 25.95) (Table 2). The pattern of change from during to after feeding differed significantly between the two groups (Table 2), with abundance decreasing at feeding properties (t = 7.58, df = 567, P < 0.0001) and increasing at nonfeeding properties (t = −2.39, df = 567, P = 0.02).
Individual Species Responses.
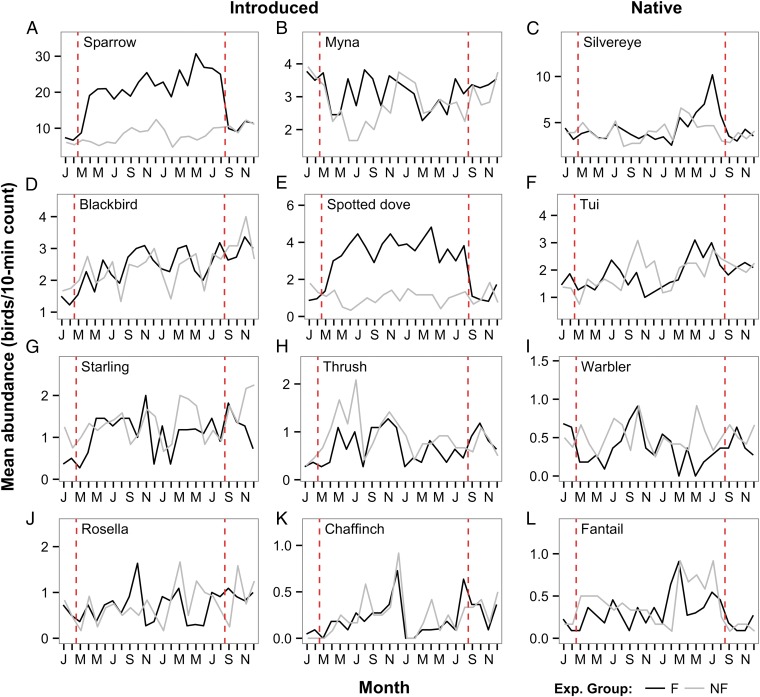
We retained the 12 most frequently observed species for GLMM analyses of individual species responses to the feeding regime (see Table S2 for full model results). Among the introduced species there was support for the feeding regime having an effect on the relative abundance of four of the eight species analyzed (Table 2). The greatest absolute change in abundance was observed in the most common species, the house sparrow (Fig. 4A and Table 3). Both feeding and nonfeeding properties had a significant increase in sparrow abundance from before to during the feeding regime (t = 11.15, df = 567, P < 0.0001; t = 2.62, df = 567, P = 0.009, respectively) but feeding properties had a significantly larger increase (Table 2). Mean abundance of house sparrow at feeding properties increased from 6.26 before the start of the feeding regime to 19.23 during feeding (Table 3). Sparrow abundance during the feeding regime was 2.4-times higher at feeding compared with nonfeeding properties (Table 3). At feeding properties there was a significant decrease in abundance from the during period to the after period (t = 9.13, df = 567, P < 0.0001), whereas nonfeeding properties had a significant increase in abundance (t = −2.61, df = 567, P = 0.009); this difference in pattern was significant (Table 2).
Fig. 4.
Relative abundance of the 12 (A–L) most commonly occurring garden bird species recorded during 10-min point counts at urban study properties in northern Auckland, New Zealand, before, during, and after the implementation of an experimental feeding regime. Experimental (Exp.) group: F, feeding properties; NF, nonfeeding properties. Within each species type (introduced/native) species are listed in order of mean abundance over all counts (n = 597). For species scientific names see Table 2. The vertical dotted lines indicate the start and end of the feeding regime. NB: y axis scale varies with species.
Table 3.
Modeled mean counts of species abundances for the 12 most common garden bird species recorded during 10-min point counts conducted at urban study properties in northern Auckland, New Zealand
| Species | Experimental period | Modeled mean count | Multiplicative factor (F/NF group) | 95% Confidence interval | t | P | |
| NF | F | ||||||
| Introduced species | |||||||
| House sparrow | Before | 6.25 | 6.26 | 1.00 | (0.71, 1.41) | 0.00 | 0.99 |
| During | 8.16 | 19.23 | 2.36 | (1.85, 2.99) | −7.46 | <0.0001 | |
| After | 10.34 | 8.31 | 0.80 | (0.59, 1.10) | 1.47 | 0.16 | |
| Common myna | Before | 3.89 | 3.58 | −0.31 | (-1.23, 0.61) | −0.71 | 0.49 |
| During | 2.82 | 3.05 | 0.23 | (-0.40, 0.87) | 0.76 | 0.46 | |
| After | 3.17 | 3.19 | 0.02 | (-0.90, 0.94) | 0.05 | 0.96 | |
| Eurasian blackbird | Before | 1.73 | 1.35 | 0.78 | (0.55, 1.11) | 1.47 | 0.16 |
| During | 2.34 | 2.43 | 1.04 | (0.85, 1.27) | 0.39 | 0.70 | |
| After | 2.99 | 2.68 | 0.90 | (0.68, 1.18) | 0.84 | 0.41 | |
| Spotted dove | Before | 1.32 | 0.72 | 0.54 | (0.26, 1.14) | −1.72 | 0.10 |
| During | 0.80 | 2.86 | 3.59 | (2.01, 6.43) | 4.59 | <0.001 | |
| After | 0.79 | 0.83 | 1.05 | (0.51, 2.13) | 0.13 | 0.90 | |
| European starling | Before | 1.00 | 0.35 | 0.35 | (0.16, 0.74) | −2.91 | <0.01 |
| During | 1.21 | 0.83 | 0.68 | (0.43, 1.08) | −1.73 | 0.10 | |
| After | 1.69 | 1.06 | 0.63 | (0.34, 1.15) | −1.61 | 0.13 | |
| Song thrush | Before | 0.42 | 0.33 | 0.78 | (0.36, 1.72) | −0.65 | 0.53 |
| During | 1.02 | 0.64 | 0.62 | (0.48, 0.81) | −3.73 | 0.002 | |
| After | 0.63 | 0.68 | 1.07 | (0.63, 1.80) | 0.27 | 0.79 | |
| Eastern rosella | Before | 0.48 | 0.64 | 1.33 | (0.38, 4.61) | 0.47 | 0.64 |
| During | 0.47 | 0.44 | 0.94 | (0.43, 2.04) | −0.17 | 0.87 | |
| After | 0.65 | 0.65 | 1.00 | (0.38, 2.68) | 0.00 | 0.99 | |
| Chaffinch | Before | 0.03 | 0.09 | 3.50 | (0.26, 4.70) | 1.01 | 0.33 |
| During | 0.24 | 0.19 | 0.80 | (0.43, 1.49) | −0.75 | 0.46 | |
| After | 0.30 | 0.24 | 0.80 | (0.31, 2.07) | −0.50 | 0.62 | |
| Native species | |||||||
| Silvereye | Before | 3.57 | 3.81 | 1.07 | (0.72, 1.59) | 0.34 | 0.74 |
| During | 3.59 | 4.49 | 1.25 | (1.01, 1.57) | 2.03 | 0.06 | |
| After | 3.77 | 4.30 | 1.14 | (0.60, 1.29) | −0.71 | 0.48 | |
| Tūī | Before | 1.11 | 1.34 | 1.21 | (0.60, 2.43) | 0.56 | 0.58 |
| During | 1.46 | 1.44 | 0.99 | (0.55, 1.78) | −0.05 | 0.96 | |
| After | 1.74 | 1.67 | 0.96 | (0.49, 1.88) | −0.13 | 0.90 | |
| Grey warbler | Before | 0.35 | 0.66 | 1.86 | (0.82, 4.25) | 1.57 | 0.13 |
| During | 0.36 | 0.29 | 0.80 | (0.42, 1.51) | −0.74 | 0.47 | |
| After | 0.31 | 0.27 | 0.89 | (0.38, 2.08) | 0.30 | 0.77 | |
| New Zealand fantail | Before | 0.12 | 0.11 | 1.04 | (0.28, 3.82) | 0.06 | 0.95 |
| During | 0.34 | 0.25 | 1.39 | (0.74, 2.60) | 1.10 | 0.29 | |
| After | 0.14 | 0.17 | 0.84 | (0.24, 2.93) | −0.28 | 0.78 | |
Species are listed by abundance (highest first). Modeled means control for levels of background feeding and vegetation as well as season, as derived from GLMMs testing the effect of an experimental feeding regime. Figures are given for each experimental period (before, during, or after the feeding regime), along with tests of significance between nonfeeding (NF) and feeding (F) groups (from GLMMs). Species are listed by mean overall abundance (highest first).
We found a similar effect for spotted dove (Fig. 4E and Table 2), with an obvious increase in abundance at feeding properties (t = 7.04, df = 567, P < 0.0001) but a decrease in abundance at nonfeeding properties (t = 3.01, df = 567, P = 0.003) from the before period to the during period, resulting in 3.6-times more doves at feeding properties during the feeding regime (Table 3). There was a significant decrease in the abundance of doves at feeding properties after feeding had stopped (t = 7.05, df = 567, P < 0.0001) but no change at nonfeeding properties (t = 0.02, df = 567, P = 0.98); this difference in the pattern of change was highly significant (Table 2).
There was also evidence of the feeding regime affecting European starling (Sturnus vulgaris) and song thrush (Turdus philomelos) abundances (Table 2). A significant increase was seen in European starling abundance from before to during feeding at feeding properties (0.35 vs. 0.83 mean individuals per count; t = 3.15, df = 567, P = 0.002) but no change at nonfeeding properties (t = 0.95, df = 567, P = 0.35). For song thrush, we only detected an interaction effect from during to after the feeding regime (Table 2), with abundance decreasing at nonfeeding properties (t = 2.31, df = 567, P = 0.02) and no significant change for feeding properties (t = −0.27, df = 567, P = 0.79) (Table 3). Song thrush abundances did not differ between experimental groups before the feeding regime (Table 3), but did differ significantly during feeding, with higher abundances at nonfeeding properties (Table 3).
Among the four common native species, there was only evidence of a feeding regime impact on one, the grey warbler, which significantly decreased in abundance at feeding properties from 0.66 mean individuals per count before feeding to 0.29 during feeding (t = −3.43, df = 567, P = 0.0007), whereas there was no significant change at nonfeeding properties (t = 0.04, df = 567, P = 0.96) (Table 2).
Discussion
Changes to Avian Community Structure.
Most of our knowledge on the impacts of feeding wild birds in urban areas derives from correlational studies or studies conducted in natural habitats (15). This study directly demonstrates that the pastime of bird feeding substantially contributes to the avian community patterns observed in urban areas. We found significant changes in community composition occurring as a result of feeding and evidence that five common garden bird species were affected by the experimental feeding regime, despite our study being carried out on a relatively small scale [11 experimental feeding stations compared with the estimated 265,000 households feeding birds across six New Zealand cities (11)]. Our findings support evidence from a number of correlational studies that have found an association between bird feeding and increased densities of feeding birds in urban areas (e.g., refs. 10, 20, and 21). From surveying feeding practices we know that ∼two of five households in New Zealand’s urban areas feed birds (11). Given this high rate of bird feeding participation, in combination with the readily observable changes to local bird communities observed in our study (based on typical feeding practices of the public), we think it likely the effects of feeding will be operating on a larger scale as well. Although we need to be cautious of making generalizations as to the overall effects of feeding on larger spatial scales, we predict that feeding has important implications for urban avifaunal assemblages.
Effects on Introduced Birds.
Enhancing the capacity of urban environments to support more species is now a growing area of research (22, 29, 30); however, not all species are equally desirable. Many of the avian species that have successfully managed to exploit urban areas are invasive or considered pests (31), such as the rock pigeon (Columba livia) (22). Identifying what promotes the success of introduced or pest species in these areas is crucial for developing strategies to enhance native biodiversity instead. Many introduced bird species in urban areas around the world are granivores or omnivores, which is ideal for capitalizing on supplementary food resources. In most countries, though, there are also native granivores and omnivores present (32, 33)—usually the primary targets of feeding—with which introduced species must compete.
Our results support the hypothesis that typical feeding practices encourage increased densities of introduced bird species in New Zealand, with obvious and substantial increases in the relative abundance of two species in particular, the house sparrow and spotted dove, and additional evidence of a positive effect on European starling. Furthermore, bird communities at feeding properties exhibited reduced variability and a shift toward communities dominated by these introduced species. The observed, rapid changes to house sparrow and spotted dove abundance occurred after the Austral breeding season, indicating that the effects, at least initially, were the product of increased juvenile survivorship and immigration of existing adults from surrounding areas, rather than an increase in reproductive success. The experimental feeding regime did encompass one breeding season; therefore, increased productivity may have contributed to the consistently higher abundances of these species for the duration of supplementary feeding (17). However, our data do not allow for this hypothesis to be tested here; it would require a multiyear study to account for interannual variation (34). Productivity certainly can be increased with food supplementation, including earlier laying dates, increased clutch size, and greater hatching and fledging success (15, 17), although this is not always the case (e.g., ref. 35).
Regardless of the mechanism of increase, the results imply that feeding promotes a higher carrying capacity for these species. House sparrows are already widespread in New Zealand, whereas spotted doves are more recent invaders and are currently in the process of expansion, radiating from Auckland City where they were first introduced in the 1920s (27, 36, 37). We propose that common feeding practices are aiding this spread, by supplementing food resources for the doves as they move into new areas and via increased pressure to disperse from areas of higher dove density, where they are already established. Feeding has been linked to range expansions of birds elsewhere, including native species moving outside of their historical ranges. For example, it has been proposed that the northward spread of the northern cardinal (Cardinalis cardinalis) and American goldfinch (Carduelis tristis) are linked to the rapid increase in bird feeding participation in the United States since the 1970s (17).
The effect of the feeding regime on song thrush abundance is not as conspicuous in comparison with the changes for the house sparrow or spotted dove. During the feeding regime, song thrush abundance was generally lower at feeding properties than at nonfeeding properties. We observed a greater decrease in abundance after the feeding regime ended at nonfeeding properties, likely because of the already lower abundance at feeding properties. These results suggest that the feeding regime had a negative effect on song thrush abundance, perhaps facilitated by the wary behavior typically exhibited by the species (38) and disturbance by dominant heterospecifics.
Contrary to our expectation, we did not see an effect of the feeding regime on common myna (henceforth myna), a key invasive species in New Zealand and globally (28, 39). A number of factors may have contributed to this result. Interspecific interactions at feeding stations may have prevented mynas from accessing supplementary food. We observed that mynas were being attracted to feeding stations, but where stations were congested with other species—particularly spotted dove—mynas were reluctant to “push in” to access the food. Therefore, mynas may have been behaviorally excluded by dominant heterospecifics when feeders were busy (40), a scenario likely compounded by the mode of food presentation. We used a seed feeder and mesh tube to dispense food, which limits access much more than simply throwing the food on the ground (the most common method of food presentation) (11), requiring individuals to contend with others. In addition, the foods we tested in this study may not have been attractive enough to encourage higher densities of mynas. Although myna are omnivores and will consume the food types we tested, other food types are more attractive to them, for example dog and cat food, which is provided by some bird-feeding participants (8, 11). Alternatively, no effect was found because changes in myna densities were too transient to be detected; that is, myna numbers increased at feeding time only with mynas leaving the area immediately after the food was gone, whereas the survey period encompassed a longer time period. Other species for which we did not detect an effect of the feeding regime may also have shown transient changes at feeding time. However, finding no evidence of an effect essentially means that the feeding regime we implemented was not capable of influencing the density of those species beyond the feeding interval.
Effects on Native Birds.
We found evidence of the feeding regime negatively affecting native biodiversity, with native species richness remaining lower at feeding properties during the feeding regime compared with before, whereas an increase was observed at nonfeeding properties. It is arguable, though, whether the effect detected is biologically significant, given that the difference in species richness was less than 0.5 species. We suggest its significance is dependent on scale; at a landscape or regional scale this effect may well be important when multiple avian assemblages are accounted for (41, 42). An important and biologically significant finding was a decline in grey warbler abundance by more than 50% at feeding properties during the feeding regime, in comparison with nonfeeding properties where grey warbler abundance remained steady. This effect is concerning in light of evidence that the grey warbler, regarded as a common species, may in fact be declining in forest habitats (43). A likely reason for the negative impact of the feeding regime on warblers is the increased disturbance by heterospecifics. The grey warbler typically forages alone or in pairs, gleaning invertebrates from foliage in the subcanopy to canopy (27). With densities of other species increasing dramatically in feeding gardens, the ability of the grey warbler to forage efficiently would be severely disrupted, especially with, for example, 50 sparrows occupying a garden on a daily basis, potentially causing displacement (44). In contrast, we did not observe any negative effects of the feeding regime on another common insectivore, the New Zealand fantail (Rhipidura fuliginosa); it is possible that their different behavioral tactics in foraging (primarily sallying and flush-pursuit) and their tendency to favor associations with other species when foraging to exploit disturbance (45) allows for flexibility or resilience where heterospecific densities increase.
Other Impacts.
There are a number of other potential consequences of our findings that directly relate to having increased densities of birds in the urban, or in fact any, environment. As with the grey warbler, high densities of one or a few dominant species can affect others through behavioral disturbance or displacement. Aggressive encounters can increase within or between species, related to higher densities, reduced territory size, or more time available for such behaviors (46–48). There may also be direct competition for other resources in the area (e.g., other food sources, nest sites, territory), which in extreme cases could lead to competitive exclusion and local extinctions (41, 44). Furthermore, a major issue associated with high bird concentrations is the increased likelihood of transmitting avian diseases (24, 49) and the associated zoonotic risks to people (50, 51). In addition, there are potential indirect effects, such as greater predation pressure on invertebrate populations. A study conducted in Michigan, United States, found that bird feeding can create areas of concentrated foraging, with experimentally placed mealworms depredated at higher rates in the presence of bird feeders (52). Not only would this affect the invertebrate prey population, but any taxa that are part of their food web. These findings suggest that the impacts of common feeding practices in New Zealand could extend beyond birds, with flow-on effects for other trophic levels.
After Feeding.
An important question to address in assessing the impacts of bird feeding is what happens to bird communities should feeding stop? In this study we found that most of the changes to local avian communities associated with feeding did not persist afterward. The rapid declines in the abundances of house sparrow and spotted dove once feeding ceased are almost certainly the result of existing individuals redistributing in the landscape. It is doubtful that the declines represent mortality because of dependence on supplementary food, as several studies have failed to establish dependence as a problem for feeder-visiting birds (13, 53). It is likely that the timeframe of our feeding regime (18 mo) was too short for more permanent community changes to occur. Effects, such as competitive exclusion, operate over much longer timeframes, possibly taking decades to become apparent (39), or may only be apparent over much larger spatial scales (41) or when environmentally stressful events occur. Similarly, population changes resulting from altered reproductive success may only be observable over multiple breeding seasons. As a consequence we cannot exclude the possibility that reversing the effects of feeding requires more than purely stopping the provision of food. This reinforces the need for and the value of long-term studies of bird-feeding impacts.
Conclusions
The findings of this study are an important step toward understanding the impacts of what is essentially one of the largest wildlife management activities in temperate regions (52). There are few studies that have experimentally investigated bird feeding, especially in an urban setting (15), perhaps because it is construed as too difficult to disentangle the impacts of feeding from the multitudes of additional variables that influence bird populations (1). We have demonstrated, however, that even with a modest-scale experimental approach the impacts of feeding can be readily observable. We stress that it is crucial to continue assessing bird feeding in situ, where all other factors determining avian community structure and ecology in urban areas still operate, to gather realistic information on its effects. Outcomes of feeding will vary with region as bird assemblages differ. We expect, though, that granivores and omnivores benefit from feeding to a greater degree than those in other dietary guilds, regardless of whether they are introduced or native species, because provisioning of grain-based foods is the prevailing practice.
Methods
Study Site.
This study was carried out at 24 urban, residential properties in northern Auckland, New Zealand (Fig. 1), between January 2012 and December 2013. The North Shore area of Auckland is largely suburban residential, with a population density of 1,600/km2 in 2006 (New Zealand Census data, www.stats.govt.nz). Properties were recruited by word-of-mouth and through local community groups. All properties offered for the study (n = 42) were visited before recruitment to assess suitability. Although the study aimed to recruit householders whose properties were representative of the whole study area, additional criteria were imposed to remove any extremely different properties: gardens were required to have a minimum lawn area of 36 m2, trees > 2 m high on at least one boundary, be sited at least one property away from any main road (experiencing constant traffic), and not newly developed (within the last 5 y). Final selection of properties was determined by the reliability of volunteers to adhere to the study guidelines, accessibility, and distance to the nearest study property. All study properties were greater than 350 m apart, to prevent repeat counts of the same birds, although most (21 of 24) were >500 m apart.
Study properties were divided into two experimental treatment groups: feeding (n = 12) or nonfeeding (n = 12). Allocation of experimental treatment was determined by a two-step process. Householder preference was first determined and strong preferences for treatment type were taken into account, as to disregard these would risk the failure of participants to comply with study guidelines. For the remaining properties (n = 16), the treatment was randomly assigned using R 3.0.2 (R Development Core Team 2013). We had expected that retaining all households over the course of the project would be difficult. However, only one feeding household withdrew (after 12 wk) and was excluded from the analysis, leaving 11 feeding and 12 nonfeeding properties for the duration of the study.
Experimental Feeding Regime.
Information obtained during the New Zealand Bird Feeding Survey 2011 (11) provided the basis for the experimental feeding regime. These results indicated that people feeding birds tended to put out more than one type of food for birds (mean 2.42 types ± 0.06 SE, n = 505), so we opted for two food types for the feeding regime. Bread, the most common food type fed to wild birds by the public (used by 88.1% of feeding participants), was chosen as the first food type. Although fruit and seed were fed by similar proportions of respondents (40.8% and 39.4%, respectively) we selected seed as the second food type as it was logistically easier to distribute to householders and to standardize quantities fed. Food quantity was also determined using Galbraith et al. (11) and through a pilot study used to assess the amount consumed in a single day. The aim was to provide an abundant and reliable source of food for birds that was within the range of that fed by the public. Hence, the experimental feeding treatment consisted of: four to five slices of bread (several compositions but excluding white bread) and 1 metric cup of seed (budgie seed mix: white millet, Hungarian millet, hulled oats, canary seed) provided on a daily basis for 18 mo. Householders were asked to put out the food between 0700 and 0800 hours.
We ensured that all existing feeding practices at the study properties had ceased 8 wk before the start of preliminary bird counts. A feeding station was set up in each feeding garden, consisting of a low feeding table (17-cm high), with a seed feeder and mesh bread tube fixed to it. Although a high proportion of bird feeding participants in New Zealand simply throw food out onto the ground (11), the design of our feeding stations reflected the need to have a structure capable of supporting a RFID antenna (for a separate part of the project) and containers to prevent food being moved. Householders were given guidelines to follow, including cleaning protocols, and were responsible for provisioning the feeding stations. Householders in the nonfeeding group were asked to refrain from putting supplementary food of any kind out for birds for the duration of the study. At the end of the feeding period, food provision was stopped immediately (i.e., no gradual decrease). This regime was approved by the University of Auckland Animal Ethics Committee (permit R921).
Avian Surveying.
The method of surveying used in this study was dictated by the nature of urban habitats. Counting birds in residential areas has a number of challenges, in particular physical barriers (e.g., fences, buildings), which prevent free movement of researchers through the landscape and reduce the probability of detecting birds (54). Because of this we used point counts from a fixed location, with a 10-min duration intended to increase detectability of birds that were blocked from sight (55, 56). A point was chosen on the study property that afforded the widest view of the surrounding area. From this point, all birds seen or heard within the surrounding radius over the 10-min survey duration were recorded. The survey radius (approximately 80 m) was restricted by the physical and auditory barriers associated with an urban setting. During the count, it was noted whether individuals were using the habitat or were in transit (i.e., flying over the survey area without stopping) with those in transit excluded from analyses. A single experienced observer (J.A.G.) performed all counts.
Four preliminary counts were conducted at each study property from January to March at 2-wk intervals, after historical feeding practices had ceased and before the start of experimental feeding. Counts were then conducted on a monthly basis for the duration of the study, and continued for 4 mo after feeding had ended [total counts, n = 597; note one count at a feeding property was abandoned because of construction noise]. Counts were conducted in the morning only, ∼1–5 h after sunrise. To visit all properties within this timeframe, counts were conducted over 2 consecutive days. Study properties were divided into four geographic blocks, with two of these blocks surveyed per morning. Block order and property order within each block were randomly assigned for each sampling round. Surveys were conducted in fine to fair weather only; particularly wet or windy days were avoided.
Statistical Analyses.
All statistical analyses were performed using R 3.0.2. The critical α level was 0.05 for all tests. For species richness and overall abundance analyses we removed incongruous species (those unlikely to use garden habitat, e.g., shorebirds and wetland birds), most of which were present in fewer than five counts. A list of all recorded species is given in Table S1, with those retained for analyses indicated.
To analyze changes in avian community composition, we used three nonparametric multivariate techniques: NMDS (57), PERMANOVA (58), and PERMDISP (59). Data were split into experimental periods (before, during, and after the feeding regime), and analyzed separately to: (i) check for differences in avian community composition between feeding and nonfeeding properties before the commencement of experimental feeding; (ii) determine whether the experimental feeding regime had an effect on community composition; and (iii) determine whether any observable changes persisted after feeding had stopped. We used the Bray–Curtis measure of dissimilarity (60) as the distance measure for all analyses, with species present in <5% of counts removed (61); see Table S1 for included species. No transformation was applied to the data before calculation of the distance-matrix, as we were specifically interested in changes involving dominant species within bird communities.
To visualize differences in bird assemblages between feeding treatments, we performed NMDS ordinations, using the metaMDS function of vegan v2.0-10 package (62) in R 3.0.2. Two-dimensional solutions were chosen and final ordinations were generated from 250 random starts. Species centroids were plotted separately to aid understanding of differences in avian community structure.
We used PERMANOVA analyses to test whether community composition varied between experimental groups in each experimental period. PERMANOVA tests for differences in the locations (centroids) of multivariate groups (63). Analyses were performed using the adonis function of vegan. P values for the test statistic (pseudo-F) are based on 999 permutations, and thus are reported down to, but not below, 0.001. We accounted for repeated measures by including property ID as a random factor and by constraining permutations to within properties (using the “strata” argument). We included survey month number (categorical factor) as a fixed effect in the models, and vegetation cover in the surrounding area (shrub/tree cover = 0–25% 26–50%, 51–75%) and background feeding level in the surrounding area (low, medium, high) as random effects. “Surrounding area” refers to properties within a 100-m radius of the focal property. Background feeding was determined in a concurrent study of local feeding practices (64). For the purposes of this modeling, we scored background feeding as low where 0–33% of surrounding households engaged in bird feeding, medium for 34–66%, and high for ≥67%. Vegetation cover was estimated using aerial photography accessed April 4, 2014 from Google Earth v7.1.2.2041 (Google Inc. 2013).
We tested for differences in the variability of bird assemblages between feeding and nonfeeding properties with PERMDISP analyses for each experimental period. Multivariate dispersions (distances of observations to their centroids) were first calculated using the betadisper function of vegan, with the mean dispersion then compared between groups via the permutest function (constraining permutations within sites; based on 999 permutations). Where designs are balanced, location vs. dispersion effects can be identified using PERMANOVA and PERMDISP, respectively, with PERMANOVA tests remaining reliable when heterogeneity in group dispersions is present (63).
Following the analysis of the community as a whole, we investigated the effects of the feeding regime on species richness (overall, native, and introduced), overall abundance, and the abundance of individual species. To do this we used a GLMM approach (65), which was appropriate given the repeated-measures structure in the data. The distribution of each count variable (species richness and abundances) was assessed and the best-fitting distribution chosen for use in the corresponding models. The negative binomial distribution was found to be the best fit in most cases (see Table S2 for exceptions). Mixed-effect models were performed in R 3.0.2 using the glmmPQL function in the MASS package (66). This function uses penalized quasi-likelihood to estimate the model. The glmmPQL function requires the dispersion parameter for the negative binomial model to be specified. This was estimated for each model by running the equivalent nonmixed-effects model with the glm.nb function, also from the MASS package.
For all models the experimental group (feeding or nonfeeding), experimental period (before, during, or after), and the experimental group × experimental period interaction were included as fixed effects. The interaction term tests the key study question of whether the feeding regime had an effect on a given response variable, by comparing patterns of change from one experimental period to another between the two experimental groups. Property ID was included as a random effect, to account for correlation of repeated measures at the same properties. The time variable was used in the error structure of the models. An autoregressive correlation structure of order 1 [AR(1)] was specified within each site, accounting for the fact that there may be correlation among counts at the same property because of close proximity in time. The corCAR1 function was used to specify this correlation with a continuous time covariate (days elapsed since the first count).
In addition, three factors with the potential to affect bird abundance were also included in the models as control variables: the level of background feeding in the surrounding area, the level of vegetation cover in the surrounding area, and season (autumn, spring, summer, winter). The model predicted (or model fitted) mean counts were calculated for each model, adjusting for these control variables. The modeled means presented are averaged across the control variables at the levels seen in the data (i.e., background feeding: 30% high, 61% medium, 9% low; vegetation cover: 26% 0–25%, 48% 26–50%, 26% 51–75%), except for season where each was represented equally (25%).
Supplementary Material
Acknowledgments
We thank the wonderful volunteer householders involved in the study; EcoStock for donating food for the study; George Perry for statistical advice and comments on the manuscript; Jessica McLay for assistance with statistical analyses; and the Galbraith Family, Ellery McNaughton, Cheryl Krull, Jo Peace, Megan Young, Sarah Wyse, Auckland Zoo staff, and all who provided assistance in the field. This work was supported in part by the University of Auckland, Auckland Council, and Centre for Biodiversity and Biosecurity.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501489112/-/DCSupplemental.
References
- 1.Chace JF, Walsh JJ. Urban effects on native avifauna: A review. Landsc Urban Plan. 2006;74(1):46–69. [Google Scholar]
- 2.Evans KL, Newson SE, Gaston KJ. Habitat influences on urban avian assemblages. Ibis. 2009;151(1):19–39. [Google Scholar]
- 3.Lack D. The Natural Regulation of Animal Numbers. Claredon; Oxford: 1954. [Google Scholar]
- 4.Newton I. Population limitation in Birds. Academic; London: 1998. [Google Scholar]
- 5.Martin TE. Food as a limit on breeding birds: A life-history perspective. Annu Rev Ecol Syst. 1987;18:453–487. [Google Scholar]
- 6.Auman HJ, Meathrel CE, Richardson A. Supersize me: Does anthropogenic food change the body condition of silver gulls? A comparison between urbanized and remote, non-urbanized areas. Waterbirds. 2008;31(1):122–126. [Google Scholar]
- 7.Jones DN, Reynolds SJ. Feeding birds in our towns and cities: A global research opportunity. J Avian Biol. 2008;39(3):265–271. [Google Scholar]
- 8.Rollinson DJ, O'Leary RA, Jones DN. The practice of wildlife feeding in suburban Brisbane. Corella. 2003;27(2):52–58. [Google Scholar]
- 9.US Fish & Wildlife Service . National Survey of Fishing, Hunting and Wildlife-Associated Recreation. US Department of the Interior, Fish and Wildlife Service, and US Department of Commerce, US Census Bureau; Arlington, VA: 2006. [Google Scholar]
- 10.Fuller RA, Warren PH, Armsworth PR, Barbosa O, Gaston KJ. Garden bird feeding predicts the structure of urban avian assemblages. Divers Distrib. 2008;14(1):131–137. [Google Scholar]
- 11.Galbraith JA, et al. Risks and drivers of wild bird feeding in urban areas of New Zealand. Biol Conserv. 2014;180(0):64–74. [Google Scholar]
- 12.Cowie RJ, Hinsley SA. The provision of food and the use of bird feeders in suburban gardens. Bird Study. 1988;35(3):163–168. [Google Scholar]
- 13.Jones D. An appetite for connection: Why we need to understand the effect and value of feeding wild birds. Emu. 2011;111(2):i–vii. [Google Scholar]
- 14.Saggese K, Korner-Nievergelt F, Slagsvold T, Amrhein V. Wild bird feeding delays start of dawn singing in the great tit. Anim Behav. 2011;81(2):361–365. [Google Scholar]
- 15.Amrhein V. Wild bird feeding (probably) affects avian urban ecology. In: Gil D, Brumm H, editors. Avian Urban Ecology: Behavioural and Physiological Adaptations. Oxford Univ Press; Oxford, UK: 2014. pp. 29–37. [Google Scholar]
- 16.Boutin S. Food supplementation experiments with terrestrial vertebrates: Patterns, problems, and the future. Can J Zool. 1990;68(2):203–220. [Google Scholar]
- 17.Robb GN, McDonald RA, Chamberlain DE, Bearhop S. Food for thought: Supplementary feeding as a driver of ecological change in avian populations. Front Ecol Environ. 2008;6(9):476–484. [Google Scholar]
- 18.Brittingham MC, Temple SA. Impacts of supplemental feeding on survival rates of black-capped chickadees. Ecology. 1988;69(3):581–589. [Google Scholar]
- 19.Chamberlain DE, et al. Avian productivity in urban landscapes: A review and meta-analysis. Ibis. 2009;151(1):1–18. [Google Scholar]
- 20.Chamberlain DE, et al. Annual and seasonal trends in the use of garden feeders by birds in winter. Ibis. 2005;147(3):563–575. [Google Scholar]
- 21.Job J, Bednekoff PA. Wrens on the edge: Feeders predict Carolina wren Thryothorus ludovicianus abundance at the northern edge of their range. J Avian Biol. 2011;42(1):16–21. [Google Scholar]
- 22.Savard J-PL, Clergeau P, Mennechez G. Biodiversity concepts and urban ecosystems. Landsc Urban Plan. 2000;48(3-4):131–142. [Google Scholar]
- 23.Pennycott TW, et al. Further monitoring for Salmonella species and Escherichia coli O86 at a bird table in south-west Scotland. Vet Rec. 2005;157(16):477–480. doi: 10.1136/vr.157.16.477. [DOI] [PubMed] [Google Scholar]
- 24.Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends Ecol Evol. 2007;22(2):95–102. doi: 10.1016/j.tree.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishigame G, Baxter GS, Lisle AT. Effects of artificial foods on the blood chemistry of the Australian magpie. Austral Ecol. 2006;31(2):199–207. [Google Scholar]
- 26.van Heezik Y, Smyth A, Mathieu R. Diversity of native and exotic birds across an urban gradient in a New Zealand city. Landsc Urban Plan. 2008;87(3):223–232. [Google Scholar]
- 27.Heather B, Robertson H. The Field Guide to the Birds of New Zealand. Viking; Auckland: 1996. [Google Scholar]
- 28.Krull CR, Galbraith JA, Glen AS, Nathan HW. Invasive vertebrates in Australia and New Zealand. In: Stow A, Maclean N, Holwell G, editors. Austral Ark. Cambridge Univ Press; Cambridge, UK: 2015. p. 680. [Google Scholar]
- 29.Goddard MA, Dougill AJ, Benton TG. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol Evol. 2010;25(2):90–98. doi: 10.1016/j.tree.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Sandström UG, Angelstam P, Mikusiński G. Ecological diversity of birds in relation to the structure of urban green space. Landsc Urban Plan. 2006;77(1–2):39–53. [Google Scholar]
- 31.McKinney ML. Urbanization as a major cause of biotic homogenization. Biol Conserv. 2006;127(3):247–260. [Google Scholar]
- 32.Kark S, Iwaniuk A, Schalimtzek A, Banker E. Living in the city: Can anyone become an ‘urban exploiter’? J Biogeogr. 2007;34(4):638–651. [Google Scholar]
- 33.Lancaster RK, Rees WE. Bird communities and the structure of urban habitats. Can J Zool. 1979;57(12):2358–2368. [Google Scholar]
- 34.Schoech SJ. Food supplementation experiments: A tool to reveal mechanisms that mediate timing of reproduction. Integr Comp Biol. 2009;49(5):480–492. doi: 10.1093/icb/icp005. [DOI] [PubMed] [Google Scholar]
- 35.Harrison TJ, et al. Does food supplementation really enhance productivity of breeding birds? Oecologia. 2010;164(2):311–320. doi: 10.1007/s00442-010-1645-x. [DOI] [PubMed] [Google Scholar]
- 36.Robertson CJR, Hyvonen P, Fraser MJ, Prichard CR. Atlas of Bird Distribution in New Zealand. Ornithological Society of New Zealand; Wellington, New Zealand: 2007. [Google Scholar]
- 37. Frost PGH (2013) Spotted dove. New Zealand Birds Online, ed Miskelly CM, Available at www.nzbirdsonline.org.nz. Accessed January 15, 2015.
- 38.Higgins PJ, Peter JM, Cowling SJ, editors. Handbook of Australian, New Zealand and Antarctic Birds. Volume 7, Boatbill to Starlings: Part 7B, Dunnock to Starlings. Oxford Univ Press; Melbourne: 2006. [Google Scholar]
- 39.Grarock K, Tidemann CR, Wood J, Lindenmayer DB. Is it benign or is it a Pariah? Empirical evidence for the impact of the common Myna (Acridotheres tristis) on Australian birds. PLoS ONE. 2012;7(7):e40622. doi: 10.1371/journal.pone.0040622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiley RH. Both high- and low-ranking white-throated sparrows find novel locations of food. Auk. 1991;108(1):8–15. [Google Scholar]
- 41.Bennett WA. Scale of investigation and the detection of competition: An example from the house sparrow and house finch introductions in North America. Am Nat. 1990;135(6):725–747. [Google Scholar]
- 42.Whittaker RJ, Willis KJ, Field R. Scale and species richness: Towards a general, hierarchical theory of species diversity. J Biogeogr. 2001;28(4):453–470. [Google Scholar]
- 43.Elliott GP, Wilson PR, Taylor RH, Beggs JR. Declines in common, widespread native birds in a mature temperate forest. Biol Conserv. 2010;143(9):2119–2126. [Google Scholar]
- 44.Betts MG, Nocera JJ, Hadley AS. Settlement in novel habitats induced by social information may disrupt community structure. Condor. 2010;112(2):265–273. [Google Scholar]
- 45.Higgins PJ, Peter JM, Cowling SJ, editors. Handbook of Australian, New Zealand and Antarctic Birds. Volume 7, Boatbill to Starlings: Part 7A, Boatbill to Larks. Oxford Univ Press; Melbourne: 2006. [Google Scholar]
- 46.Józkowicz A, Górska-Kłęk L. Activity patterns of the mute swans Cygnus olor wintering in rural and urban areas: A comparison. Acta Ornithol. 1996;31(1):45–51. [Google Scholar]
- 47.Ydenberg RC. The conflict between feeding and territorial defence in the great tit. Behav Ecol Sociobiol. 1984;15(2):103–108. [Google Scholar]
- 48.Tamm S. Breeding territory quality and agonistic behavior: Effects of energy availability and intruder pressure in hummingbirds. Behav Ecol Sociobiol. 1985;16(3):203–207. [Google Scholar]
- 49.Brittingham MC, Temple SA. Avian disease and winter bird feeding. The Passenger Pigeon. 1988;50(3):195–203. [Google Scholar]
- 50.Alley MR, et al. An epidemic of salmonellosis caused by Salmonella Typhimurium DT160 in wild birds and humans in New Zealand. N Z Vet J. 2002;50(5):170–176. doi: 10.1080/00480169.2002.36306. [DOI] [PubMed] [Google Scholar]
- 51.Lawson B, et al. Epidemiological evidence that garden birds are a source of human salmonellosis in England and Wales. PLoS ONE. 2014;9(2):e88968. doi: 10.1371/journal.pone.0088968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinson TJ, Flaspohler DJ. Winter bird feeding and localized predation on simulated bark-dwelling arthropods. Wildl Soc Bull. 2003;31(2):510–516. [Google Scholar]
- 53.Brittingham MC, Temple SA. Does winter bird feeding promote dependency? J Field Ornithol. 1992;63(2):190–194. [Google Scholar]
- 54.van Heezik Y, Seddon PJ. Accounting for detectability when estimating avian abundance in an urban area. N Z J Ecol. 2012;36(3):391–397. [Google Scholar]
- 55.Galbraith JA, Fraser EA, Clout MN, Hauber ME. Survey duration and season influence the detection of introduced eastern rosella (Platycercus eximius) in New Zealand. NZ J Zool. 2011;38(3):223–235. [Google Scholar]
- 56.MacLeod CJ, Greene T, MacKenzie DI, Allen RB. Monitoring widespread and common bird species on New Zealand’s conservation lands: A pilot study. N Z J Ecol. 2012;36(3):300–311. [Google Scholar]
- 57.Kruskal JB. Nonmetric multidimensional scaling: A numerical method. Psychometrika. 1964;29(2):115–129. [Google Scholar]
- 58.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26(1):32–46. [Google Scholar]
- 59.Anderson MJ. Distance-based tests for homogeneity of multivariate dispersions. Biometrics. 2006;62(1):245–253. doi: 10.1111/j.1541-0420.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- 60.Bray JR, Curtis JT. An ordination of upland forest communities of southern Wisconsin. Ecol Monogr. 1957;27:325–349. [Google Scholar]
- 61.McCune B, Grace JB. Analysis of Ecological Communities. MjM Software Design; Gleneden Beach, OR: 2002. [Google Scholar]
- 62.Oksanen J, et al. 2013 vegan: Community Ecology Package. R package version 2.0-10. Available at cran.r-project.org/web/packages/vegan/index.html. Accessed July 20, 2014.
- 63.Anderson MJ, Walsh DCI. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol Monogr. 2013;83(4):557–574. [Google Scholar]
- 64.McNaughton EJ. 2013. Supplementary Bird Feeding Practices in Urban Auckland and Patterns of Use by Common Myna (Acridotheres tristis). Bachelor of Science dissertation (University of Auckland, Auckland, New Zealand)
- 65.Bolker BM, et al. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol Evol. 2009;24(3):127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Venables WN, Ripley BD. Modern Applied Statistics with S. 4th Ed Springer; New York: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.