Significance
We report the chemical basis for a critical question in ocean science: how do single-celled algae, which are responsible for almost half of Earth's photosynthesis, sense their environment to respond appropriately to the lethal threat of predation? The increasing frequency of toxic algal blooms, with worldwide consequences to human health, fisheries, and marine ecosystem functioning, has garnered much attention in recent years, but it has remained unclear how algal toxicity is regulated. With the current paper, we show that substantial (20×) induction of toxicity occurs when one species of algae is exposed to a family of previously unknown chemical cues from predatory zooplankton (copepods). The copepodamides represent the first discovery, to our knowledge, of chemical cues mediating interactions between marine zooplankton and their prey.
Keywords: lipid signaling, Alexandrium, inducible defense, harmful algal bloom, paralytic shellfish toxin
Abstract
Interactions among microscopic planktonic organisms underpin the functioning of open ocean ecosystems. With few exceptions, these organisms lack advanced eyes and thus rely largely on chemical sensing to perceive their surroundings. However, few of the signaling molecules involved in interactions among marine plankton have been identified. We report a group of eight small molecules released by copepods, the most abundant zooplankton in the sea, which play a central role in food webs and biogeochemical cycles. The compounds, named copepodamides, are polar lipids connecting taurine via an amide to isoprenoid fatty acid conjugate of varying composition. The bloom-forming dinoflagellate Alexandrium minutum responds to pico- to nanomolar concentrations of copepodamides with up to a 20-fold increase in production of paralytic shellfish toxins. Different copepod species exude distinct copepodamide blends that contribute to the species-specific defensive responses observed in phytoplankton. The signaling system described here has far reaching implications for marine ecosystems by redirecting grazing pressure and facilitating the formation of large scale harmful algal blooms.
Phytoplankton form the base of marine food webs by contributing half of Earth’s carbon fixation (1, 2). Interactions between phytoplankton and their zooplankton grazers are critical for regulating oceanic nutrient cycles and productivity, yet we know little about the signaling mechanisms mediating these interactions. A striking example is the chemical cues from zooplankton that provoke pivotal changes in prey traits. The responding phytoplankton induce toxin production, life history transitions, colony size changes, and alternations in swimming behavior to evade predation (3–7). The presence of zooplankton consequently modulates marine food webs in unforeseen ways beyond the direct consumption of prey. These planktonic cues contribute to a complex chemical language whose words are still poorly understood by humans (7–9).
Here we explore the chemical interplay between zooplankton copepods and the harmful algal bloom-forming dinoflagellate Alexandrium minutum. Through unknown chemosensory mechanisms, toxin-producing members of the Alexandrium genus including A. minutum experience dramatically increased toxicity when exposed to grazing copepods or chemical signals from copepods (10). The more toxic cells are subsequently less susceptible to grazers, which leads to increased relative abundance of the defended species and thereby contributes to the formation of algal blooms (5). Alexandrium spp. cells produce a mixture of potent neurotoxic alkaloids of the saxitoxin family. These toxins accumulate in food webs and intoxicate zooplankton, bivalves, fish, seabirds, marine mammals, and humans via fish and shellfish consumption (11). We report the isolation and identification of the cueing compounds. In addition, we verify the emission of the compounds from living copepods and measure their presence in oceanic water in proportion to copepod densities.
Results
We followed the toxin-inducing activity in extracts of the potently inducing copepod Centropages typicus (10) through several steps of chemical separation, yielding an active fraction containing six polar lipids (later named copepodamides A–F) with molecular weights of 707–761 Da (Fig. 1 and Fig. S1). Yields of copepodamides were not sufficient for structure elucidation, but the same compounds were present in the commercially fished copepod Calanus finmarchicus, providing us with sufficient material for full structure elucidation and detailed dose–response experiments. Members of the genus Calanus are also known to induce toxin production in Alexandrium sp. (12).
Fig. 1.
Structures of copepodamides A–H (MW, molecular weight), with effective copepodamide concentrations for doubling of saxitoxins concentrations calculated from dose–response data after 2-d exposure of Alexandrium cells to each individual copepodamide (Fig. 2).
The copepodamides were each found to contain a fatty acyl group derived from ω-3, all-cis fatty acids docosahexaenoic acid 22:6Δ4,7,10,13,16,19 (DHA), eicosapentaenoic acid 20:5Δ5,8,11,14,17 (EPA), or stearidonic acid 18:4Δ6,9,12,15 (SDA; Fig. 1). Mass spectrometry (MS) and degradative chemical experiments revealed that the remaining part of the compounds consisted of one of two scaffolds represented by copepodamides G and H (Figs. S1 and S2). Copepodamides A–C each possess the scaffold of copepodamide G plus DHA, EPA, or SDA, respectively, whereas copepodamides D–F each share the scaffold of copepodamide H plus one of the same three fatty acyl groups. Targeted MS analysis revealed that copepodamides G and H were also present in Calanus extracts, and their greater quantities in frozen C. finmarchicus allowed structure elucidation of copepodamides G–H from purified material.
We established the lipid side chain of copepodamide H as a hydroxylated analog of the isoprenoid phytanic acid based on NMR and MS data, with a carbon-carbon double bond evident at C-3, confirmed by reductive ozonolysis (Fig. 1 and Figs. S3 and S4). Additional NMR data indicated hydroxyl groups at C-5 and C-15, with 1H and 13C NMR data for the distal portion of the isoprenoid portion matching closely those of a hydroxylated phytanic acid analog from a terrestrial plant (13) (Fig. S5). Copepodamide G differed from H by two atomic mass units (Fig. S2), with only slight differences in NMR spectral data indicative of an absence of the carbon-carbon double bond in copepodamide G (Fig. S6). Tandem MS analysis, chemical degradation, derivatization, and synthesis of an analog in the degradation pathway confirmed that the oxidation state of the C-3-C-17 bond represented the sole difference between copepodamides G and H (Figs. S7 and S8).
Dose–response experiments showed that purified copepodamides A–F induce toxin production in A. minutum at pico- to nanomolar concentrations (Fig. 2A). Copepodamides A–C each exhibited ∼10 times greater potency than copepodamides D–F, respectively, which differ only by one carbon-carbon single vs. double bond. The enhanced potencies of copepodamides A–C suggest that the electronic or steric properties of this portion of the molecule are crucial for toxin induction. In contrast, the identity of the fatty acyl group (DHA vs. EPA vs. SDA) appears to be functionally less important. Some kind of substituent attached via the oxygen at C-5 is, however, required for substantial toxin-inducing activity because copepodamides A–F exhibited 10- to 2,000-fold greater potency than copepodamides G–H, which lack a fatty acyl group at C-5 (Fig. 2A).
Fig. 2.
(A) Dose–response curves showing induction of toxicity following exposure of Alexandrium cells to individual, pure copepodamides A–H. Each symbol represents the mean of three independent replicates ± SE. Curves represent nonlinear least square fits to the Michaelis–Menten equation. At doses near 2,000 nM, copepodamide H was even less active than at 60–300 nM (n = 3 independent replicates per copepodamide per concentration; symbols represent mean values ± SE). (B) PCA representation of copepodamides A–F in whole body extracts of common marine copepods Calanus sp., Centropages typicus, and Pseudocalanus sp. (n = 4 extracts of 5–10 individuals for each species).
Comparison among copepod species showed that the most potent copepodamides A–C represent a substantial proportion (41 ± 1%) of the lipid blend of strong inducers such as Centropages typicus (10), whereas the weaker inducers Calanus sp. (12) and Pseudocalanus sp. (10) produce 13 ± 1% and 15 ± 1%, respectively, of copepodamides A–C.
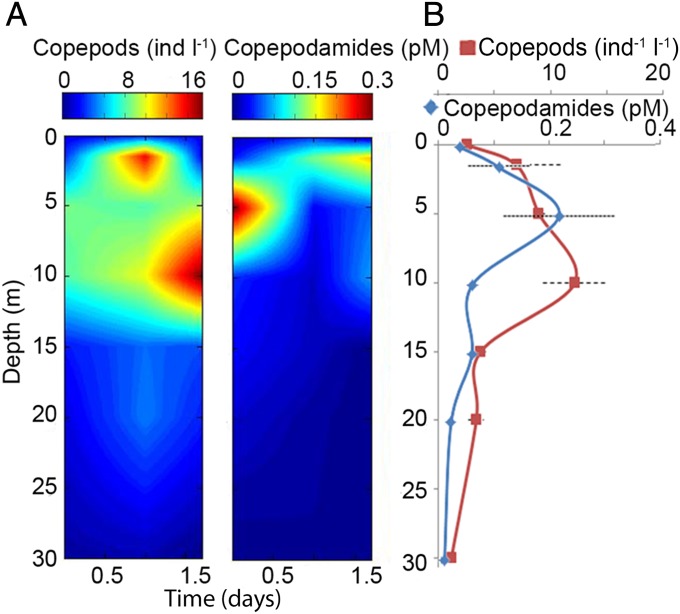
Measurements of natural exudation rates of toxin-inducing compounds from live, field-collected Calanus sp. showed a total exudation rate of copepodamides A–F of 120 pmol copepodamides/individual per day (Fig. 3). We were also able to measure copepodamides in field-collected seawater from seven distinct depths (0–30 m) at three times of day in early summer at one near-shore site in the Northeast Atlantic Ocean. Total copepododamide (A–F) concentrations ranged from below detection to 0.34 pM. Both copepodamide concentrations and copepod densities consistently showed subsurface maxima and small-scale patchiness (Fig. 4A). Averaged values for each depth over the sampling period are shown in Fig. 4B and show a stronger correlation between copepod density and copepodamides (r2 = 0.34).
Fig. 3.
Exudation rate of copepodamides into seawater by live, field-collected Calanus copepods. Each bar represents the mean of three experimental units of two to three copepods each + SE.
Fig. 4.
(A) Copepod density and copepodamide concentration interpolated from three replicate depth profiles (0–30 m depth) at the same location, obtained over a 1.5-d time period (time = 0 equals 1600 hours). Both copepods and copepodamides were sampled at seven depths (0, 1.5, 5, 10, 15, 20, and 30 m) for each profile. (B) Averaged values of copepod density and copepodamides over the 1.5-d time period for each depth ± SE of mean (n = 3, r2 = 0.34).
Discussion
This study introduces a family of chemical messengers that marine phytoplankton use to perceive lethal threats from zooplankton. The algae respond by triggering a metabolic cascade, leading to production of secondary metabolites to resist that threat. Copepodamides represent, to our knowledge, the first characterized chemical cues that mediate interactions between marine zooplankton and their prey. Other abundant and bloom-forming phytoplankton like the diatom Skeletonema marinoi and the prymnesiophyte Phaeocystis globosa also respond to unknown grazer cues by splitting colonies into smaller units that benefit from reduced losses to predators (3, 4), suggesting that the ability to respond adaptively to grazer cues may be a common feature in bloom-forming algae.
The copepod species tested here had specific blends of copepodamides A–F (Fig. 2B). A. minutum cells show species specific responses to copepod cues, where some copepods induce a several-fold increase in toxicity and others trigger weaker responses (10, 12). The most potent inducer, Centropages typicus, was confirmed to have the highest proportion of the more potent copepodamides A–C, whereas weaker inducers had approximately three times lower proportion of copepodamides A–C. Thus, the copepodamide fingerprint contributes to the response of A. minutum cells to different copepod species. In addition, differences in exudation rate between copepods may enhance or reduce the effect of copepodamide composition.
Natural copepod densities vary by several orders of magnitudes on both spatial and temporal scales (14), primarily due to seasonal patterns and diurnal vertical migration (15). Predator-induced traits evolve in response to such variable threats, if a reliable cue can be associated with predator presence (16). As shown in Fig. 4B, copepodamides provide such a proxy for copepod density at relevant spatial and temporal scales. The maximum total concentration of copepodamides A–F observed in the field measurements, 0.34 pM, is conservative as recovery of pure copepodamides in seawater from solid phase extraction columns at low picomolar concentrations was 40% or lower. Furthermore, copepod biomass on the sampling occasion was relatively low with <1–18 individuals/L and dominated by juvenile stages of the genera Acartia, Temora, Centropages, Oithona, and the harpactocoid Microsetella. The experiment shows the presence of copepodamides in natural seawater and its positive relation to copepod densities, but more detailed experiments on the concentration and turnover of copepodamides in the field are needed in the future. Standing stock of Calanus finmarchicus of 0.6–11 g dry mass/m2 have been reported in this area, peaking in late autumn as the most abundant copepod species (17). The corresponding daily exudation of copepodamides by Calanus (Fig. 3), corrected for the different potency of the emitted copepodamides (Fig. 2A), should alone be sufficient to double toxicity of A. minutum cells in a 7- to 140-m water column, showing that copepodamides are produced in relevant amounts in the field.
The copepodamides are the only defense elicitors isolated from copepods, but Yasumoto and colleagues (7) identified six short-chain aliphatic sulfates from the freshwater water fleas Daphnia pulex, which induce morphological changes that reduces susceptibility to predation in the green alga Scenedsmus (18). Apart from the presence of sulfur in both classes of lipids, there is little similarity between these aliphatic sulfates and the copepodamides. The presence of a taurine moiety attached to hydroxylated phytanic acid via an amide bond (Fig. 1A and Fig. S2) makes copepodamides members of a little-known class named taurolipids, previously only found in the protozoans Tetrahymena (19) and Euglena (20), as well as in marine sponges (21) and sea urchins (22). The function of copepodamides is not known, but taurolipids in protozoans have been suggested to act as emulsifiers in digestive vacuoles (23), and taurine-conjugated compounds are involved in digestion in many other animals (24). The exudation rate of copepodamides decreased rapidly (hours) when copepods were starved, consistent with a digestive role. The presence of other signaling lipids in aquatic microbes (9, 25) suggest that polar lipids may be an important group of chemical signals in the aquatic microenvironment.
Given that copepods constitute the most important link from primary producers to higher trophic levels in marine food webs, the ecological, environmental, and economic consequences of copepodamide signaling and the up to 20-fold induction in algal toxicity may be profound. Paralytic shellfish toxins are concentrated through marine food webs, reaching neurotoxic doses to many nontarget animals (26). The dramatic predator-induced change in phytoplankton toxin content shows that the chemical interplay between marine zooplankton, and their prey is key to understanding predator prey interactions and population dynamics in the pelagic ecosystem. The copepodamides reported here should provide a strong model system to experimentally pursue the role of chemical signals in the pelagic ecosystem.
Materials and Methods
Phytoplankton Culturing.
Alexandrium minutum GUMACC strain 83 was originally isolated from Ria de Vigo, Spain. The cultures were reinoculated (∼1:5) in freshly prepared K medium prepared with natural, pasteurized, filtered seawater at 33 psu salinity, under 16-h light/8-h dark cycles at 18 °C in a Termaks incubator.
Collection of Copepods.
Centropages typicus, hereafter Centropages, and Calanus sp. were collected outside Sven Loven Center for Marine Sciences, Kristineberg, Sweden, using a WP-3 or Mantatrawl plankton tow net with 450-µm mesh size. Pseudocalanus sp. was collected by bucket. Adult copepods were separated from smaller plankton by filtering with a submerged 200-µm plankton mesh, picked under microscope by glass pipette, and were either frozen, extracted, or used live in experiments within 1 h. Calanus finmarchicus (hereafter Calanus) used for bulk extraction was purchased frozen from Calanus AS.
Toxin Induction Assay.
A. minutum cells in exponential growth phase were diluted with freshly prepared K medium to a final concentration 10,000–20,000 cells/mL before addition to the experimental units of 1.0 mL each in 1.5-mL Eppendorf tubes or 5-mL glass test tubes. Extracts or pure compounds (n = 3 for each) were added in 5 µL DMSO for bioassay-guided fractionation experiments or coated onto the inside of the culture tubes by addition in methanol and evaporating the solvent under a stream of nitrogen before the culture was added for dose–response experiments. Both additions were run with the appropriate controls, i.e., DMSO without copepod compounds, or methanol evaporated on the walls of the culture vessels in triplicate. Samples were incubated for 48 h at the conditions described above. Cells were harvested by centrifugation at 12,000 × g for 5 min. The supernatant was gently removed by pipet and discarded; the cells pellet were frozen and then freeze-dried in a Heto LyoPro lyophilizer. Acetic acid (200 µl, 0.05 M) was added to each tube, which was subjected to three cycles of freezing and thawing to lyse cells and extract toxins (27). The lysed cells were then centrifuged at 4,000–12,000 × g for 5 min, and the supernatant containing dissolved toxins was transferred to an HPLC vial. The A. minutum isolate used produces gonyautoxins (GTXs) 1–4 (5), which are sulfated analogs of saxitoxin. Separation and analysis of GTXs was achieved by standard methods (28), with a Hitachi LaChrom l-7100 HPLC pump coupled to a post column reaction coil followed by an L-7485 fluorescence detector. Compounds were separated on a Genesis C8 silica, 4-μm, 150 × 3.0-mm column. Analysis of standard solutions of GTX 1–4 (CRMP-NRC) were interspersed with experimental samples, and peak areas were recorded for each GTX. Concentrations of GTXs 1–4 were summed to produce total GTX concentration, because the proportion of individual GTXs among experimental tubes did not vary.
Extraction and Bioassay-Guided Fractionation of Compounds from Centropages.
Live adult Centropages (∼800 individuals, males and females) were stored frozen until extraction, which was performed with 5 mL methanol three times. Methanol extracts were combined and fractionated by liquid–liquid partitioning between methanol/water (9:1) and hexanes (15 mL each). The methanol/water portion was adjusted to 3:2 by addition of water and partitioned with chloroform (24 mL), after which the methanol was removed from the methanol/water portion, more water was added (to 15 mL), and the residual aqueous extract was partitioned with ethyl acetate (15 mL). The chloroform-soluble extract was separated further by silica solid phase extraction (SPE; Isolute Si, 2 g; Sorbent AB), eluting with hexanes followed by hexane/ethyl acetate (1:1), ethyl acetate, ethyl acetate/methanol (1:1), methanol, and finally deionized water (each 15 mL). The ethyl acetate/methanol fraction was fractionated by size exclusion chromatography using an Sephadex LH-20 with ethyl acetate/methanol/water (20:5:2) at 0.2 mL/min. The active fraction, as determined using the toxin induction assay described above, was then further separated with an Agilent 1100 diode array UV HPLC with a Phenomenex Luna silica 3-µm column (150 mm length, 2 mm diameter) heated to 30 °C, eluting at 300 µL/min with a gradient from 100% ethyl acetate to 100% methanol. Fractions were collected according to UV absorption characteristics at 280 nm. Because the isolated yields of copepodamides were not sufficient to allow structural elucidation, we proceeded with isolation of these compounds from larger quantities of Calanus, described below.
Extraction and Isolation of Compounds from Calanus.
Freeze-dried Calanus (612 g dry mass) was extracted successively with methanol, chloroform, and hexanes. Crude extracts were combined and subjected to liquid–liquid partitioning using a scaled-up approach of that described for Centropages. Chloroform-soluble materials which induced Alexandrium toxicity were chromatographed on silica gel using gradient elution with hexanes and ethyl acetate. Active fractions were combined and separated by reversed phase SPE (ENVI-18, 10 g; Supelco) using a step gradient of aqueous methanol, followed by normal phase SPE (Si, 20 g; Isolute) eluting with hexanes and isopropanol. Copepodamides A–F were purified from the active fraction by reversed phase HPLC using an Agilent Zorbax SB-C18 column (5 μm, 250 mm length, 4.6 mm diameter) on a Hitachi LaChrom l-7100 HPLC pump coupled to a Hitachi LaChrom l-7455 diode array UV detector, with 10% (vol/vol) of column output split for time-of-flight (TOF) mass spectral analysis described below. The mobile phase was 88% (vol/vol) solution A (A = acetonitrile/methanol/water 40:45:15 with 0.2% formic acid, 0.1% ammonia, and 5 μM phosphoric acid) and 12% (vol/vol) solution B (B = isopropanol with 0.2% formic acid, 0.1% ammonia, and 5 μM phosphoric acid), at 1.0 mL/min. The optimal method for purifying copepodamides G and H differed from that of A–F: freeze-dried Calanus tissue (62.2 g) was extracted with methanol five times and extracts were separated by liquid–liquid partitioning with heptane/methanol (98:2) vs. methanol/water/aqueous ammonium hydroxide (94:5:1) (29). Copepodamides G and H were localized to the aqueous fraction, which was then subjected to reversed phase SPE (ENVI-18, 10 g; Supelco), eluting with a step gradient of aqueous methanol (30–100%). Copepodamides G and H eluting with 50% aqueous methanol were purified by reversed phase HPLC using the procedure as described for A–F above, except for the mobile phase, which was isocratic with 32% (vol/vol) aqueous acetonitrile (with 0.1% formic acid) performed at 1.0 mL/min. Plastic-derived phthalates were removed from purified copepodamides G and H by HPLC using a Waters 1525 binary HPLC pump coupled with a Waters 2487 dual wavelength detector at 210 and 254 nm [column: Grace Alltima C18 silica 5 μm, 250 mm length, 10 mm diameter; mobile phase: 32% (vol/vol) aqueous acetonitrile with 0.1% formic acid at 3.0 mL/min].
Structure Determination of Copepod Compounds.
The structures of copepodamides A–H were pursued by a combination of NMR spectroscopy, MS, and degradation experiments and verified by synthesis of a degradation analog.
Chemicals.
Phytanic acid was purchased from Lipidox. Chemicals including 9-decen-1-ol, methyl propiolate, 6-oxoheptanoic acid, BF3 diethyl etherate, pyridinium dichromate, butyllithium, methyllithium, 1,2-diheptadecanoyl-sn-glycero-3-phosphocholine, and N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) were purchased from Sigma-Aldrich. The methyl esters of DHA and EPA were obtained from Larodan, and SDA methyl ester was analyzed in a standard mixture (Marine PUFA III; Supelco). Methoxide solution used for alkaline transesterification was produced by dissolving metallic sodium (Sigma-Aldrich) in dry methanol.
NMR spectroscopy.
NMR spectra of copepodamides D, G, and H were acquired on a Bruker Avance 500 MHz spectrometer equipped with a 5-mm broadband or inverse detection probe or using a 900-MHz Agilent DD2 NMR spectrometer equipped with a 5-mm cold probe (Figs. S3, S5, and S6). Compounds were analyzed in DMSO-d6 (Cambridge Isotope Labs) using Shigemi tubes with spectra referenced to 2.49 and 39.5 ppm for 1H and 13C, respectively. 13C chemical shifts for copepodamide H were determined using a distortionless enhancement by polarization transfer (DEPT-135) experiment and heteronuclear multiple bond correlation (HMBC) data rather than 13C NMR spectroscopy due to low compound concentration. Assignment of 1H and 13C NMR signals for copepodamide H was aided by 1H-1H correlation spectroscopy (COSY), heteronuclear single quantum coherence (HSQC), HMBC experiments, and comparison of chemical shifts with literature values for a related compound (26).
MS.
Accurate mass spectra and MS-MS fragmentation data for copepodamides A–F were acquired on an Agilent Q-TOF 6430 mass spectrometer coupled to an Agilent 1100 series HPLC. The ion source (Agilent Jet Stream) was operated at 300 °C and 3,500 V with a nitrogen gas flow of 8 L/min at 35 psi. Fragmentor voltage was 150–176 V and collision energy was 30–50 V (negative mode) or 5–15 V (positive mode). Chromatography was achieved using an Agilent Poroshell EC-C18 column (2.7 μm; 2.1 × 150 mm, 0.25 mL/min) with either isocratic [32% (vol/vol) aqueous acetonitrile, 0.1% formic acid] or gradient [30–100% (vol/vol) acetonitrile, 0.1% formic acid] mobile phase.
Derivatization experiments.
Copepodamides D–F were subjected to alkaline or acidic transmethylation using freshly prepared 0.5 M sodium methoxide or 2.5% (vol/vol) H2SO4, respectively, in dry methanol (30). The resulting fatty acid methyl esters were analyzed by GC/MS on a 30-m HP-5ms column using an Agilent 7820 gas chromatograph connected to a 5975 mass selective detector. The oven temperature was raised by 4 °C/min from 80 °C to 300 °C, and injector and transfer line temperature was maintained at 250 °C. The identity of EPA, DHA, and SDA methyl esters was confirmed by retention time and mass spectrum in comparison with authentic methyl ester standards.
The structures of copepodamides G–H were confirmed by degradation chemistry and synthesis of an analog compound described in detail in Figs. S4, S7, and S8. GC/MS in these experiments was performed with a Hewlett-Packard model 5970B mass selective detector connected to a Hewlett-Packard model 5890 gas chromatograph equipped with a 12-m phenylmethylsilicone capillary column. Helium was used as the carrier gas. The oven temperature was raised from 120 °C to 320 °C at 10 °C/min.
Dose–Response Experiments.
The dose–response relationship for copepodamides A–H was measured using the toxin induction assay with each pure compound tested at six to eight concentrations in triplicate. The increase in toxicity (total concentration of GTXs 1–4 relative to controls) was plotted against the concentration of each copepodamide, and a nonlinear least-square line fit (nlinfit MATLAB) to the Michaelis–Menten equation (Eq. 1) was performed
| [1] |
where toxin content T is the concentration of GTXs in the experimental units as a function of C, the compound concentration (M), and the two constants Tmax, the maximum toxicity that can be provoked (relative to negative controls set at 0%), and Km, the half saturation constant (M). For each compound, the curve fit R2 was ≥ 0.9.
Quantification of Copepodamides A–H.
Because isolated yields of pure copepodamides A–F were low (∼1–20 μg of each compound from 612 g dry mass Calanus), their actual concentrations for dose–response measurement were determined by acidic transmethylation [2.5% (vol/vol) H2SO4 in methanol at 80 °C for 16 h] of copepodamides E–F together with an internal standard (1,2-diheptadecanoyl-sn-glycero-3-phosphocholine). The concentration of each resulting fatty acid methyl ester was determined by comparison with the methyl esters of the internal standard by GC/electron ionization (EI)-MS as described earlier. The remaining samples of intact copepodamides E–F, whose concentrations were now known, were subsequently used as analytical standards to determine the concentrations of A–F by LC/MS using an Agilent 1260 HPLC system coupled to an Agilent 6410 triple quadrupole detector equipped with an electrospray interface. The copepodamides were separated on an RP-MS Accucore 150 × 2.1-mm, 2.6-μm column (Thermo Scientific) thermostated to 50 °C using a gradient from methanol/acetonitrile/water (35:35:30, A) to 2-propanol (B) at a constant flow of 0.25 mL/min. Both solvents were supplemented with 0.2% formic acid, 0.1% ammonia, and 5 µM phosphoric acid. A 5-min isocratic elution (95% A) was followed by a linear increase of B to 95% (vol/vol) in 18 min followed by 5-min isocratic elution, reversal, and re-equilibration of the column for 6 min. The ion source was operated at 250 °C and 4,500 V in negative mode with a nitrogen gas flow of 11 L/min at 40 psi. A multiple reaction monitoring (MRM) method was optimized to increase sensitivity using the MassHunter optimizer software (diagnostic fragments: m/z 432.2 for A–C; 430.2 for D–F; 124.0 for G–H; fragmentor voltage 250 V; collision energy 44 for A–F and 40 for G–H). Quantification of G–H was achieved by removal of the taurine group from copepodamide H (at two concentrations) as described earlier together with an internal standard (1,2-diheptadecanoyl-sn-glycero-3-phosphocholine). The resulting lactones from copepodamide H and the internal standard were extracted by liquid–liquid partitioning with water and heptane followed by trimethylsilylation using N,O-bis(trimethylsilyl)trifluoroacetamide-pyridine 1:1 (vol/vol) and measurement by GC/EI-MS. The remaining sample of intact copepodamide H was subsequently used as an analytical standard to determine the concentrations of copepodamide G–H.
Exudation Rates from Live Copepods.
To test whether copepodamides are released into ambient seawater at relevant concentrations, exudates were collected from live, field-collected Calanus sp. copepods using a closed loop SPE device. Initial incubations lasted 16 h, but exudation rates decreased rapidly over time, probably as a result of starvation, and short-term incubations (≤30 min) of two to three copepods (n = 3 replicate incubations) were used to obtain relevant exudation rates. Copepods were carefully pipetted together with seawater into the reservoir of SPE columns (ENV+ Biotage 10–100 mg sorbent in 1- to 3-mL reservoirs). The incubation water was previously filtered (0.2-µm syringe filter VWR) and purified through the same type of SPE column to remove background concentrations of organic compounds and to simplify the sample matrix. Copepod-free incubations were included as controls (n = 2). The SPE column was connected to a peristaltic pump set to 0.75 mL/min. After 20- to 30-min incubation, the copepods were removed, the exact incubation time noted, and the columns desalted with 1 mL deionized water. The copepodamides were eluted with 1.5 mL methanol, concentrated by evaporating the methanol under a stream of nitrogen at 40 °C, and redissolved in 50 µL methanol for LC/MS analysis (Agilent 6410 triple quadrupole) as described above.
Field Sampling of Copepods and Copepodamides in Seawater.
To verify the presence of copepodamides in the field, we sampled seawater and zooplankton at different depths in the Skagerrak Strait of the Northeast Atlantic Ocean, outside Sven Lovén Centre for Marine Sciences, Tjärnö (N 58°52′934″, E 11°05′835″). Three replicate depth profiles were obtained at 1600 hours on June 16, 0500 hours on June 17, and 2300 hours on June 17, 2014. At each time point, a line with seven 100-mL syringes interspersed at 0, 1.5, 5, 10, 15, 20, and 30 m depth was deployed. The line was attached to a float and left drifting for ∼20 min. A concrete weight served to keep the line vertical and to pull the plunger of each syringe by gravitational force through connection to the line. Seawater at each depth drawn through the syringe passed through an SPE column (Isolute 100 mg ENV+). The procedure was repeated once to maximize the volume sampled at each depth (33–225 mL). Zooplankton in 4 L seawater were sampled at the same time with a Ruttner bottle at the same seven depths. Zooplankton were concentrated through a 25-µm mesh plankton net, transferred to 15-mL centrifuge tubes, and preserved with Lugols solution for counting using an inverted microscope at 40× magnification (Nikon Diaphot). Each SPE column was desalted with 1 mL deionized water, and copepodamides were eluted with 2 mL MeOH into glass vials. The extracts were dried under a stream of nitrogen gas at 50 °C, redissolved in 50 µL MeOH, and analyzed by LC-MS using the Agilent triple quadrupole system with standard copepodamides as described above.
Copepodamide Profiling of Copepod Species.
To compare the proportions of copepodamides among copepods species, we prepared four replicate methanol extracts of Centropages, Calanus, and Pseudocalanus for which 5–50 copepods of each species were transferred to individual glass vials, seawater was removed using a glass pipette, and the copepods were extracted in 1–5 mL methanol for >24 h. Copepodamides A–F were quantified in each extract using the LC/MS-MS method described above, and the proportions of A–F for each copepod extract were compared by principal component analysis (PCA) using SIMCA software (Umetrics).
Supplementary Material
Acknowledgments
Per Jonsson, Mark Hay, and Thomas Kiørboe contributed valuable insights to earlier drafts of the manuscript, and J. Glushka, T. Alexander, D. Brumley, and R. Poulin provided assistance with NMR spectroscopy. This work was supported by The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) Grants 21.0/2003-1122 and 217-2007-955 (to H.P.) and FORMAS Grant 223-2012-693 (to E.S.), with additional support from the Waernska Guest Professorship at University of Gothenburg, Royal Swedish Academy of Sciences, and US National Science Foundation Award OCE-1060300 (to J.K.); Swedish Research Council Grant 2011-5803 (to M.H.); a grant from the Olle Engkvist Byggmästare Foundation (to E.S. and M.X.A.); and the Centre for Marine Chemical Ecology at the University of Gothenburg.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420154112/-/DCSupplemental.
References
- 1.Behrenfeld MJ, Falkowski P. Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol Oceanogr. 1997;42(1):1–20. [Google Scholar]
- 2.Falkowski PG, Barber RT, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281(5374):200–207. doi: 10.1126/science.281.5374.200. [DOI] [PubMed] [Google Scholar]
- 3.Bergkvist J, Thor P, Jakobsen HH, Wängberg SA, Selander E. Grazer-induced chain length plasticity reduces grazing risk in a marine diatom. Limnol Oceanogr. 2012;57(1):318–324. [Google Scholar]
- 4.Long JD, Smalley GW, Barsby T, Anderson JT, Hay ME. Chemical cues induce consumer-specific defenses in a bloom-forming marine phytoplankton. Proc Natl Acad Sci USA. 2007;104(25):10512–10517. doi: 10.1073/pnas.0611600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selander E, Thor P, Toth GB, Pavia H. Copepods induce paralytic shellfish toxin production in marine dinoflagellates. Proc R Soc Lond Ser B-Biol Sci. 2006;273(1594):1673–1680. doi: 10.1098/rspb.2006.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selander E, Jakobsen HH, Lombard F, Kiørboe T. Grazer cues induce stealth behavior in marine dinoflagellates. Proc Natl Acad Sci USA. 2011;108(10):4030–4034. doi: 10.1073/pnas.1011870108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasumoto K, et al. Aliphatic sulfates released from Daphnia induce morphological defense of phytoplankton: Isolation and synthesis of kairomones. Tetrahedron Lett. 2005;46(28):4765–4767. [Google Scholar]
- 8.Gillard J, et al. Metabolomics enables the structure elucidation of a diatom sex pheromone. Angew Chem Int Ed Engl. 2013;52(3):854–857. doi: 10.1002/anie.201208175. [DOI] [PubMed] [Google Scholar]
- 9.Vardi A, et al. Viral glycosphingolipids induce lytic infection and cell death in marine phytoplankton. Science. 2009;326(5954):861–865. doi: 10.1126/science.1177322. [DOI] [PubMed] [Google Scholar]
- 10.Bergkvist J, Selander E, Pavia H. Induction of toxin production in dinoflagellates: The grazer makes a difference. Oecologia. 2008;156(1):147–154. doi: 10.1007/s00442-008-0981-6. [DOI] [PubMed] [Google Scholar]
- 11.Landsberg JH. The effects of harmful algal blooms on aquatic organisms. Rev Fish Sci. 2002;10(2):113–390. [Google Scholar]
- 12.Wohlrab S, Iversen MH, John U. A molecular and co-evolutionary context for grazer induced toxin production in Alexandrium tamarense. PLoS ONE. 2010;5(11):e15039. doi: 10.1371/journal.pone.0015039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feld H, Zapp J, Becker H. Secondary metabolites from the liverwort Tylimanthus renifolius. Phytochemistry. 2003;64(8):1335–1340. doi: 10.1016/j.phytochem.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Fransz HG, Colebrook JM, Gamble JC, Krause M. The zooplankton of the North-Sea. Neth J Sea Res. 1991;28(1-2):1–52. [Google Scholar]
- 15.Bollens SM, Frost BW. Predator induced diel vertical migration in a planktonic coepod. J Plankton Res. 1989;11(5):1047–1065. [Google Scholar]
- 16.Tollrian R, Harvell CD. The Ecology and Evolution of Inducible Defences. Princeton Univ Press; Princeton: 1999. [Google Scholar]
- 17.Bamstedt U. The macrozooplankton community of Kosterfjorden, western Sweden -abundance, biomass, and preliminary data on the life-cycles of dominant species. Sarsia. 1988;73(2):107–124. [Google Scholar]
- 18.Hessen DO, Van Donk E. Morphological-changes in Scenedesmus induced by substances released from Daphnia. Arch Hydrobiol. 1993;127(2):129–140. [Google Scholar]
- 19.Kaya K. Chemistry and biochemistry of taurolipids. Prog Lipid Res. 1992;31(1):87–108. doi: 10.1016/0163-7827(92)90017-d. [DOI] [PubMed] [Google Scholar]
- 20.Saidha T, Stern AI, Schiff JA. Taurine conjugates in the lipid fraction of euglena cells and their mitochondria. J Gen Microbiol. 1993;139(2):251–257. [Google Scholar]
- 21.Huang R, Peng Y, Zhou X, Yang X, Liu Y. A new taurine derivative from South China Sea marine sponge Axinella sp. Nat Prod Res. 2013;27(17):1537–1541. doi: 10.1080/14786419.2012.733389. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, et al. New N-acyl taurine from the sea urchin Glyptocidaris crenularis. Biosci Biotechnol Biochem. 2010;74(5):1089–1091. doi: 10.1271/bbb.90848. [DOI] [PubMed] [Google Scholar]
- 23.Kaya K, Keiko NU. Distribution of taurolipids in Tetrahymena cells. Biochim Biophys Acta. 1986;878(2):281–283. [Google Scholar]
- 24.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72(1):101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 25.Alegado RA, et al. A bacterial sulfonolipid triggers multicellular development in the closest living relatives of animals. eLife. 2012;1:e00013. doi: 10.7554/eLife.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson DM, White AW. Marine biotoxins at the top of the food-chain. Oceanus. 1992;35(3):55–61. [Google Scholar]
- 27.Flynn K, Flynn KJ. An automated HPLC method for the rapid analysis of paralytic shellfish toxins from dinoflagellates and bacteria using precolumn oxidation at low temperature. J Exp Mar Biol Ecol. 1996;197(1):145–157. [Google Scholar]
- 28.Asp TN, Larsen S, Aune T. Analysis of PSP toxins in Norwegian mussels by a post-column derivatization HPLC method. Toxicon. 2004;43(3):319–327. doi: 10.1016/j.toxicon.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Löfgren L, et al. The BUME method: A novel automated chloroform-free 96-well total lipid extraction method for blood plasma. J Lipid Res. 2012;53(8):1690–1700. doi: 10.1194/jlr.D023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christie WW. Lipid Analysis. Pergamont Press; Oxford: 1976. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.